Abstract
Malignant gliomas including glioblastoma multiforme (GBM) and anaplastic astrocytomas are the most common primary brain tumors. Despite multimodal treatment including surgery, chemotherapy and radiation, median survival for patients with GBMs is only 12–15 months. Identifying molecules critical for glioma progression is crucial for devising effective targeted therapy. In the present study, we investigated the potential contribution of Astrocyte Elevated Gene-1 (AEG-1) in gliomagenesis and explored the possibility of AEG-1 as a therapeutic target for malignant glioma. We analyzed the expression levels of AEG-1 in 9 normal brain tissues and 98 brain tumor patient samples by Western blot analysis and immunohistochemistry. AEG-1 expression was significantly elevated in > 90% of diverse human brain tumor samples including GBMs and astrocytic tumors, and also in human glioma cell lines as compared to normal brain tissues and normal astrocytes. Knockdown of AEG-1 by siRNA inhibited cell viability, cloning efficiency, invasive ability of U87 human glioma cells and 9L rat gliosarcoma cells. We also found that matrix metalloproteases (MMP-2 and MMP-9) are involved in AEG-1-mediated invasion of glioma cells. In an orthotopic nude mouse brain tumor model using primary human GBM12 tumor cells, AEG-1 siRNA significantly suppressed glioma cell growth in vivo. Taken together these provocative results indicate that AEG-1 may play a crucial role in the pathogenesis of glioma and that AEG-1 could represent a viable potential target for malignant glioma therapy.
Keywords: AEG-1, brain tumor, glioma, invasion, angiogenesis
Introduction
Globally, cancers of the brain and nervous system account for 1.7% of new disease, representing 189,000 cases annually and resulting in 142,000 deaths (1). More than one third of these cases are gliomas that arise from glial cells (1). Malignant gliomas, including glioblastoma multiforme (GBM) and anaplastic astrocytomas are the most common primary brain tumors in the United States (2). GBM is a highly aggressive and neurologically destructive tumor that frequently colonizes the cerebral hemispheres. Its ability to rapidly infiltrate the surrounding brain parenchyma contributes to its pathogenicity and resistance to conventional therapies. Glioma develops as a result of stepwise accumulations of multiple genetic alterations, which result in the activation of oncogenes and the inactivation of tumor suppressor genes (3). The differential expression of these critical genes and their downstream effectors enables cells to override growth controls and undergo tumorigenic conversion and progression. Although, significant progress has been made in understanding the molecular mechanisms involved in the genesis and progression of glioma, prognosis and treatment of this tumor type continue to be bleak (4–6). The current treatment for GBM includes a combination of debulking surgery when possible with chemotherapy and radiation therapy. Even with this multimodal regimen, prognosis for GBM patients remains poor with a mean survival of only 12–15 months (1, 7). Consequently, it is of great clinical value to identify specific molecular targets involved in glioma progression as well as novel therapeutic strategies that can exploit these altered pathways that regulate the development and evolution of this aggressive cancer.
Astrocyte elevated gene-1 (AEG-1), was originally cloned in our laboratory as a novel human immunodeficiency virus (HIV)-1- and tumor necrosis factor-α-inducible gene in primary human fetal astrocytes (PHFA) (8, 9). Mouse AEG-1 was cloned as a protein mediating metastasis of breast cancer cells to lung and was named metadherin (10). Mouse/rat AEG-1 was also cloned by gene-trapping techniques and was named 3D3/lyric (11). AEG-1 is a downstream target molecule of Ha-ras that mediates its tumor promoting effects (12). Over-expression of AEG-1 augments anchorage independent growth and invasion of HeLa, malignant glioma, prostate cancer, neuroblastoma and hepatocellular carcinoma cells (13–17). Conversely, inhibition of AEG-1 significantly inhibits these phenotypes in malignant glioma, neuroblastoma and prostate cancer cells and in vivo metastasis of breast cancer cells to lung (10, 15, 17, 18). Expression analysis revealed that AEG-1 expression is significantly elevated in melanoma, breast, and prostate cancer cells compared with their normal counterparts (9). Recent studies indicate that AEG-1 expression is elevated in some solid tumors including prostate, breast, esophageal cancer, hepaocellular carcinoma and neuroblastoma (15–17, 19, 20). AEG-1 is also over-expressed at both mRNA and protein levels in human glioma cell lines compared with PHFA (9). Additionally, AEG-1 expression is elevated in adult astrocytes displaying an aggressive glioma-like phenotype when injected into nude mice, resulting from sequential expression of SV40 T antigen, telomerase (hTERT), and T24 Ha-ras (9, 12).
We recently observed that AEG-1 is frequently over-expressed in human brain tumors (18). In the present study we show that the expression of AEG-1 is a common event in CNS tumor development. AEG-1 is upregulated in various human glioma cells and surgical specimens of human brain tumors of diverse origin including malignant glioma and anaplastic astrocytomas, and its expression is increased with the progression of malignant glioma. Interestingly, AEG-1 expression is more profound at the invasive margin of malignant glioma tumors and is associated with markers of invasion and angiogenesis such as matrix metalloproteases (MMP)-2 and 9 and CD31. Additionally, we provide in vivo evidence that inhibition of AEG-1 prevents growth of primary human GBM cells orthotopically implanted in athymic nude mice. In total, our results suggest that AEG-1 could be a potential therapeutic target for malignant glioma.
