Abstract
The glycosides of flavonoid, anthocyanins and A type proanthocyanidins in cranberry concentrate were characterized and quantified using liquid chromatography–tandem mass spectrometry (LC–MS/MS). Cranberry concentrate (1 g/body weight) was orally gavaged to Fischer-344 rats (n = 6), and blood and urine samples were collected over 24 h periods. Quercetin, 3′-O-methylquercetin (isorhamnetin), myricetin, kaempferol, and proanthocyanidin dimer A2, together with thirteen conjugated metabolites of quercetin and methylquercetin and intact peonidin 3-O-galactoside and cyanidin 3-O-galactoside were identified in the rat urine after cranberry treatment. Very low levels of isorhamnetin (0.48 ± 0.09 ng/mL) and proanthocyanidin dimer A2 (0.541 ± 0.10 ng/mL) were found in plasma samples after 1 h of cranberry administration. Although no quercetin was detected in plasma, MRM analysis of the methanolic extract of urinary bladder showed that chronic administration of cranberry concentrate to rats resulted in accumulation of quercetin and isorhamnetin in the bladder. These results demonstrate that cranberry components undergo rapid metabolism and elimination into the urine of rats and are present in the urinary bladder tissue potentially allowing them to inhibit urinary bladder carcinogenesis.
Keywords: cranberry, metabolites, LC–MS/MS, analysis, rats, urine recovery
INTRODUCTION
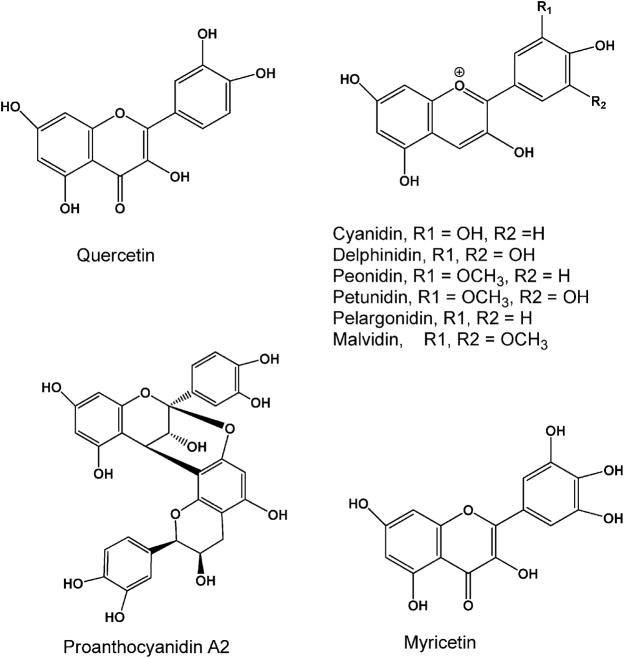
The fruit of the American cranberry (Vaccinium macrocarpon), a rich source of dietary flavonoids and anthocyanins, has attracted much public interest as a functional food, due to its reported ability to decrease Escherichia coli urinary tract infections (UTIs).1–5 Cranberry fruit has a diverse phytochemical profile of flavonols, anthocyanins, and proanthocyanidins (Figure 1), and cranberries are one of the leading fruit sources of quercetin on a weight basis.6,7 Several lines of evidence have also suggested antitumor activity of cranberry in breast, cervical and prostate cancer cell lines.8–11 Our preliminary data indicate that cranberry juice may be effective in preventing urinary bladder carcinogenesis,12 leading us to the hypothesis that cranberry juice is an effective chemopreventive agent for bladder cancer and its effect is due to the cranberry phytochemicals and their urinary metabolites stored in the bladder.
Figure 1.
Structures of major abundant flavonols, proanthocyanidins and anthocyanins in cranberry concentrate.
Given the increasing role of cranberry in prevention and treatment of UTI and cancers, there is a need for a comprehensive chemical analysis of all classes of compounds present in cranberry preparations, but the current incomplete compositional and bioavailablility information leaves considerable uncertainty as to the appropriate dietary intake levels and the interpretation of the effectiveness of this dietary supplement. This lack of information also confounds inferences about epidemiological relationships in health and disease. In addition to rigorously characterizing the plant product, it is essential to understand the dynamics of the compounds in the body and their route of excretion. Further data are needed to determine which cranberry metabolites are responsible for the inhibitory effects on urinary bladder carcinogenesis. To the best of our knowledge, there is no comprehensive assessment of the uptake and metabolism of cranberry components in rats. This study analyzed the major components of cranberry concentrate to provide a quantitative description of compounds present in standardized cranberry materials. It profiled phytochemicals available in cranberry concentrate and identified the metabolites of cranberry in the urine, plasma and urinary bladder of rats by using different modes of liquid chromatography tandem mass spectrometry (LC–MS/MS).
