Abstract
The impact of nonideal sorption on atrazine transport was investigated for two sandy porous media with 0.38 and 0.03% organic-carbon contents. In contrast to prior investigations, effluent atrazine concentrations were monitored over a range of five orders of magnitude to examine long-term elution behavior. As characterized by batch experiments, atrazine experienced nonlinear sorption for both media. The results of the column experiments showed that atrazine exhibited extensive elution tailing (delayed approach to relative concentration of zero). This non-ideal transport was more pronounced for the medium with higher organic-carbon content. A mathematical model incorporating nonlinear, rate-limited sorption/desorption described by a continuous distribution function was used to successfully simulate atrazine transport.
Keywords: sorption kinetics, continuous-distribution, transport modeling, pesticide
INTRODUCTION
Atrazine is a widely applied herbicide used to increase crop production by targeting pre-and post-emergent grassy weeds. The widespread national and global use of this pesticide has contributed to its prevalence as a contaminant of concern in drinking water and waterways. For example, atrazine was detected in all of the 146 water samples collected at 8 locations from the Mississippi, Missouri, and Ohio Rivers, with 27% of those samples containing atrazine concentrations above the EPA's maximum contaminant level (MCL) of 3 µg L−1 (USGS, 1991). It is the second most commonly found pesticide in domestic and rural water wells (U.S.NLM, 1995). With such widespread use and persistence in the environment, understanding its transport and fate behavior is of critical importance.
The sorption and transport behavior of atrazine has been investigated in numerous studies (e.g., Hayes, 1970; Rao et al., 1979; Nkedi-Kizza, et al, 1989; Brusseau and Rao, 1991; Brusseau, 1993; Gaber et al., 1995; Xing et al., 1996; Chen and Wagenet, 1997; Lechon et al., 1997; Sluszny et al., 1999; Mao and Ren, 2004; Sonon and Schwab, 2004; Pang et al., 2005; Montoya et al., 2006; Deng et al., 2008). However, potential low-concentration tailing phenomena, associated with nonlinear and/or rate-limited sorption/desorption processes, has not been examined for atrazine transport to a significant extent. Such tailing behavior has been typically observed for other organic compounds when analytical methods with sufficient detection limits have been employed, as shown by the results of aqueous miscible-displacement (e.g., Piatt and Brusseau, 1998; Johnson et al., 2003, 2009; Deng et al., 2008) and gas-purge (e.g., Werth et al., 1997; Lorden et al., 1998) experiments. The objective of this research is to investigate the impact of non-ideal sorption on atrazine transport, under the hypothesis that.nonlinear and rate-limited desorption will result in extensive elution tailing.
MATERIALS AND METHODS
Two natural sandy porous media were used for the experiments. Borden aquifer sediment was collected from the Canadian Air Force Base in Borden, Ontario. Eustis sub-soil was collected from The University of Florida, Gainesville, Florida. Properties of each media are listed in Table 1. Miscible-displacement experiments were conducted using analytical-grade atrazine [2-chloro-4-(ethylamine)-6-(isopropylamine)-s-triazine], which was dissolved in a background electrolyte solution of 0.02 M CaCl2. Pentafluorobenzoic acid in a 0.02 M CaCl2 solution was used as a non-reactive tracer to characterize the hydrodynamic properties of packed columns.
Table 1.
Porous media properties.
| Porous Media | Particle Size Distribution, % | Organic Carbon, % | ||
|---|---|---|---|---|
| Sand | Silt | Clay | ||
| Eustis | 95.6 | <1.0 | 4.4 | 0.38 |
| Borden | 96.2 | 2.0 | 1.8 | 0.03 |
Miscible-displacement experiments were conducted with stainless steel (Alltech) columns that were 7 cm long, with internal diameters of 2.1 cm. All tubing and fittings were stainless steel to minimize sorption to the experiment apparatus. The columns were packed uniformly with dry porous media, and then saturated with electrolyte solution. Two sets of experiments were conducted, using solutions with a lower and a higher atrazine concentration (Co) for injection. A constant flow rate of approximately 0.50 ml min−1, equivalent to a mean pore-water velocity (v) of 25 cm h−1, was used for all experiments. All injection pulses were approximately 30 pore volumes, after which the columns were flushed with electrolyte solution to effect elution. Replicate experiments were conducted for the Borden media at both initial concentrations, and for Eustis media at the higher injection concentration. To confirm that effluent concentrations reached the injection concentration (C/Co = 1), all experiments were stopped for a minimum of 12 hours between injection and elution to test for changes in concentrations (i.e., a flow interruption test, Brusseau et al., 1989). The results confirmed system equilibration for each experiment.