Materials and Methods
Cell lines, Cell Culture and Human Tissue Samples
Human malignant glioma cells U87, U251, U373, H4 and T98G cells were purchased from American Type Culture Collection (Manassas, VA). GBM-18 (G18) is an established cell line derived from a primary GBM patient sample (21). Human primary GBM 6 and 12 cells were previously described and provided by Dr. C. David James (University of California, San Francisco) (22). 9L is a rat gliosarcoma cell (23). Cells were cultured in Dulbecco's modified Eagle's essential medium + F12 (DMEM+F12) supplemented with 10% FCS at 37°C incubator supplemented with 5% CO2. Normal primary human fetal astrocyte cells, PHFA, have been described previously (9). Archival frozen human tissue samples, including 25 GBMs, eighteen astrocytomas, eighteen meningiomas, nineteen oligodendrogliomas and oligoastrocytomas, eighteen other brain tumor samples, and nine samples of morphologically normal brain tissue, were obtained from the Department of Neurological Surgery, Columbia University. The use of these archival tissues in this study was approved by the Institutional Review Board of Columbia University.
Immunostaining
Frozen human tissue samples fixed in formalin, embedded in paraffin, sectioned and mounted on glass slides. The sections were deparaffinized and were permeabilized with 0.1% Triton X-100 in PBS for 30 minutes. Sections were then blocked for 1 hour at room temperature with 5% goat serum and 1% BSA in PBS and incubated with anti-AEG-1, anti-Ki67 and anti-MMP-9 antibodies overnight at 4°C. Sections were then rinsed in PBS and incubated with either Alexa Fluor 488 or Alexa Fluor 546–conjugated anti-chicken antibodies for detection of AEG-1. Ki-67 was detected with Alexa Fluor 546–conjugated anti-rabbit antibodies and MMP-9 was detected with Alexa Fluor 488-conjugated anti-goat IgG (Molecular Probes; Invitrogen). The sections were mounted in VECTASHIELD Fluorescence Mounting Medium containing DAPI (Vector Laboratories). Images were analyzed using an Olympus immunofluorescence microscope. AEG-1, MMP-2 and CD31 were also detected in the sections of normal brain and brain tumor samples by avidin-biotin-peroxidase complexes with DAB (brown/purple) substrate solution (Vector Laboratories). For tissue microarray (CS17-01-001, Cybrdi, Bethesda, MD), the slides were processed according to the manufacturer's instructions and labeled with AEG-1 antibody as described above. Alexa Fluor 488–conjugated anti-chicken antibody was used for detection of AEG-1. The primary antibodies used were anti-AEG1 (1:500, chicken polyclonal, used in single and double immunofluorescence labeling), anti-AEG-1 (1:200, rabbit polyclonal; Zymed, used in single IHC staining), anti–ki67 (1:100, rabbit polyclonal; Abcam), anti-MMP-2 (1:100, rabbit polyclonal; Abcam), anti-MMP-9 (1:200, goat polyclonal Santa Cruz Biotechnology Inc.), and anti-CD31 (1:100, mouse monoclonal; Vector Laboratories).
Preparation of whole cell lysates and Western Blot analysis
Preparation of whole cell lysates and Western Blot analysis was performed as described (13). The antibodies used were anti-AEG-1 (1:1000, chicken polyclonal) (9) and anti-EF1α (1:1000), Upstate Biotechnology, Lake Placid, NY). For densitometric evaluation X-ray films were scanned and analyzed with ImageJ software (NIH).
siRNA and lentivirus construction
AEG-1 siRNA (12) was generated by using the Silencer siRNA Construction kit (Ambion, Austin, TX) according to the manufacturer's instructions. The 19-bp AEG-1 sequence used to generate AEG-1 shRNA is 5' CAGAAGAAGAAGAACCGGA 3'. Detailed description of lentivirus vector production is described previously (24).
Anchorage-independent growth assay in soft agar
Anchorage-independent growth assay was performed by seeding 1 × 105 cells in 0.4% Noble agar on a 0.8% agar base layer with growth medium. Colonies were counted 2 weeks after seeding, and the data from triplicate determinants were expressed as mean ± SEM.
Colony formation in monolayer
9L rat gliosarcoma cells were plated at a density of 1 × 106 cells per 6-cm dish, and 1 day later were transfected with 50 nM of control or AEG-1 siRNA. After 2 days, the cells were trypsinized and counted, and 200 cells were plated in 6-cm dishes. Colonies of >50 cells were scored after 14 days.
Invasion assay
Invasion was measured by using 24-well BioCoat cell culture inserts (BD) with an 8-μ-porosity polyethylene terephthalate membrane coated with Matrigel Basement Membrane Matrix (100 μg/cm2; BD). In brief, the Matrigel was allowed to rehydrate for 2 hours at room temperature by addition of warm, serum-free DMEM. The wells of the lower chamber were filled with medium containing 5% FBS. Cells (5 × 104) were seeded in the upper compartment (6.25-mm membrane size) in serum-free medium. The invasion assay was performed at 37°C in a 5% CO2 humidified incubator for 22 hours. At the end of the invasion assay, filters were removed, fixed, and stained with the Diff-Quick Staining Kit (IMEB). Cells on the upper surface of the filters were removed by wiping with a cotton swab, and invasion was determined by counting the cells that migrated to the lower side of the filter using a microscope at 100× magnification.