MATERIALS AND METHODS
Materials
Standards of quercetin, quercetin 3-O-galactoside, quercetin 3-O-rhamnoside, isorhamnetin, myricetin, kaempferol, peonidin 3-O-glucoside, kuromanin (cyanidin 3-O-glucoside) chloride, ideanin (cyanidin 3-O-galactoside) chloride and proanthocyanidin dimer A2 were purchased from Indofine Chemicals Inc. (Hillsborough, NJ, USA). Rat liver microsomes were obtained from BD Biosciences (Woburn, MD, USA). High performance liquid chromatography (HPLC) solvents (methanol, and acetonitrile) and reagents were purchased from Fisher (Norcross, GA, USA) and were of HPLC grade. Cranberry concentrate powder CranStar 90 was supplied by ShanStar Biotech, Inc. (Dunkirk, NY, USA) and stored at −20°C in light-tight containers until used.
Cranberry Powder Administration and Sample Collection
Female Fischer-344 rats were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN, USA). They were maintained in a controlled environment at 23°C and 55% relative humidity under a 12 h dark–light cycle, with free access to soy-free custom diet TD86369 (Harlan Teklad, Wisconsin, MD, USA) and tap water. All experimental procedures were conducted in accordance with Institutional Animal Care and Use Committee of the University of Alabama, Birmingham, and National Institutes of Health guidelines. After overnight (4 p.m. to 8 a.m.) fasting, cranberry concentrate powder in aqueous solution was administered by gavage (1 g/kg, body weight) to the rats (n = 6). The rats were treated for 10 months with cranberry extract via gavage (1 g/kg body weight, 1 gavage/day, five days a week). Blood and urine samples were collected at different time points (blood 1, 2, 3, 4 h, and urine 18, 25,42,48 h) after cranberry administration (1 gavage). At the end of the study, isoflurane anesthetized rats were euthanized by cervical dislocation. The urinary bladder was dissected after a 10 min perfusion with ice-cold normal saline and immediately frozen in liquid nitrogen.
To obtain total aglycon metabolites in plasma and urine, samples (100 μL) were hydrolyzed by incubating with β-glucuronidase/sulfatase from Helix pomatia (Sigma, St, Louis, MO, USA) at 37°C overnight to hydrolyze β-glucuronide and sulfate conjugates.13 Phenolphthalein β-glucuronide and 4-methylumbelliferone sulfate were added to monitor completeness of hydrolysis with β-glucuronidase/sulfatase. Hydrolyzed samples were extracted with diethyl ether, evaporated to dryness, and finally reconstituted in methanol–water (80:20 v/v) prior to LC–MS/MS analysis. For the analysis of conjugated metabolites of cranberry, a protein precipitation method was used by treating each sample with 5 volumes of methanol containing 0.1% acetic acid. Samples were centrifuged, and the supernatant directly transferred to an autosampler for LC–MS/MS analysis. Chrysin was used as an internal standard.
The urinary bladder tissue (0.05 g) was homogenized with liquid nitrogen chilled mortar and pestle and extracted by adding 1 mL of methanol–water (80:20 v/v) containing 0.1% acetic acid. The samples were vortex mixed, and the homogenate was centrifuged at 3000g for 10 min. The supernatant was removed, dried under air and reconstituted in 100 μL of methanol–water (80:20 v/v). The reconstituted solutions were transferred to HPLC auto sampler vials for LC–MS/MS analysis.
Glucuronidation of Quercetin and Isorhamnetin in Vitro
Rat microsomes were used to prepare glucuronidated derivatives of quercetin or isorhamnetin. 500 μg of rat liver total microsomal protein was reacted with 300 μmol/L quercetin or isorhamnetin, 5 μg/mL alamethicin, 4.5 mmol/L uridine 5′-diphosphoglucuronic acid (UDPGA), 10 mmol/L MgCl2, and 50 mmol/L Tris-HCl, pH 7.5. The reaction mixtures were incubated at 37°C for 4 h with gentle mixing and terminated by the addition of equal volumes of methanol followed by centrifugation (Eppendorf Centrifuge 5415D, Humberg, Germany) at 16100g at 4°C to pellet the microsomes. The reaction supernatants were transferred to autosampler vials and analyzed by LC–MS/MS.
Extraction of Anthocyanin Metabolites
A previously reported method was followed with minor modification to extract anthocyanin metabolites.14 Briefly, acidified urine samples (1/60 volume of 12 mol/L HCl) were extracted with a solid phase extraction (SPE) cartridge (Sep-Pak C18, waters, Milford, MA, USA). The cartridges were washed with methanol and equilibrated with 10 mL of 12 mmol/L HCl. After the acidified urine samples were loaded onto the cartridges, the cartridges were washed with acidified water and eluted with methanol containing 12 mmol/L HCl. The methanolic extract was evaporated to dryness, dissolved with 200 μL of 0.12 mol/L aqueous HCl and transferred to autosample vials for LC–MS/MS analysis.