Atrazine was analyzed by ultraviolet-visible spectrophotometry (UV-Vis, lower quantifiable detection limit (QDL) = 0.05 mg L−1) for higher concentrations, and by enzyme-linked immunosorbent assay ((ELISA), QDL = 0.05 µg L−1)) for low concentrations. Recently, consumer-based ELISA kits have become available for atrazine analysis, including one manufactured by Abraxis™. This ELISA kit is based on the principles of competitive ELISA reactions to determine atrazine concentrations ranging from 0.05–5.0 µg L−1 (Abraxis, 2004). This kit has been verified for atrazine analysis under the EPA Environmental Testing Verification Program (EPA, 2004), and has received comparative evaluation (Graziano et al., 2006; Jiang et al., 2006). Use of the ELISA method provides a detection limit that is two to three orders of magnitude lower than those associated with standard UV-Vis or gas chromatography. An error analysis was conducted and it was determined that the analytical error associated with each data point is smaller than the size of the data-point symbols used in plotting the breakthrough curves.
Batch experiments were conducted with both the Eustis and Borden media to characterize atrazine sorption. Each soil was pulverized using a ball mill to eliminate aggregates and to reduce the time required to attain sorption equilibrium. The Eustis medium was combined with the solution aliquots at a 1:1 solids-to-solution ratio, while the Borden medium was combined at 2:1. The initial solution concentrations of atrazine ranged from 1.7 µg L−1 to 18.9 mg L−1. The experiments were conducted in triplicate for each of the five solution concentrations, with triplicate standards for each high and low dilution. Samples were rotary mixed for 72 hours, and then removed from the mixer and centrifuged at 2750 rpm for 1 hour. Batch experiments were analyzed by ELISA due to fine particles in the sample supernatant causing interference with analysis by UV-Vis; however, stock and standard solutions were crosschecked by UV-Vis.
Atrazine transport was simulated with a mathematical model that uses a probability density function to describe a log-normal distribution of sorption/desorption domains and their associated rate coefficients. The model was calibrated to the measured data to obtain best fits, with optimized values for the mean first-order sorption/desorption rate coefficient (mean ln-k2) and its variance ln (σ2ln-k2), and the fraction of instantaneous sorption/desorption. The model also includes nonlinear sorption/desorption. The governing equations are presented elsewhere (Li and Brusseau, 2000; Johnson et al., 2003).
RESULTS AND DISCUSSION
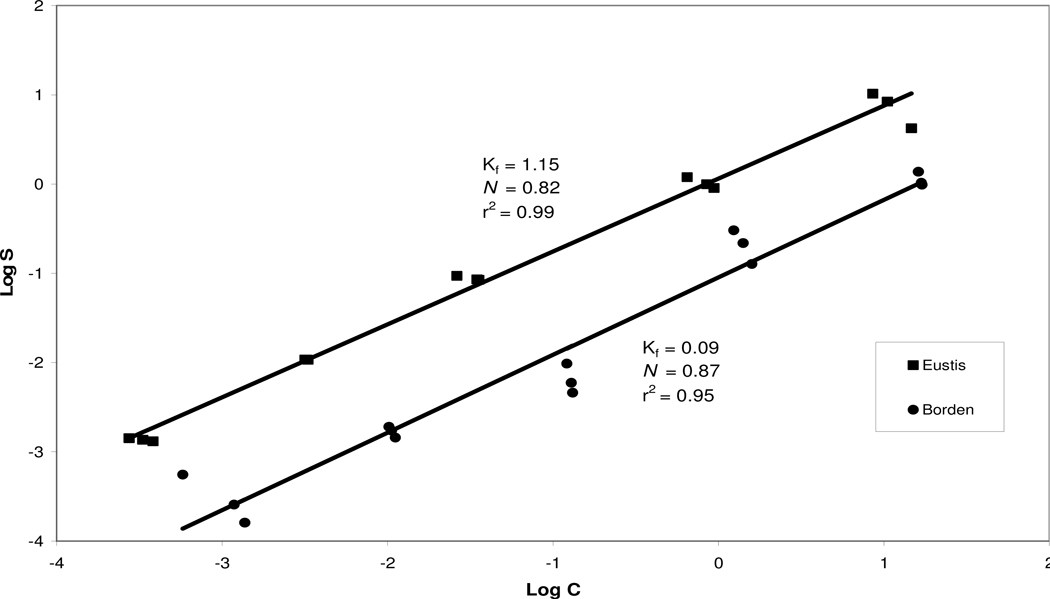
Results of the batch experiments show that atrazine exhibits nonlinear sorption for both Eustis and Borden media (Figure 1). The Freundlich coefficients were Kf = 1.15 (0.91–1.5, 95% confidence intervals) and N = 0.82 (0.77–0.87) for Eustis soil, and Kf = 0.09 (0.06–0.14), and N = 0.87 (0.76–0.98) for the Borden sediment (Table 2). These results indicate that atrazine sorption is greater for the Eustis soil, most likely due to the higher organic-carbon content.