Gelatin Zymography
MMP activities were determined by gelatin zymography. Fifty micrograms of protein were incubated at 37°C for 30 min in SDS sample buffer without reducing agent and then electrophoresed on a 9% SDS-polyacrylamide gel containing 0.2% gelatin at 4°C (25). After electrophoresis, the gel was washed in 2.5% Triton X-100 for 1 hour at room temperature to remove SDS, and then incubated for 24 hours at 37°C in 50 mmol/L Tris-HCl (pH 7.5) containing 0.15 mM NaCl, 10 mM CaCl2, and 0.02% NaN3. The gel was stained with 0.5% Coomassie brilliant blue R250, destained with 30% methanol and 10% acetic acid in water and photographed.
Transient Transfection and Luciferase Assays
A total of 1 × 105 9L cells were seeded per well in 24-well plates and transfected with 1 μg of total DNA (either pGL3-Basic, MMP-2 or MMP-9 and β-galactosidase) by using LipofectAMINE 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Luciferase assays were performed as described (13). Luciferase activity was normalized by β-galactosidase activity and the data presented are the fold activation relative to pGL3-Basic. Data presented as mean ± SD from three independent experiments performed in duplicate or triplicate.
Intracranial implant of GBM 12 cells in nude mice
Athymic female NCr-nu/nu mice (NCI-Fredrick) (n=5 for each group) weighing ~20 g, were used for this study. Mice were maintained under pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U.S. Department of Agriculture, Washington, DC, the U.S. Department of Health and Human Services, Washington, DC, and the National Institutes of Health, Bethesda, MD. GBM12 glioma cell lines were originally derived from patients at the Mayo Clinic (Rochester, MN) (22). GBM12 cells were cultured in DMEM supplemented with 5% (v/v) fetal calf serum and 100 μg/ml (1% v/v) penicillin-streptomycin. Cells were incubated in a humidified atmosphere of 5% (v/v) CO2 at 37°C. Mice were anesthetized via i.p. administration of (ketamine, 40 mg/kg; xylazine, 3 mg/kg) and immobilized in a stereotactic frame (KOPF). A 24-gauge needle attached to a Hamilton syringe was inserted into the right basal ganglia to a depth of 3.5-mm and then withdrawn 0.5-mm to make space for tumor cell accumulation. The entry point at the skull was 2-mm lateral and 1-mm dorsal to the bregma. Intra-cerebral injection of 0.5 × 106 GBM12 glioma cells infected with lenti virus expressing either control siRNA or AEG-1 siRNA (~10 mice per treatment group) in 2 μl of DMEM medium was performed over 10 min. The skull opening was enclosed with sterile bone wax and the skin incision was closed using sterile surgical staples.
Luciferase imaging of GBM12-luciferase cells implanted in athymic mice brains
GBM12 cells were stably transfected to express luciferase and were kindly provided by Dr. C. David James (University of California, San Francisco). Five hundred thousand GBM12-luciferase (LUC) cells were implanted into every athymic mouse brain. Mice were scanned at day 21, 28, and 42 after implantation using an Olympus OLY-750 color CCD camera with imaging software.
Statistical analysis
All of the experiments were performed at least three times. The results are expressed as mean ± SD. Statistical comparisons were made using an unpaired two-tailed Student t test and analysis of variance. Differences with a P-value of <0.05 were considered statistically significant.
Results
AEG-1 is highly expressed in glioma cell lines and patient-derived brain tumor samples, including GBMs
To analyze expression of AEG-1 in brain tumor cell lines, Western Blot analysis was performed in a panel of glioma cell lines. AEG-1 was highly expressed in the established glioma cell lines U87, U251, U373, H4 and T98G, and also in two primary GBM cell lines (GBM6 and GBM12) as compared to PHFA (Fig. 1A). To determine the expression pattern of AEG-1 in primary brain tumors, protein was isolated from quick-frozen brain samples obtained at initial surgery from 107 patients, including 9 normal, 25 with GBM, 18 with astrocytomas, 18 with meningiomas, 19 with oligodendrogliomas, and 18 with other types of brain tumors. The relative level of AEG-1 expression was quantified by Western Blot Analysis and was expressed as a ratio between AEG-1 and the loading control protein EF1α (12–14) to correct for variation in the amount of protein. The expression level of AEG-1 protein present in brain tumors varied but was increased (3- to 10-fold as compared to normal brain) in >90% of the brain tumors with the highest median observed in GBM tissues (Fig. 1B and C). Immunohistochemical staining was used to evaluate AEG-1 expression in formalin-fixed paraffin embedded sections of normal brain and various brain tumors. As shown in Fig. 1D, the normal brain tissue was negative for AEG-1 except for sparse cytoplasmic staining in a few glial cells and neurons. In contrast, strong staining for AEG-1 occurred in the malignant glioma cells and also in tumor cells in recurrent meningioma, ependydoma, and others (Fig. 1D). These results indicate that AEG-1 expression is notably increased in diverse brain tumors including gliomas.
Figure 1.