LC–MS/MS Analyses
Cranberry powder (10 mg) was dissolved in water (20 mL), extracted with ethyl acetate (10 mL × 2) using a separating funnel. The ethyl acetate soluble fraction was evaporated to dryness and reconstituted with methanol:water (80:20). Profiling of cranberry components in water and ethyl acetate soluble fractions was performed using an Acquity uPLC HSST3 (100 × 2.1 mm), 1.8 μm column with the mobile phase consisting of a linear gradient of 10–100% aqueous methanol in 5% formic acid. The gradient started with 10% B and increased to 100% B over 5 min, and returned to the starting condition at 6 min. A 20 μL injection volume was used. Cranberry components were tentatively identified using different modes of LC–MS/MS. Quantification of known flavonols, anthocyanins and proanthocyanidin dimer A2 was performed by comparison with standard curves obtained using known concentrations (10–0.001 μg/mL) of standards: quercetin 3-O-galactoside, quercetin 3-O-rhamnoside, cyanidin 3-O-galactoside, peonidin 3-O-glucoside and proanthocyanidin A2.
LC–MS/MS analyses of rat urine, plasma and urinary bladder samples were performed using a system consisting of a model SIL-HT refrigerated Shimadzu autosampler, an HPLC instrument (Shimadzu Scientific Instruments, Inc. Columbia, MD, USA), and an API 4000 mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada). Chromatography was carried out on a reverse-phase Phenomenex Fusion C18 column (150 × 2.0 mm i.d., Torrance, CA, USA) with the mobile phase consisting of solvent A (0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid). A 15 min gradient was established running from 80 to 100% B over the first 6 min; this was maintained for a further 3 min, and the system was returned to the initial 5% B at 10 min.
The column effluent was introduced into the mass spectrometer using electrospray ionization (ESI) in the negative and positive ion modes. Nitrogen was used as nebulizer and curtain gas. The nebulizer current and temperature were 5 A and 250°C, respectively. The collision gas (N2) was set at high, and collision energy was 30 eV with electron multiplier voltage (1900 V). The following mass transitions were used for multiple reaction monitoring (MRM) analysis: quercetin m/z 301/151, isorhemnetin m/z 315/151, proanthocyanidin dimer A2 m/z 577/287, peonidine glucoside/galactoside m/z 463/301, cyanidin glucoside/galactoside m/z 449/287, quercetin glucoside/galactoside m/z 465/303, quercetin rhamnoside m/z 447/301, kaempferol m/z 285/185, myricetin m/z 317/179, myricetin hexoside m/z 491/317, methylquercetin glucuronide sulfate m/z 571/491, quercetin monoglucuronide m/z 477/301, methylquercetin sulfate m/z 395/315, quercetin sulfate m/z 381/301, quercetin diglucuronide m/z 653/477 and methylquercetin diglucuronide m/z 667/491. Isorhemnetin and proanthocyanin A2 were quantified by comparison with known standard curves. Calibration curves were prepared by spiking blank plasma with working solution to obtain final concentrations (10–0.001 μg/mL). The LC–MS/MS system was controlled by BioAnalyst 1.4.2 software.
RESULTS AND DISCUSSION
Profiling Components in Cranberry Concentrate
Since several components in the fruit juice maybe capable of producing complementary biological effects, the chemical composition of cranberry concentrate powder was characterized to better interpret the diversity of available phytochemicals. Quantitative analysis of the major components was carried out to identify a complete, quantitative description of standard cranberry materials. A high sensitive ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) method was developed for profiling constituents of cranberry. Initial MS/MS analyses were performed on standard compounds to develop characteristic product ion or neutral loss information for multiple reaction monitoring (MRM), precursor ion and neutral loss scans.
A combined analysis of neutral loss of 132 (pentosides), 146 (rhamnosides), 162 (hexosides, galactosides and glucosides), 294 (containing both pentosides and hexosides), precursor ion scans m/z 271 (pelargonidin), m/z 287 (cyanidin or proanthocyanidins), m/z 317 (petunidin), m/z 301 (peonidin) and MRM analyses in positive ion mode allowed for the detection and characterization of several flavonols and anthocyanin glycosides. A series of glycoside derivatives of quercetin, myricetin, peonidin, cyanidin, petunidin and malvidin were detected and tentatively characterized (Table 1). Quercetin 3-O-galactoside was the most abundant component in cranberry fruit powder. 15,16 Many isomeric components possibly differing in substitution of their rings with different groups such as sugars were observed. For example, three MRM peaks corresponding to quercetin pentose sugar with mass transition m/z 435/303 (positive ion mode) appeared at retention times 4.40, 4.47, and 4.59 min (Table 1). Based on previous report, these compounds are most likely to contain arabinose sugar linked to different positions of quercetin.16 Previous reports indicate that A type proanthocyanidins that have C2–O–C7 or C2–O–C5 linkages between monomeric units are present in cranberries.17 MRM analysis of ethyl acetate extract of cranberry showed the presence of proanthocyanidin dimer A2 m/z 577/287, and trimer m/z 865/577 (Table 1), and their structures were further confirmed by the interpretation of product ions obtained from LC–MS/MS analysis of m/z 577 and 865.