Figure 1.
Isotherms for atrazine sorption for Eustis and Borden media.
Table 2.
Batch experiment results.
| Media | Kf | N | Regression r2 |
|---|---|---|---|
| Eustis | 1.15 (0.90–1.48) |
0.82 (0.77–0.87) |
0.99 |
| Borden | 0.09 (0.06–0.14) |
0.87 (0.76–0.98) |
0.95 |
95% confidence intervals are shown in ( )
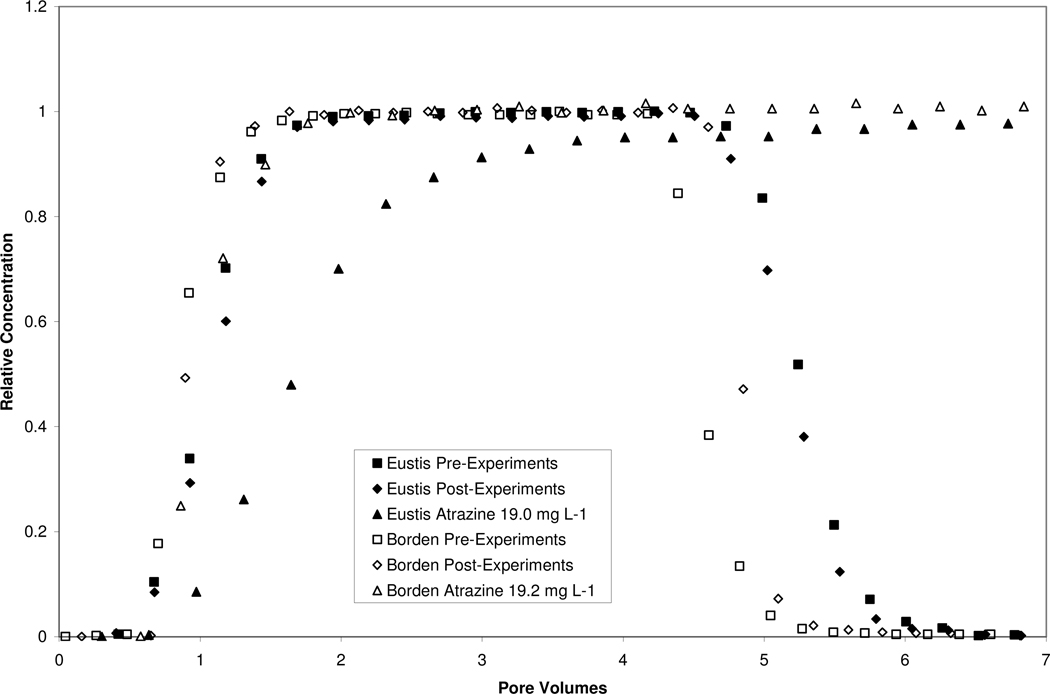
The breakthrough curves for the non-reactive tracer (PFBA) exhibit minimal spreading and tailing, indicating relatively ideal hydrodynamic transport conditions (Figure 2). Breakthrough curves from tracer tests conducted before and after the atrazine transport experiments are essentially identical. The atrazine breakthrough curves are delayed to varying degrees in relation to the PFBA curves, as illustrated in Figure 2. Mass recoveries for the atrazine experiments ranged from 96–105%, indicating no significant transformation or irreversible sorption effects for atrazine transport.