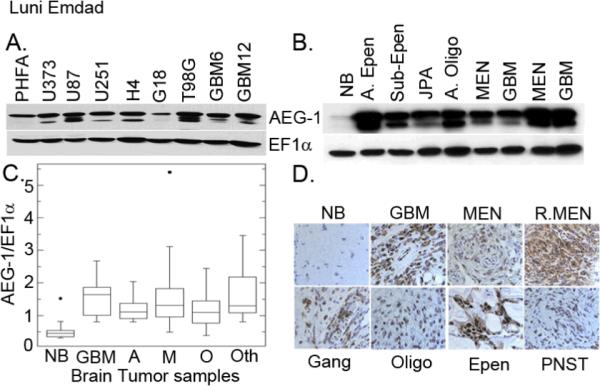
AEG-1 expression is upregulated in diverse brain tumors including malignant glioma. (A) Expression of AEG-1 in PHFA and in a panel of human malignant glioma cells is analyzed by Western blot analysis. EF1α was used as an internal control to ascertain equal loading. (B) Expression of AEG-1 protein in normal brain tissue and archival clinical human brain tumor samples by Western blot analysis. EF1α was used as an internal control for protein loading. A.Epen: anaplastic ependymoma, JPA-juvenile pilocytic astrocytoma, A. Oligo- anaplastic oligoastrocytoma. (C) Relative expression of AEG-1 in normal brain tissue and primary brain cancer samples. The expression levels are presented as a ratio between AEG-1 and loading control EF1α. NB, normal brain; GBM, glioblastoma multiformes; A, anaplastic astrocytoma (grade II and grade III); M, meningiomas; O, oligo-dendroglioma and oligo-astrocytoma; Oth, others such as ependymoma, ganglioma, peripheral neural sheath tumor etc. (D) Immunohistochemical staining for AEG-1 in normal brain tissue and formalin-fixed paraffin-embedded tissue sections of various brain tumors. Signals were developed with DAB chromogen (brown- AEG-1) and counterstained with hematoxylin.
The expression of AEG-1 is more intense in GBM cells than in astrocytoma cells
Next we analyzed whether there is any association of AEG-1 expression with glioma progression. As shown in Fig. 2A, AEG-1 expression is stronger in GBM samples than in astrocytoma samples. These results were confirmed in a glioma tissue microarray (Fig. 2B). Immunofluorescence studies demonstrate that while there is almost no staining for AEG-1 in normal brain, there is significant expression of AEG-1 in grade III astrocytoma with a further increase in GBMs. To further examine the role of AEG-1 expression in cancer progression, the expression patterns of AEG-1 and Ki67 were analyzed in normal and GBM tissues. As shown in Figure 2C, Ki67 was barely expressed in the low AEG-1 expressed area, while areas with high AEG-1 levels showed intense expression of Ki67.
Figure 2.
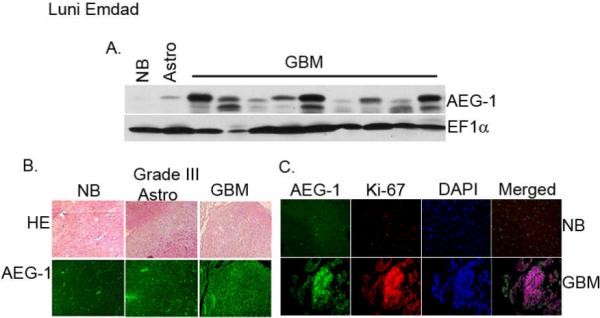
AEG-1 expression is more profound in the clinical samples of GBM. (A) Expression of AEG-1 protein in normal brain tissue and in tissues samples of astrocytoma and GBMs by Western blot analysis. EF1α was used as an internal control for protein loading. (B) Immunofluroscence staining of AEG-1 in normal brain and in astrocytic tumor tissue array (lower panel). Upper panel: H&E stain. (C) Immunofluroscence staining of AEG-1 (green) and ki67 (red) in normal brain and in GBM. Nuclei were stained with DAPI.
The expression of AEG-1 in GBM is more profound in the invasive margin of tumors and its aberrant expression is associated with elevated levels of the invasive molecules MMP-2 and MMP-9 and the angiogenic molecule CD31
A hallmark of highly malignant human glioma is their rapid invasive growth into surrounding brain parenchyma. To analyze the expression of AEG-1 in the invasive margin of malignant gliomas we established a syngeneic rat glioma model. Rat 9L glioma cells were implanted in rat brain and sections containing the implanted tumor and normal adjacent rat brain tissue were stained for AEG-1. The expression of AEG-1 was significantly increased in the invasive margin of the tumor when compared to the normal brain (Fig. 3A) indicating that AEG-1 expression is higher in the invading tumor cells. Next we performed immunofluorescence and IHC staining of AEG-1, MMP-2 and MMP-9 (key regulators of glioma invasion) in sections of normal brain and human GBM. As shown in Fig. 3B and C, both MMP-2 and MMP-9 were significantly over-expressed in glioma sections as previously reported (26, 27). Staining of AEG-1 in consecutive sections clearly indicated that AEG-1 expression strongly associated with expression of MMP-2 and MMP-9 in human glioma tissues (Fig. 3B and C).
Figure 3.