Table 1.
Profiling of Compounds in Cranberry Concentrate Using LC–MS/MS
| tR (min) | parent/product ion m/z | tentatively identified compounds |
|---|---|---|
| 2.80 | 865/577 | proanthocyanidin trimer |
| 3.44 | 449/287 | cyanidin 3-O-galactoside |
| 3.61 | 577/287 | proanthocyanidin dimer A2 |
| 3.64 | 419/287 | cyanidin arabinoside |
| 3.73 | 463/301 | peonidin 3-O-galactoside |
| 3.75 | 493/331 | malvidin hexoside |
| 3.89 | 433/301 | peonidin arabinoside |
| 3.95 | 463/331 | malvidin arabinoside |
| 4.01 | 627/303 | quercetin diglucoside |
| 4.03 | 481/319 | myricetin hexoside |
| 4.28 | 465/303 | quercetin 3-O-galactoside |
| 4.35 | 435/303 | quercetin pentoside |
| 4.40 | 435/303 | quercetin pentoside |
| 4.47 | 435/303 | quercetin pentoside |
| 4.59 | 479/317 | petunidin hexoside |
| 4.60 | 449/303 | quercetin 3-O-rhamnoside |
| 4.70 | 509/347 | dimethoxymyricetin hexoside |
| 4.55 | 479/317 | monomethoxyquercetin hexoside |
| 4.82 | 449/317 | petunidin arabinoside |
| 4.90 | 479/347 | monomethoxymyricetin pentoside |
| 5.01 | 303/153 | quercetin |
The water-soluble fraction of cranberry showed polar glycosides, mainly anthocyanin glycosides. Anthocyanins are responsible for the attractive red color of cranberry fruits and are typically observed in ESI-MS positive mode as protonated molecular ion [M + H]+. Using different modes of LC–MS/MS, eight anthocyanins were tentatively identified (Table 1). Six types of anthocyanins (cyanidin, peonidin, pelargonididn, malvididn, delphinidin and petunidin) have been reported in cranberry juice18. Identification of these components in cranberry is important since the specificity of the sugar moiety is reported to have a predominant role in the bioavailability.19,20 MRM based quantitative analysis revealed that the major components of cranberry are quercetin 3-O-galactoside (25 μg/mg), quercetin 3-O-rhamnoside (15.31 μg/mg), cyanidin 3-O-galactoside (5.02 μg/mg), peonidin 3-O-galactoside (2.16 μg/mg) and proanthocyanidin A2 (1.50 μg/mg). A very low level of aglycon quercetin (0.85 μg/mg) was also present in the cranberry fruit powder.
Plasma Metabolites
To determine identities and concentrations of cranberry metabolites in rat plasma following cranberry treatment, MRM analysis was performed after enzymatic hydrolysis. Very low concentrations of isorhamnetin and no quercein were detected in the plasma samples. The highest plasma concentration of isorhamnetin (0.48 ± 0.09 ng/mL) was detected 1 h (Figure 2) after cranberry administration. The area under the curve (AUC 0–4 h) of isorhamnetin in plasma was 0.92 ng · h/mL. This indicates its very low bioavailability in rats. Our data are consistent with a previous report that little or no quercetin is detectable in the plasma after the ingestion of either pure aglycon or grape juice.21 Poor bioavailability of quercetin (mostly in the glycoside form) from red wine has also been reported.22
Figure 2.
Isorhamnetin concentrations in rat plasma following cranberry administration (1 g/kg body weight). Values are mean ± SE, n = 4–5. *p < 0.05 compared to all other time points via one way ANOVA analysis.