Figure 2.
Breakthrough curves for transport of Pentafluorobenzoic Acid (non-reactive tracer) in Eustis and Borden media. Selected breakthrough curves for atrazine are included for comparison.
The retardation factors for atrazine determined from moment analysis of the breakthrough curves are presented in Table 3. For the Eustis medium, the retardation factor determined for the experiment conducted with a lower injection concentration is larger than those obtained for the experiments conducted with a higher injection concentration. This is consistent with nonlinear sorption, supporting the batch experiment results. Such an analysis can not be done for the Borden data, given the uncertainty associated with the low retardation factors (1.02 and 1.04) obtained for the 1 mg L−1 experiments. Values for the sorption distribution coefficient back-calculated from the column retardation factors are presented in Table 3. Also presented are equivalent Kd values determined from the batch-experiment results by the following: Kd Batch = Kf C(N−1), where C is the injection concentration for the comparable column experiment. A comparison shows similar values were obtained with the two methods.
Table 3.
Summary of atrazine distribution coefficients from batch and column experiments.
| Media | Column Co(ppm) | Column Ra | Column Kd | Batch Kdb |
|---|---|---|---|---|
| Eustis | 0.9 | 7.51 | 1.37 | 1.17 (0.92–1.5) |
| Eustis | 18.7 | 3.83 | 0.6 | 0.68 (0.45–1.01) |
| Eustis | 19.0 | 3.52 | 0.53 | 0.68 (0.46–1.01) |
| Borden | 1.0 | 1.04 | nmc | 0.09 (0.06–0.14) |
| Borden | 1.1 | 1.02 | nmc | 0.09 (0.06–0.14) |
| Borden | 18.9 | 1.27 | 0.05 | 0.06 (0.03–0.13) |
| Borden | 19.2 | 1.46 | 0.08 | 0.06 (0.03–0.13) |
Retardation values determined via moment analysis
