Abstract
Elevated levels of reactive oxygen species are found in most oncogenically transformed cells and are proposed to promote cellular transformation through mechanisms such as inhibition of phosphatases. BCR-ABL, the oncoprotein associated with the majority of chronic myelogenous leukemias, induces accumulation of intracellular ROS causing enhanced signaling downstream of PI3K. Previously we have shown that the transcription factor NF-κB is activated by BCR-ABL expression and is required for BCR-ABL-mediated cellular transformation. Inhibition of IKKβ and NF-κB leads to cell death through an unknown mechanism. Here, we analyze the potential involvement of NF-κB in moderating BCR-ABL-induced ROS levels to protect from death in response to cell stress. The data confirm that BCR-ABL promotes ROS levels and demonstrate that NF-κB prevents excessive ROS levels. Inhibition of NF-κB leads to an increase in ROS levels and to cell death controlled through ROS-induced JNK activity. The data demonstrate that one function for NF-κB in oncogenesis is the suppression of oncoprotein-induced ROS levels and that inhibition of NF-κB in some cancers, including CML, will increase ROS levels and promote cell death.
Introduction
Chronic myeloid leukemia is a malignant clonal disorder of hematopoietic stem cells that results in increased and deregulated growth of myeloid cells (Sawyers, 1999). Approximately 95% of CML cases arise from the formation of the Philadelphia (Ph) chromosome, a product of a chromosomal translocation that brings together the c-abl gene on chromosome 9 and the bcr gene on chromosome 22. This translocation results in the creation of the BCR-ABL fusion protein, which is a constitutively active tyrosine kinase (Sawyers, 1999). As a consequence of increased tyrosine kinase activity, BCR-ABL phosphorylates substrates including Grb2, Crkl and Shc, and activates signaling cascades, such as the Ras pathway, PI3K/Akt and Stat5, affecting the growth and differentiation of myeloid cells (Diaz-Blanco et al., 2007).
NF-κB is a transcription factor comprised of five family members: p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2). These proteins share a conserved Rel homology domain, which controls DNA binding, dimerization and interaction with inhibitory IκB proteins (Bassères and Baldwin, 2006; Courtois and Gilmore, 2006). NF-κB activation typically occurs through one of two distinct pathways. In the classical pathway, the p50-p65(RelA) heterodimer is activated by the IκB kinase (IKK) complex, which contains two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ. IKK phosphorylates IκBα, an inhibitory protein that normally sequesters p50-p65 in the cytoplasm, causing it to become ubiquitinated and subsequently degraded, allowing NF-κB to accumulate in the nucleus. In the alternative pathway, IKKα homodimers are activated and subsequently phosphorylate p100. This results in the proteolytic processing of p100 to p52 and allows p52-RelB dimers to translocate to the nucleus (Hayden and Ghosh, 2004). Once in the nucleus, NF-κB is known to regulate the expression of a variety of genes, including those encoding cytokines and cytokine receptors, inflammatory mediators, and antiapoptotic proteins (Bassères and Baldwin, 2006).
NF-κB is activated in many solid tumors (Basséres and Baldwin, 2006) and hematologic malignancies, including CML (Braun et al., 2006), where it provides proliferative and cell survival mechanisms. NF-κB is activated by BCR-ABL and is required for cellular transformation and tumor formation induced by this oncoprotein (Hamdane et al., 1997; Reuther et al. 1998). Inhibition of IKK in BCR-ABL-expressing cells induces death (Cilloni et al., 2006; Duncan et al., 2008). Interestingly, Imatinib- and/or Dasatinib-resistant cells were shown to be susceptible to IKKβ inhibition (Duncan et al, 2008), suggesting a novel therapeutic option for CML. However, the mechanism whereby IKKβ inhibition induces death of BCR-ABL-expressing cells has not been determined.