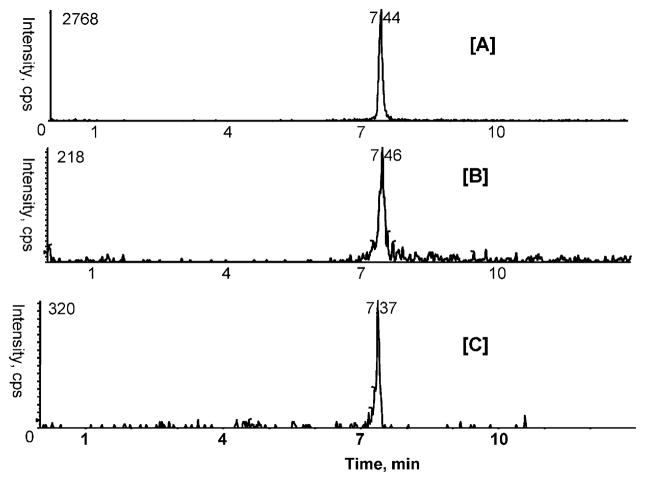
Since A type proanthocyanidins constitute one of the major phenolic components in cranberries and may have implications in UTIs and cancers, we examined whether the major proanthocyanidins such as proanthocyanidin dimers and trimers are absorbed and metabolized in vivo. MRM analysis of plasma samples was carried out to detect proanthocyanidins and anthocyanins after dosing. Intact proanthocyanidin dimer A2 was found in rat plasma with a concentration of 0.54 ± 0.10 ng/mL (Figure 3B). No anthocyanins were present in rat plasma. Since the absorption of intact anthocyanin glycosides from the gastrointestinal track is rapid, reaching the circulatory system within 30 min, it is possible that anthocyanins are already cleared from the plasma samples before the 1 h collection time point.23,24 These results are consistent with previous results.23
Figure 3.
Representative MRM ion chromatograms obtained from [A] standard proanthocyanidin dimer A2 (10 ng/mL); [B] and [C] detection of proanthocyanidin dimer A2 in plasma and urine after 1 h and over 14 h cranberry gavage to rats, respectively. Mass transition m/z 577/287 in positive ion mode was used.
Urinary Metabolites
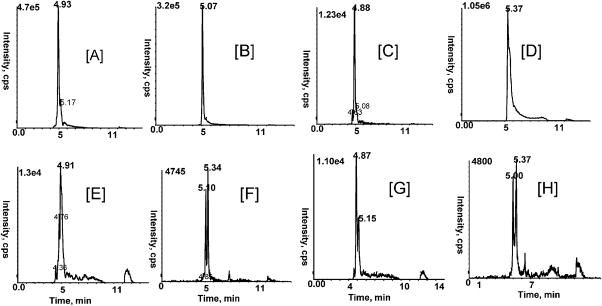
LC–MS/MS analysis of urinary metabolites of cranberry was performed using multiple MS/MS experiments (MRM and MS/MS). MRM analysis with the mass transitions m/z 317/179, 285/185, 301/151 and 315/151 (negative ion mode) indicated the presence of myricetin, kaempferol, quercetin, and methylquercetin, respectively, in the enzymatically hydrolyzed urine samples (data not shown). Previous studies have shown that, prior to reaching the systemic circulation, these flavonoids are metabolized by UDP-glucuronyltransferase, sulfotransferases, and methyl transferase to form several glucuronidated, sulfated, and methylated conjugates, respectively.25–28 Glucuronidation appears to be the major route for absorption and metabolism of flavonols such as quercetin, and they can be glucuronidated even before absorption.28 Therefore, we analyzed urine samples for aglycons as well as conjugated metabolites of flavonols using MRM. Following cranberry treatment, 13 conjugated metabolites (including isomers) of quercetin and methylquercetin were detected by MRM in rat urine. Two mono- and diglucuronides of quercetin and methylquercetin and two monosulfate conjugates of quercetin and three monosulfate conjugates of methylquercetin were detected (Figure 4E–H). Glucuronidated metabolites of quercetin and methylquercetin were identified by comparing MRM chromatograms with those of synthesized by rat liver microsomes in vitro (see below).
Figure 4.
MRM chromatograms showing the formation of glucuronide derivatives of quercetin and isorhamnetin. Synthesized glucuronides are shown in [A–D] and compared with urinary metabolites [E–H]. [A] diglucuronide of quercetin, [B] monoglucuronide of quercetin, [C] diglucuronide of isorhamnetin, [D] monoglucuronide of isorhamnetin. Mass transitions m/z 477/301 (monoglucuronide of quercetin), m/z 653/301 (diglucuronide of quercetin), m/z 491/315 (monoglucuronide of isorhamnetin) and m/z 667/491 (diglucuronide of isorhamnetin) were used.
Our previous studies showed the presence of proanthocyanidin dimer B2 in blood and urine in rats after oral administration of grape seed extract.13 In this study, MRM analysis of the urine samples collected after cranberry administration identified intact proanthocyanidin dimer A2. Unlike monomeric catechins, proanthocyanidins appeared to undergo no phase II metabolism and remain intact in both plasma and urine (Figure 3) 13,29. Very little is known about the uptake and metabolism of proanthocyanidins in rats and humans. Like monomeric flavanol, proanthocyanidins may undergo intestinal microbial metabolism.