Equivalent Kd values are calculated as Kd Batch = Kf C(N-1), where C is the injection concentration for the column experiment used for comparison; 95% confidence intervals are shown in ( ).
Retardation factors are too low to reliably determine Kd values
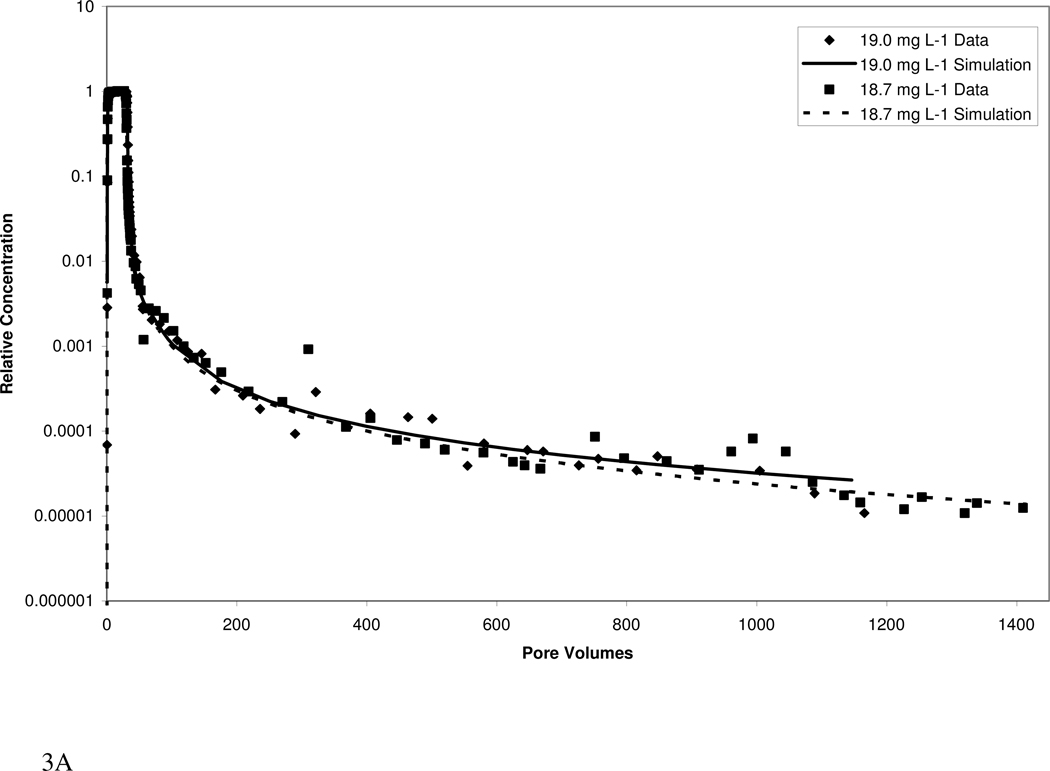
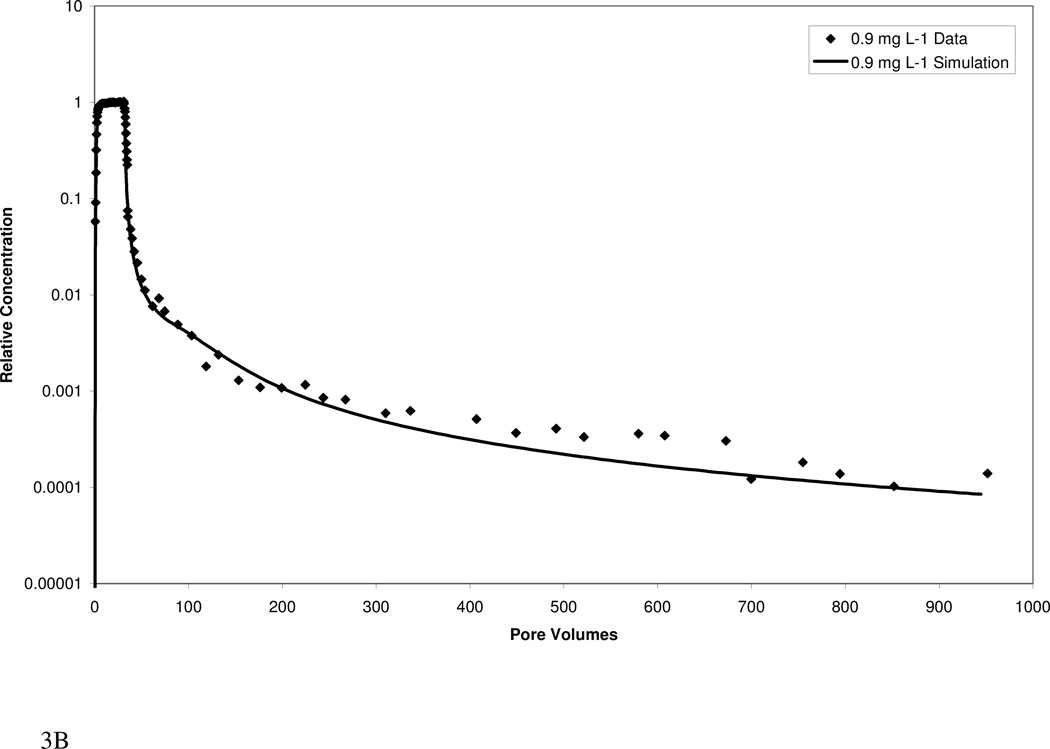
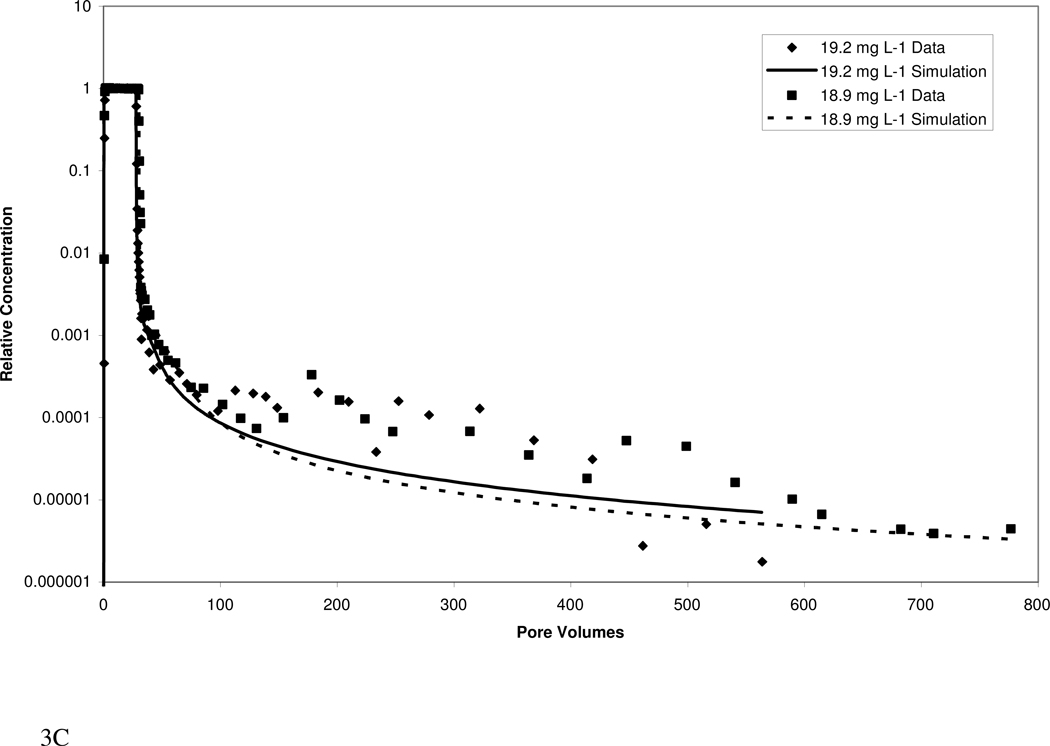
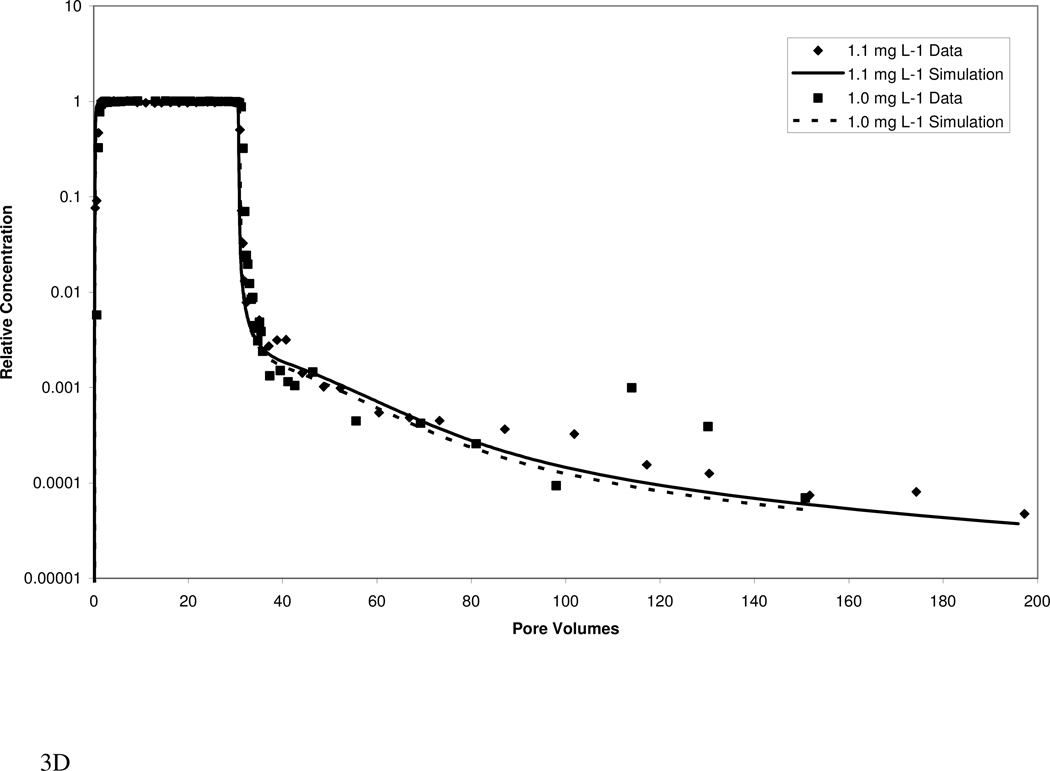
Atrazine transport in both media exhibited extensive elution tailing (Figure 3). Approximately 1000 pore volumes of water flushing were required to reach the QDL, despite the relatively low retardation factors. The elution tailing is more extensive for the higher organic-carbon content Eustis soil than for the Borden material. The extensive tailing observed herein would have largely been missed if analysis had been limited to standard methods.