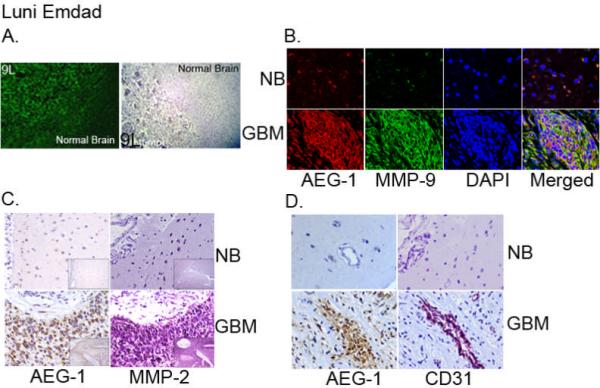
AEG-1 expression is more prominent in the invasive margin of rat glioma and AEG-1 expression is associated with MMP-2, MMP-9 and CD31 in human glioma tissue. (A) L9 rat glioma cells were implanted in syngeneic rats. Immunofluorescence studies (left panel, green) and immunohistochemical studies (right panel, brown) were performed in sections containing tumor and adjacent normal brain. (B) The sections of normal brain and GBM were immunostained for AEG-1 (red) and MMP-9 (green). Nuclei were stained with DAPI. (C) Serial sections of formalin-fixed, paraffin-embedded normal brain and GBM were stained with a rabbit polyclonal AEG-1, and a rabbit polyclonal MMP-2. Signals were developed with DAB chromogen (brown - AEG-1) or Vector VIP (purple - MMP-2) and counterstained with hematoxylin. (D) The sections of normal brain and GBM were immunostained for AEG-1 and the endothelial cell marker CD31. Immunohistochemical analysis demonstrated complete overlap of AEG-1 and Cd31 in tumor blood vessel.
Recent studies suggest that AEG-1 might induce angiogenesis in vitro and in vivo (28, 29). To examine the expression of AEG-1 in glioma tumor vessels we performed IHC for AEG-1 and CD31 (a marker of angiogenesis) in consecutive tissue sections of normal brain and human GBM. Overlapping AEG-1 and CD31 staining was observed in tumor vessels (Fig. 3D) further supporting a potential role of AEG-1 in tumor angiogenesis.
Knockdown of AEG-1 in glioma cells inhibits cloning efficiency and enhances sensitivity to chemotherapeutic agents
We next examined the effects of loss-of-expression of AEG-1 using gene-specific siRNA on the growth of U87 and 9L glioma cells. Earlier studies from our group and others established the involvement of AEG-1 in cellular growth and invasion in various cancer cells (13–17, 20). To study whether similar activities occurred in glioma cells, we blocked AEG-1 expression in 9L rat glioma and U87 human malignant glioma cells using siRNA. As shown in Fig. 4B and C, AEG-1 siRNA significantly decreased cloning efficiency and colony formation in monolayer cultures of U87 and 9L cells, respectively. Additionally we tested if knockdown of AEG-1 could sensitize glioma cells to chemotherapeutic agents. T98G and U87 cells were exposed to docetaxel after transfection with control and AEG-1 siRNA for 96 hours. As shown in Fig. 4D, glioma cells transfected with AEG-1 siRNA were more sensitive to docetaxel than cells transfected with control siRNA. These results suggest that AEG-1 could be involved in glioma cell proliferation as well as drug resistance of glioma.
Figure 4.
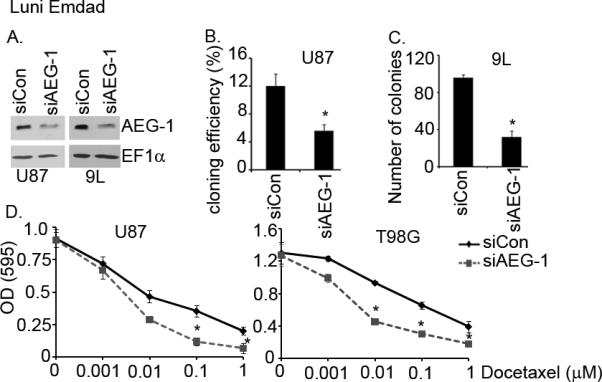
AEG-1 siRNA (siAEG-1) significantly inhibits cloning efficiency, cell growth, and invasion of glioma cells. (A) Knockdown of AEG-1 by siRNA in glioma cells. EF1α was used as an internal control to ascertain equal loading. (B) AEG-1 siRNA inhibits cloning efficiency of U87 human glioma cells. *P<0.05 vs siCon-treated cells. (C) Knockdown of AEG-1 by siRNA inhibits colony formation in monolayer of 9L rat gliosarcoma cells. *P<0.05 vs siCon-treated cells. (D) Knockdown of AEG-1 increases the sensitivity of U87 and T98G to a chemotherapeutic, docetaxel. Cell viability was evaluated by MTT assay. *P<0.05 vs siCon-treated cells.