Identification of Glucuronidated Metabolites
For the identification of urinary glucuronidated metabolites of quercetin and isorhamnetin, quercetin or isorhamnetin was incubated with rat liver microsomes and UDPGA. MRM analysis of the incubates with mass transitions m/z 477/301 and 653/301 and m/z 667/315 and 491/315 showed prominent peaks corresponding to mono- and diglucuronides of quercetin and isorhemnetin, respectively (Figure 4A–D). The monoglucuronidated metabolites formed in microsomal incubation were tentatively identified as 7-O-glucuronosyl derivatives of quercetin and isorhemnetin (Figure 4A–D) because they were the major glucuronide metabolites formed after incubation of quercetin or isorhemnetin with rat liver microsomes in the previous studies.30 The diglucuronidated metabolites appeared to be formed based on MRM analysis, but their structures could not be assigned in the present study. The MRM peaks corresponding to the urinary monoglucuronide metabolites as can be seen in Figure 4F,H closely resemble to those formed in microsomal incubations (Figure 4B, D), indicating them to be 7-O-glucuronosyl derivatives of quercetin and isorhemnetin.
Anthocyanin Metabolites in Urine
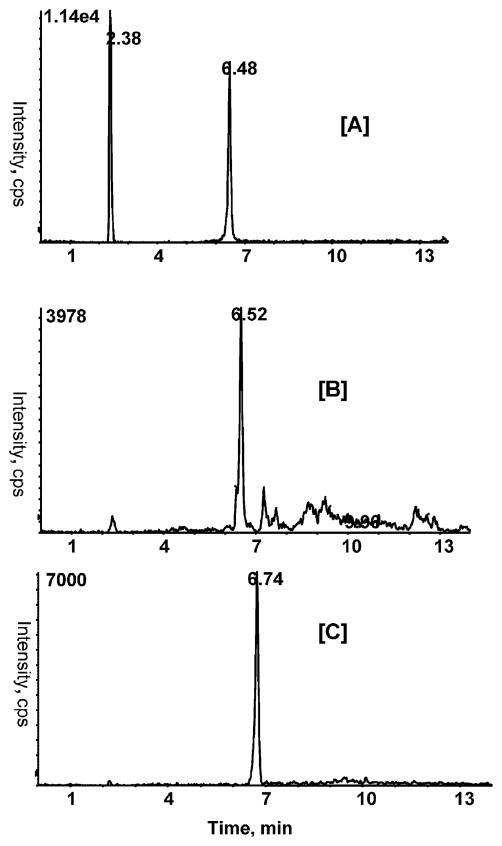
Compared to flavonols, anthocyanins are reactive compounds whose stability depends on pH, temperature, light, oxygen and enzymes.24 Human studies investigating the bioavailability of anthocyanins indicated that anthocyanin glycosides can be absorbed rapidly within 2 h of ingestion and are excreted in urine. The overall bioavailability of anthocyanins in both animals and humans is low.31 In our study, peonidin and cyanidin galactoside are the major anthocyanins present in cranberry powder given to animals. MRM analysis of extracted urine samples indicated the presence of unmetabolized peonidin 3-O-galactoside and cyanidin 3-O-galactoside (Figure 5). The urinary level of peonidin 3-O-galactoside was 0.13 ± 0.01 μg/mL. Cyanidin 3-O-galactoside was detected, but could not be quantified due to its very low concentration. These observations are consistent with the previous reports.32,33 Studies have shown that anthocyanin glycosides can be extensively metabolized to their corresponding phenolic acids and aldehydes through the cleavage of the C-ring by the gut microflora.34 The detection of only major anthocyanins in the urine may reflect their microbial metabolism and possible chemical degradation during sample preparation or thawing of frozen samples.14
Figure 5.
Representative MRM chromatogram in positive ion mode showing anthocyanins in rat urine following cranberry treatment. [A] Cyanidin 3-O-galactose standard (10 ng/mL); [B] and [C] corresponded cyanidin 3-O-glactoside and peonidin 3-O-glactoside, respectively, in urine. The urinary level of peonidin 3-O-galactoside was 0.13 ± 0.01 μg/mL. Mass transitions m/z 463/301 (peonidine 3-O-galacto-side/glucoside), m/z 449/287 (cyanidin galactoside/glucoside) were used.
Identification of Cranberry Metabolites in Rat Urinary Bladder
Urinary bladder samples were extracted (as described in Materials and Methods) and analyzed by MRM. Interestingly, the MRM analysis of the methanolic extracts of rat bladder chronically fed with cranberry showed the presence of unconjugated quercetin and isorhamnetin (Figure 6). Their structures of were further confirmed by comparing MRM with those of standards. These data indicate that chronic treatment of cranberry components concentrates in the urine and urinary bladder. Our previous studies on chemopreventive effects of cranberry have demonstrated that chronic administration of cranberry juice concentrate protects against chemically induced bladder cancer in rats.12 Cranberry metabolites are almost exclusively excreted and concentrated in the urine, and storage of urine containing cranberry metabolites in the bladder likely further enhances the exposure of bladder to these compounds. As a result, the bladder, especially the bladder epithelium that gives rise to the majority of bladder cancers, is likely the most exposed tissue to cranberry components and their metabolites in vivo. Therefore, urinary metabolites of cranberry may have role(s) in prevention against the progression of carcinogenesis.