Figure 3.
Measured and simulated breakthrough curves for atrazine transport in Eustis (A, B) and Borden (C, D) media at two injection concentrations. Simulations produced with a mathematical model employing a continuous distribution of sorption domains (represented by a log-normal probability density function) are shown.
The transport model incorporating a continuous-distribution function for sorption produced good simulations of the measured data, as illustrated in Figure 3. Calibrated parameters included mean ln-k2, ln-variance k2, and the fraction of instantaneous desorption. Values for the three parameters for Eustis were 0.10, 18, and 0.0 respectively, while values for Borden were 0.25, 29.5, and 0.25, respectively. For both media, the calibrated parameters were similar for the high and low injection-concentration experiments, supporting the robustness of the modeling. A comparison of model simulations including and excluding nonlinear sorption indicates that nonlinear sorption has a relatively minor impact on the observed elution tailing. Thus, the extensive tailing is attributed primarily to the impact of rate-limited desorption. A model incorporating the widely used two-domain or two-site description of sorption kinetics could not accurately simulate the measured data (data not shown).
SUMMARY
A series of column and batch experiments was conducted to characterize the sorption and transport of atrazine in two natural porous media. The improved detection limits associated with the ELISA method provided an opportunity to characterize tailing phenomenon, which appears to have not been done to such an extent in prior studies for atrazine. The results of the column experiments showed that atrazine exhibited extensive elution tailing. This non-ideal transport was more pronounced for the medium with a higher organic-carbon content. A mathematical model incorporating nonlinear, rate-limited sorption/desorption described by a continuous distribution function was used to successfully simulate the measured data. The extensive tailing was due primarily to rate-limited desorption, with a minor contribution from nonlinear sorption. Such behavior is expected to have an impact on persistence and long-term bioavailability of atrazine residues in soils and sediments.
ACKLOWLEDGEMENTS
This research was funded by The National Institute of Environmental Health Sciences Superfund Basic Research Program (ES04940).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- ABRAXIS. Abraxis atrazine kit 100T instructions manual. 2004 [Google Scholar]
- Brusseau M. Using QSAR to evaluate phenomenological models for sorption of organic compounds by soil. ENVIRON SCI TECHNOL. 1993:1835–1846. [Google Scholar]
- Brusseau M, Rao P. Influence of sorbate structure on nonequilibrium sorption of organic compounds. ENVIRON SCI TECHNOL. 1991:1501–1506. [Google Scholar]
- Brusseau M, Rao P, Jessup R, Davidson J. Flow interruption: A method for investigating sorption nonequilibrium. J CONTAM HYDROL. 1989:223–240. [Google Scholar]
- Chen W, Wagenet R. Description of atrazine transport in soil with heterogeneous nonequilibrium sorption. SOIL SCI SOC AM J. 1997:360–371. [Google Scholar]
- Deng J, Jiang X, Zhang X, Hua W, Crawford JW. Continuous time random walk model better describes the tailing of atrazine transport in soil. CHEMOSPHERE. 2008:2150–2157. doi: 10.1016/j.chemosphere.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gaber H, Inskeep W, Comfort S, Wraith J. Nonequilibrium transport of atrazine through large intact soil cores. SOIL SCI SOC AM J. 1995:60–67. [Google Scholar]
- Graziano N, McGuire M, Roberson A, Adams C, Jiang H, Blute N. 2004 National atrazine occurrence monitoring program using the Abraxis ELISA method. ENVIRON SCI TECHNOL. 2006:1163–1171. doi: 10.1021/es051586y. [DOI] [PubMed] [Google Scholar]