Knockdown of AEG-1 inhibits invasion and MMP-2 and MMP-9 activity in glioma cells
The central role of AEG-1 in invasion of various cancers has been reported (13–17). In agreement with the previous reports we show here that AEG-1 siRNA significantly inhibited the invasive ability of 9L and U87 glioma cells (Fig. 5A and B). Studies from both in vitro and in vivo tumor model systems have suggested a link between increased expression and activation of several MMPs such as MMP-2 and MMP-9 and malignant glioma invasiveness (30, 31). The MMPs produced by the tumor, endothelial, and/or stromal cells degrade extracellular matrix at the invasive front of the glioma cells, thus removing the extracellular matrix barrier allowing subsequent tumor cell migration into newly created, more permissive spaces in the adjacent brain structures (32, 33). To investigate the effects of AEG-1 on MMP activation we performed gelatin zymography assays. As shown in Fig. 5C AEG-1 siRNA significantly decreased MMP-2 and MMP-9 activity in both glioma cells. Additionally we analyzed the effects of AEG-1 siRNA on MMP-2 and MMP-9 promoter activity in 9L glioma cells. Knockdown of AEG-1 by siRNA significantly inhibited the promoter activity of MMP-2 and MMP-9 in 9L glioma cells, suggesting that the effect of AEG-1 on these genes involved transcriptional activation (Fig. 5D). These results indicate that AEG-1 may play a key role in glioma invasion by modulating MMPs.
Figure 5.
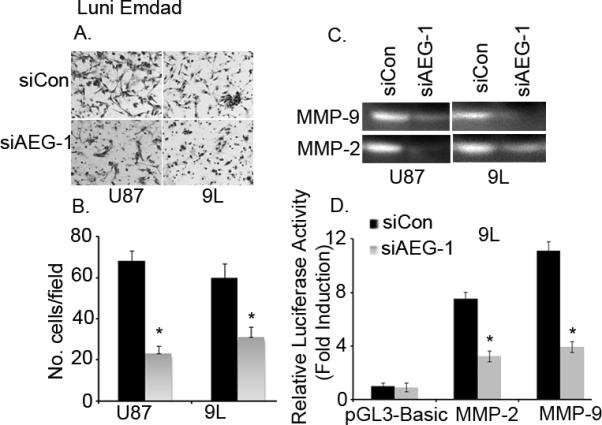
AEG-1 inhibition by siRNA decreases matrigel invasion of glioma cells by modulating MMP-2 and MMP-9. (A) U87 and 9L cells were transfected with either control siRNA (siCon) or AEG-1 siRNA (siAEG-1), and then 5 × 104 cells were seeded onto the upper chamber of a matrigel invasion chamber system in the absence of serum. Twenty-four hr after seeding, the filters were fixed, stained, and photographed. (B) Quantitation of the invasion assay. The data expressed in the graph is the mean ± SE of three independent experiments. *P<0.05 vs siCon-treated cells. (C) Zymography analyses of MMP-2 and MMP-9 activities in supernatants collected from control or AEG-1 siRNA-treated U87 and L9 glioma cells. (D) 9L cells were transfected with either control or AEG-1 siRNA. The next day cells were transfected with pGL3-Basic, pGL3-MMP-2 promoter or pGL3-MMP-9 promoter and pSV-β-gal vectors, and 48 h later, luciferase and β-gal activities were determined as described in materials and methods. Values are presented as fold normalized activity relative to that of empty vector pGL3-Basic taken as 1. The data expressed in the graph is the mean ± SE of three independent experiments. *P<0.05 vs siCon-treated cells.
Effects of AEG-1 siRNA on human primary GBM cell tumorigenicity in a mouse intracranial xenograft model
To examine the in vivo function of AEG-1 in gliomagenesis, athymic nude mice were injected intracranially with GBM12 cells that had been genetically engineered to express luciferase and transduced with lentivirus expressing either control or AEG-1 siRNA. Mice were scanned twice a week for 6 weeks after intracranial implant. Treatment of GBM12 tumors with AEG-1 siRNA significantly reduced the luciferase activity within the tumor earliest at 3 weeks after implant and in one animal implanted with AEG-1 siRNA-transduced GBM12 cells, the tumor was almost undetectable (Fig. 6A). Further scanning at 4 and 6 weeks after implant revealed a similar reduced luciferase activity as observed at 3 weeks. To confirm the efficacy of AEG-1 siRNA in vitro the remainder of implanted lenti-AEG-1 siRNA-transduced GBM12 cells was plated for cloning efficiency and Western blot analysis. Western blot analysis revealed that treatment with lenti-AEG-1 siRNA significantly reduced AEG-1 expression (~50% of lenti-Conrol siRNA-treated cells). Knockdown of AEG-1 also significantly inhibited the cloning efficiency of GBM12 cells in soft agar (Fig. 6C and D). These results suggest that AEG-1 could be a therapeutic target for glioma.
Figure 6.
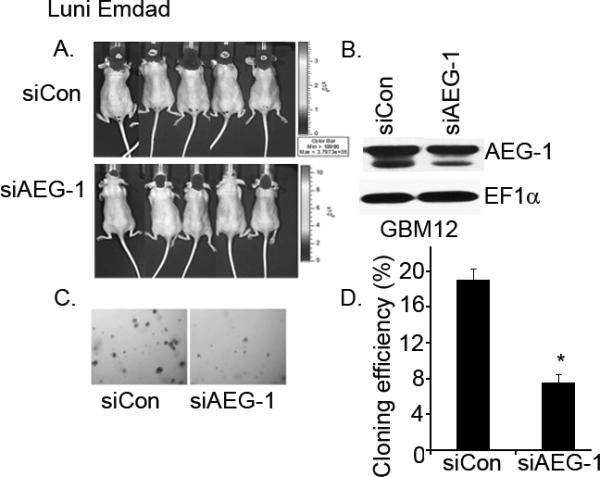
Knockdown of AEG-1 in GBM12 cells reduces intracranial tumor growth in vivo. (A) GBM12 cells stably transfected with a plasmid expressing luciferase transduced with lentivirus expressing control siRNA (siCon) or AEG-1 siRNA (siAEG-1) were implanted into athymic nude mice brains. Animals were injected IP with 175 mg/kg luciferin 21, 28 and 42 d after GBM12 cell implantation and light emanating from the implanted tumors visualized in a CCD camera apparatus with software 5 min after injection for 10 min. (B) Knockdown of AEG-1 in lentivirus-siAEG-1-transduced GBM12 cells. EF1α was used as an internal control to ascertain equal loading. (C and D) Cloning efficiency of GBM12 cells treated with lentivirus-siCon or lentivirus-siAEG-1. A total of 1 × 105 cells were seeded in 0.4% agar on 0.8% base agar. Two weeks later, colonies >0.1-mm were counted under a dissection microscope. *P<0.05 vs lentivirus-siCon-treated cells.