Figure 6.
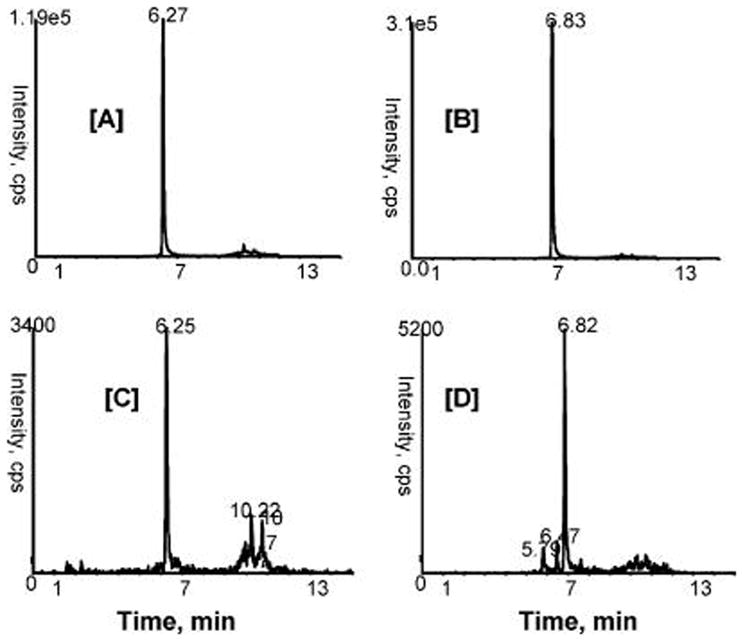
Representative MRM ion chromatograms in negative ion mode from [A] standard quercetin m/z 301/151 (100 ng/mL); [B] standard isorhemnetin m/z 315/151 (100 ng/mL); [C] and [D] detection of quercetin and isorhemnetin in the urinary bladder, respectively, following chronic cranberry administration to rats.
Taken together, we have profiled a wide range of cranberry components using a combination of the LC–MS/MS techniques. Our data demonstrate that unmodified proanthocyanidin dimer A2 can be absorbed and detected in plasma as early as 1 h after oral gavage of cranberry in rats. No quercetin or anthocyanins are detected in plasma, but they are detected in the urine. Among the anthocyanins identified, only peonidin 3-O-galactose and cyanidin 3-O-galactoside were found in the urine of cranberry treated rats, indicating the extensive metabolism of the anthocyanins after absorption or degradation of metabolites during sample preparation or digestion.
Acknowledgments
Funding Sources
These studies were supported by NIH (R21CA137519-01; J.K.P., principal investigator). Operation of the UAB Targeted Metabolomics and Proteomics Laboratory was supported in part by NCCAM P50 AT00477, NCI U54 CA100949, NIAMS P30 AR50948 and NIDDK P30 DK079337, and the in vivo work by NS57098 (J.M.W.). Funds for the mass spectrometer were provided by a grant from the UAB Health Services Foundation General Endowment Funded.
We are grateful to ShanStar Biotech Inc. (Dunkirk, NY) for providing cranberry fruit powder. Thanks are due to Dr. Kalpana Paudel (University of Kentucky) for her help in calculating AUC of isorhamnetin in plasma.