- Hayes M. Adsorption of triazine herbicides on soil organic matter, including a short review on soil organic matter chemistry. RESIDUE REV. 1970:131–174. doi: 10.1007/978-1-4615-8464-3_6. [DOI] [PubMed] [Google Scholar]
- Jiang H, Adams C, Graziano N, Roberson A, McGuire M, Khiari D. Enzyme-linked immunosorbent analysis (ELISA) of atrazine in raw and finished drinking water. ENVIRON ENG SCI. 2006:357–366. [Google Scholar]
- Johnson G, Zhang Z, Brusseau M. Characterizing and quantifying the impact of immiscible-liquid dissolution and nonlinear, rate-limited sorption/desorption on low-concentration tailing elution tailing. WATER RESOUR RES. 2003:1120–1127. [Google Scholar]
- Johnson G, Norris DK, Brusseau M. Mass removal and low-concentration tailing of trichloroethene in freshly-amended, synthetically-aged, and field-contaminated aquifer material. CHEMOSPHERE. 2009:542–548. doi: 10.1016/j.chemosphere.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechon Y, Garcia-Valcarcel A, Matienzo T, Sanchez-Brunete C, Tadeo J. Comparison of analytical procedures for determination of soil sorption coefficients of some triazine herbicides. COMMUN SOIL SCI PLAN. 1997:1835–1844. [Google Scholar]
- Li Z, Brusseau ML. Nonideal transport of reactive solutes in heterogeneous porous media 6. Microscopic and macroscopic approaches for incorporating heterogeneous rate-limited mass transfer. WATER RESOUR RES. 2000:2853–2867. [Google Scholar]
- Lorden SW, Chen WL, Lion LW. Experiments and modeling of the transport of trichloroethene vapor in unsaturated aquifer material. ENVIRON SCI TECHNOL. 1998:2009–2017. [Google Scholar]
- Mao M, Ren L. Simulating nonequilibrium transport of atrazine through saturated soil. GROUND WATER. 2004:500–508. doi: 10.1111/j.1745-6584.2004.tb02618.x. [DOI] [PubMed] [Google Scholar]
- Montoya J, Costa J, Liedl R, Bedmar F, Daniel P. Effects of soil type and tillage practice on atrazine transport through intact soil cores. GEODERMA. 2006:161–173. [Google Scholar]
- Nkedi-Kizza P, Brusseau M, Rao P, Hornsby A. Nonequilibrium sorption during displacement of hydrophobic organic chemicals and 45Ca through soil columns with aqueous and mixed solvents. ENVIRON SCI TECHNOL. 1989:814–820. [Google Scholar]
- Pang L, Close M, Flintoft M. Degradation and sorption of atrazine, hexazinone and procymidone in costal sand aquifer media. PEST MANAG SCI. 2005:133–143. doi: 10.1002/ps.952. [DOI] [PubMed] [Google Scholar]
- Piatt JJ, Brusseau ML. Rate-limited sorption of hydrophobic organic compounds by soils with well-characterized organic matter. ENVIRON SCI TECHNOL. 1998:1604–1608. [Google Scholar]
- Rao P, Davidson J, Jessup R, Selim H. Evaluation of conceptual models for describing nonequilibrium adsorption-desorption of pesticides during steady-flow in soils. Soil Science SOIL SCI SOC AM J. 1979:22–28. [Google Scholar]
- Sluszny C, Graber ER, Gerstl Z. Sorption of s-triazine herbicides in organic matter amended soils: fresh and incubated systems. WATER AIR SOIL POLL. 1999:395–410. [Google Scholar]
- Sonon L, Schwab A. Transport and persistence of nitrate, atrazine, and alachlor in large intact soil columns under two levels of moisture content. SOIL SCI. 2004:541–553. [Google Scholar]
- U.S. EPA. 2004 http://www.epa.gov/etv/verifications/vcenter-28.html.
- U.S. National Library of Medicine. Hazardous substances databank. Bethesda, Maryland: 1995. pp. 8–17. [Google Scholar]
- U.S. Department of Interior (US Geologic Survey) Spring sampling finds herbicides throughout Mississippi River and tributaries. Virginia: Reston; 1991. pp. 8–25. [Google Scholar]
- Werth CJ, Cunningham JA, Roberts PV, Reinhard M. Effects of grain-scale mass transfer on the transport of volatile organics through sediments: 2. Column results. WATER RESOUR RES. 1997:2727–2740. [Google Scholar]
- Xing B, Pignatello J, Gigliotti B. Competitive sorption between atrazine and other organic compounds in soils and model sorbents. ENVIRON SCI TECHNOL. 1996:2432–2440. [Google Scholar]