Discussion
Although the diverse roles of AEG-1 in tumor progression in multiple cancers are now being elucidated, investigations of its function in brain tumorigenesis remains unclear. We presently provide definitive evidence that elevated AEG-1 expression is a common event in brain tumors of diverse origin, including GBM. Moreover, knockdown of AEG-1 expression significantly inhibits the growth of human and rat glioma cells in vitro and significantly suppresses the invasive ability of glioma cells by modulating MMP-2 and MMP-9 activity. Additionally, AEG-1 inhibition significantly abrogates intracranial tumor growth in nude mice. Taken together, these results indicate that AEG-1 overexpression is a frequent alteration in brain tumors of multiple origins and overexpression of AEG-1 correlates with increased glioma cell proliferation and tumorigenicity. Moreover, inhibiting AEG-1 expression translates into suppression of transformation-associated properties and decreased intracranial tumor growth in vivo suggesting that AEG-1 could be a novel target for the therapy of malignant glioma and other types of brain cancers.
Recent studies have implicated AEG-1 overexpression as an important event in multiple types of cancer. Aberrant AEG-1 expression has been observed in a number of solid tumors including breast, prostate, esophageal carcinoma, and neuroblastoma (15, 17, 19, 20). In addition, we showed that AEG-1 expression is increased with the stages and grades of the disease in more than 90% of the hepatocellular carcinoma samples analyzed (16). Additionally, AEG-1 can enhance survival of normal cells under conditions of serum-starvation, induce an aggressive tumorigenic phenotype in immortal cloned rat embryo fibroblasts and promote tumor angiogenesis (29, 34). All these observations suggest that AEG-1 may potentially act as an oncogene in various cancer types. To investigate whether the upregulation of AEG-1 is also related to the progression of malignant glioma, we presently performed immunohistochemistry and Western blot analysis to characterize AEG-1 expression in commercially available tissue microarrays and in clinical archival astrocytoma and glioma tissues. These results demonstrate that AEG-1 is expressed at higher levels in glioma tissues as compared with astrocytoma tissues. These studies are now being expanded with a larger number of clinical samples to validate the prognostic significance of AEG-1 in glioma.
In addition to vasculature remodeling and destruction of the surrounding normal brain tissue, local invasive infiltration and growth are key features of GBM. The invasive character of glioma appears to depend partly on the proteolytic destruction of the estracellular matrix. Several studies have indicated that proteases are involved in tumor growth and invasion at the primary and metastatic sites (33, 35). Previous observations suggest that MMP-2, MMP-9 and MT1-MMP are present at elevated levels in several glioma cell lines and surgical specimens. In the present study we demonstrate that elevated AEG-1 expression in glioma specimens is associated with increased expression of MMP-2 and MMP-9. Additionally, we found that AEG-1 siRNA significantly inhibits MMP-2 and MMP-9 activation blocking glioma invasion. These data provide a direct link between induction of these proteolytic enzymes by AEG-1 and the induction of invasive properties in GBM cells.
Previously we have reported that AEG-1 is a significant positive regulator of nuclear factor kappa-β (NF–κB) and activation of NF-κB by AEG-1 represents a key molecular mechanism by which AEG-1 promotes anchorage-independent growth and invasion in malignant glioma cells (13, 14). Aberrant or constitutive activation of NF-κB has been documented in human malignant gliomas (36–38). The observation that in many glioma cells NF–κB is constituvely active emphasizes a vital role of AEG-1 in the induction of NF-κB and in regulation of aggressive properties of malignant gliomas. When AEG-1 was inhibited by siRNA, p65 could associate with the IL-8 promoter, whereas the association of AEG-1 as well as CBP was significantly reduced indicating that AEG-1 might function as a bridging molecule between p65 NF-κB and CBP and the basal transcription machinery, thus facilitating transcriptional activation of NF-κB downstream genes necessary for migration and invasion (14).
Since AEG-1 might play an important role in glioma cell growth and survival, we explored the potential therapeutic role of AEG-1 in combination with a chemotherapeutic drug. We found that knockdown of AEG-1 significantly enhanced the cytotoxicity of docetaxel in human glioma cells. Docetaxel is a tubulin-stabilizing agent currently used for the therapy of metastatic breast cancer, prostate cancer and non-small cell lung cancer (39). Our results suggest that docetaxel chemotherapy could be efficacious in glioma cells when administered in combination with AEG-1 siRNA. This possibility remains to be determined, since docetaxel has not been used clinically in GBM. Studies are in progress to ascertain if there are beneficial effects of AEG-1 inhibition when combined with temozolomide, a chemotherapeutic widely used in malignant glioma cells.