References
- 1.Howell AB, Foxman B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA, J Am Med Assoc. 2002;287:3082–3083. doi: 10.1001/jama.287.23.3082. [DOI] [PubMed] [Google Scholar]
- 2.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–1562. [PubMed] [Google Scholar]
- 3.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ [Br Med J] 2001;322:1571–1573. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobota AE. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol. 1984;131:1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Pinzón-Arango PA, Gallardo-Moreno AM, Camesano TA. Direct adhesion force measurements between E. coli and human uroepithelial cells in cranberry juice cocktail. Mol Nutr Food Res. 2010;54:1744–1752. doi: 10.1002/mnfr.200900535. [DOI] [PubMed] [Google Scholar]
- 6.Bilyk A, Sapers GM. Varietal differencies in the quercetin, kaempferol, and myricetin contents of high bush blueberry, cranberry, and thorn less blackberry fruits. J Agric Food Chem. 1986;34:585–588. [Google Scholar]
- 7.Hakkinen SH, Karenlampi SO, Heinonen M, Mykkanen HM, Torronen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47:2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 8.Seeram NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cells. J Agric Food Chem. 2004;52:2512–2517. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- 9.Neto C. Cranberry and Its Phytochemicals: A Review of In Vitro Anticancer Studies. J Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson P, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J Nutr. 2004;134:1529–1535. doi: 10.1093/jn/134.6.1529. [DOI] [PubMed] [Google Scholar]
- 11.Bomser J, Madhavi DL, Singletary K, Smith MA. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62:212–216. doi: 10.1055/s-2006-957862. [DOI] [PubMed] [Google Scholar]
- 12.Prasain JK, Jones K, Moore R, Barnes S, Leahy M, Roderick R, Juliana MM, Grubbs CJ. Effect of cranberry juice concentrate on chemically-induced urinary bladder cancers. Oncol Rep. 2008;19:1565–1570. [PMC free article] [PubMed] [Google Scholar]
- 13.Prasain JK, Peng N, Dai Y, Moore R, Arabshahi A, Wilson L, Barnes S, Wyss JM, Kim H, Watts RL. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felgines C, Talavéra S, Gonthier MP, Texier O, Scalbert A, Lamaison JL, Rémésy C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J Nutr. 2003;133:1296–1301. doi: 10.1093/jn/133.5.1296. [DOI] [PubMed] [Google Scholar]
- 15.Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2002;50:5844–5849. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]
- 16.Vvedenskaya IO, Rosen RT, Guido JE, Russell DJ, Mills KA, Vorsa N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J Agric Food Chem. 2004;52:188–195. doi: 10.1021/jf034970s. [DOI] [PubMed] [Google Scholar]
- 17.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 18.Ohnishi R, Ito H, Kasajima N, Kaneda M, Kariyama R, Kumon H, Hatano T, Yoshida T. Urinary excretion of anthocyanins in humans after cranberry juice ingestion. Biosci, Biotechnol Biochem. 2006;70:1681–1687. doi: 10.1271/bbb.60023. [DOI] [PubMed] [Google Scholar]
- 19.Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:52–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 20.Hollman PC, Buijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moeity is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radical Res Commun. 1999;31:569–573. doi: 10.1080/10715769900301141. [DOI] [PubMed] [Google Scholar]
- 21.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 22.de Vries JH, Hollman PC, van Amersfoort I, Olthof MR, Katan MB. Red wine is a poor source of bioavailable flavonols in men. J Nutr. 2001;131:745–748. doi: 10.1093/jn/131.3.745. [DOI] [PubMed] [Google Scholar]
- 23.Felgines C, Texier O, Besson C, Fraisse D, Lamaison JL, Rémésy C. Blackberry anthocyanins are slightly bioavailable in rats. J Nutr. 2002;132:1249–53. doi: 10.1093/jn/132.6.1249. [DOI] [PubMed] [Google Scholar]
- 24.McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 25.Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-β-d-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radical Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 26.Wittig J, Herderich M, Graefe EU, Veit M. Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr, B: Biomed Sci Appl. 2001;753:237–43. doi: 10.1016/s0378-4347(00)00549-1. [DOI] [PubMed] [Google Scholar]
- 27.Hong YJ, Mitchell AE. Metabolic profiling of flavonol metabolites in human urine by liquid chromatography and tandem mass spectrometry. J Agric Food Chem. 2004;52:6794–801. doi: 10.1021/jf040274w. [DOI] [PubMed] [Google Scholar]
- 28.Mullen W, Graf BA, Caldwell ST, Hartley RC, Duthie GG, Edwards CA, Lean ME, Crozier A. Determination of flavonol metabolites in plasma and tissues of rats by HPLC-radiocounting and tandem mass spectrometry following oral ingestion of [2-(14)C]quercetin-4′-glucoside. J Agric Food Chem. 2002;50:6902–6909. doi: 10.1021/jf020598p. [DOI] [PubMed] [Google Scholar]
- 29.Appeldoorn MM, Vincken JP, Gruppen H, Hollman PC. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J Nutr. 2009;139:1469–1473. doi: 10.3945/jn.109.106765. [DOI] [PubMed] [Google Scholar]
- 30.van derWoude H, Boersma MG, Vervoort J, Rietjens IM. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol. 2004;17:1520–1530. doi: 10.1021/tx049826v. [DOI] [PubMed] [Google Scholar]
- 31.Felgines C, Texier O, Besson C, Fraisse D, Lamaison JL, Rémésy C. Blackberry anthocyanins are slightly bioavailable in rats. J Nutr. 2002;132:1249–1253. doi: 10.1093/jn/132.6.1249. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J Agric Food Chem. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- 33.McGhie TK, Ainge GD, Barnett LE, Cooney JM, Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J Agric Food Chem. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- 34.Aura AM, Martin-Lopez P, O’Leary KA, Williamson G, Oksman-Caldentey KM, Poutanen K, Santos-Buelga C. In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr. 2005;44:133–142. doi: 10.1007/s00394-004-0502-2. [DOI] [PubMed] [Google Scholar]