A key finding of the present study is that knockdown of AEG-1 by a lentivirus-based siRNA approach significantly inhibits GBM12 cell growth in vivo. Further studies using lentivirus or adenovirus expressing AEG-1 siRNA will be necessary in established tumors in the brain of nude and syngeneic animals to confirm potential therapeutic efficacy of this approach. Targeting AEG-1 by lentivirus-based siRNA approaches or by small molecule inhibitors in combination with radiotherapy and chemotherapy might produce extended survival benefits in malignant glioma patients and significantly improve the outcome of patients afflicted with this aggressive and invariably fatal disease.
Acknowledgements
DS is a Harrison Research Scholar in Cancer Research at the VCU Massey Cancer Center. PBF holds the Thelma Newmeyer Corman Chair in Cancer Research at the VCU Massey Cancer Center and is a Samuel Waxman Cancer Research Foundation (SWCRF) Investigator.
Financial Support: The present study was funded in part through NIH grants R01 CA134721 (PBF), P01 CA104177 (PBF and PD) and the Samuel Waxman Cancer Research Foundation (PBF); the Goldhirsh Foundation and the Dana Foundation (DS); and a Joelle Syverson Fellowship from the American Brain Tumor Association (LE).
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.CBTRUS, statistical report: primary brain tumors in the United States, 1998–2002. Central Brain Tumor Registry of the United States (CBTRUS); Chicago: 2006. [Google Scholar]
- 3.Cavenee WK. Accumulation of genetic defects during astrocytoma progression. Cancer. 1992;70:1788–93. doi: 10.1002/1097-0142(19920915)70:4+<1788::aid-cncr2820701621>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2:616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 6.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 7.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 8.Su ZZ, Kang DC, Chen Y, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 9.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland HG, Lam YW, Briers S, Lamond AI, Bickmore WA. 3D3/lyric: a novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Exp. Cell Res. 2004;294:94–105. doi: 10.1016/j.yexcr.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emdad L, Sarkar D, Su ZZ, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 15.Kikuno N, Shiina H, Urakami S, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 16.Yoo BK, Emdad L, Su ZZ, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–77. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SG, Jeon HY, Su ZZ, et al. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene. 2009;28:2476–84. doi: 10.1038/onc.2009.93. [DOI] [PubMed] [Google Scholar]
- 18.Emdad L, Sarkar D, Su ZZ, et al. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Zhang N, Song LB, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–26. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 20.Yu C, Chen K, Zheng H, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 21.Guarini L, Temponi M, Bruce JN, et al. Expression and modulation by cytokines of the intercellular adhesion molecule-1 (ICAM-1) in human central nervous system tumor cell cultures. Intl J Cancer. 1990;46:1041–1047. doi: 10.1002/ijc.2910460616. [DOI] [PubMed] [Google Scholar]
- 22.Yacoub A, Hamed H, Emdad L, et al. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7:917–33. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]
- 23.Gutin PH, Hilton J, Walker MD. Experimental brain tumor chemotherapy: DNA damage in the rat gliosarcoma 9L treated with CCNU. Clin Neurosurg. 1977;24:653–64. doi: 10.1093/neurosurgery/24.cn_suppl_1.653. [DOI] [PubMed] [Google Scholar]
- 24.Yoo BK, Gredler R, Vozhilla N, et al. Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci USA. 2009 Aug 4;106(31):12938–43. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emdad L, Sarkar D, Su ZZ, Boukerche H, Bar-Eli M, Fisher PB. Progression elevated gene-3 (PEG-3) induces pleiotropic effects on tumor progression: modulation of genomic stability and invasion. J Cell Physiol. 2005;202:135–46. doi: 10.1002/jcp.20097. [DOI] [PubMed] [Google Scholar]
- 26.Munaut C, Noël A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer. 2003;106:848–55. doi: 10.1002/ijc.11313. [DOI] [PubMed] [Google Scholar]
- 27.Guo P, Imanishi Y, Cackowski FC, et al. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–90. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar D, Emdad L, Lee SG, Yoo BK, Su ZZ, Fisher PB. Astrocyte elevated gene-1 (AEG-1): far more than just a gene regulated in astrocytes. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1846. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emdad L, Lee SG, Su ZZ, Boukerche H, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci USA. doi: 10.1073/pnas.0910936106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 31.Lakka SS, Rajan M, Gondi C, et al. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011–9. doi: 10.1038/sj.onc.1205894. [DOI] [PubMed] [Google Scholar]
- 32.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 34.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–21. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 35.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 36.Raychaudhuri B, Han Y, Lu T, Vogelbaum MA. Aberrant constitutive activation of nuclear factor B in glioblastoma multiforme drives invasive phenotype. J Neurooncol. 2007;85:39–47. doi: 10.1007/s11060-007-9390-7. [DOI] [PubMed] [Google Scholar]
- 37.Kondo Y, Hollingsworth EF, Kondo S. Molecular targeting for malignant gliomas. Int J Oncol. 2004;24:1101–9. [PubMed] [Google Scholar]
- 38.Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NF-κB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941–51. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 39.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–42. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]