c-Jun N-terminal kinase (JNK), also known as stress-activated protein kinase (SAPK), is a member of the MAPK family and is involved in the regulation of c-jun, a component of the AP-1 family of transcription factors (Leppä and Bohmann, 1999). JNK is predominately activated by cellular stress mechanisms, including increased levels of reactive oxygen species (ROS), but can also be activated by other stimuli including cytokines and oncogenic transformation. JNK is actived by MAPKKs through the phosphorylation of threonine 183 and tyrosine 185. JNK then phosphorylates c-Jun at serines 63 and 73 causing an increase in c-Jun transcriptional activity (Gupta et al., 1996). c-Jun activity is implicated in cell transformation, proliferation and death downstream of JNK (Leppä and Bohmann, 1999; Vogt, 2001). Interestingly, both c-jun and JNK are required for transformation of hematopoietic cells by BCR-ABL (Raitano et al., 1995) as well as their survival after transformation (Hess et al., 2002). However, under stimuli that induce cell stress, JNK activation can lead to death (Shen and Liu, 2006; Dhanasekaran and Reddy, 2008). JNK becomes activated by stimuli in a constitutive manner through increased intracellular ROS and activates apoptotic and necrotic death pathways (Tang et al, 2002; Pham et al, 2004; Ventura et al., 2004; Kamata et al, 2005).
It has been demonstrated that oncogenic transformation results in increased levels of intracellular ROS, which are used as secondary signaling molecules to increase proliferation and to promote the oncogenic potential of transformed cells (Pelicano et al., 2004; Benhar et al., 2002). For example, oncogenic Ras leads to increased levels of ROS, which are important in oncogenic transformation and proliferation (Irani et al., 1997). Previous reports have shown that hematopoietic cell lines transformed with BCR-ABL have increased levels of intracellular ROS (Sattler et al, 2000; Kim et al, 2005; Naughton et al., 2009). ROS promotes PI3K-induced signaling downstream of BCR-ABL by inhibiting phosphatases which normally limit signal transduction cascades (Naughton et al., 2009), thereby increasing tumorigenicity.
Here we have explored the potential involvement of NF-κB in moderating intracellular ROS levels downstream of BCR-ABL. The results indicate that NF-κB activity functions to suppress BCR-ABL-induced ROS levels. Additionally, inhibition of IKK or NF-κB leads to enhanced ROS levels and elevated JNK activity to promote cell death. The experiments reveal a key pro-oncogenic mechanism and demonstrate a mechanism whereby inhibition of NF-κB activity promotes cytotoxicity of certain cancer cells.
Materials and Methods
Cell lines
32D and Ba/F3 hematopoietic murine cells were maintain in RPMI 1640 medium (Gibco) supplemented with 10% FBS and 10% Wehi-conditioned media as a source of IL-3. 32D and Ba/F3 cells stably expressing p185 or p210 BCR-ABL, respectively, were maintained in RPMI 1640 supplemented with 10% FBS. 293Ts were maintained in DMEM supplemented with 10% FBS.
Chemicals
2′,7′-Dichlorodihydrofluorescein Diacetate (DCF-DA; Calbiochem) was dissolved in DMSO. Catalse and n-acetyl-cysteine (Sigma) were dissolved in culture media. The pH of NAC was then adjusted to 7.2 and the stock was subsequently passed through a 0.2μm filter. Butylated hydroxyanisole (Sigma) was dissolved in ethanol. Compound A, SP600125 (Sigma) and Z-VAD-FMK (Sigma) were dissolved in DMSO. All stocks were diluted to working dilutions in culture media.
Detection of ROS
Cells were harvested, washed twice with PBS, and then incubated with DCF-DA at a final concentration of 10μM for 15 minutes at 37°C in the dark. Cells were then washed once with PBS and analyzed immediately by flow cytometry.
Cell death staining
Cells were harvested and washed twice with cold PBS. 5×105 cells were resuspended in 100 μl Annexin binding buffer (BD Pharmagen) and stained with Annexin V (BD Pharmagen; 1:20) and 7-Amino-actinomycin D (BD Pharmigen; 1:50) or Propidium Iodide (BD Pharmigen; 1:20) at RT in the dark for 15 minutes. 400μl binding buffer was subsequently added and the cells were analyzed immediately by flow cytometry.
Antibodies
Phospho-JNK (T183/Y185), JNK, Phospho-c-jun (S73), c-jun, and cleaved caspase 3 (Asp175), caspase 3 and IκBα were obtained from Cell Signaling Technologies. β-tubulin was obtained from Santa Cruz Biotechnology. β-actin was obtained from Calbiochem.
Western blotting
Cells were harvested, washed twice with cold PBS and resuspended in lysis buffer (50 mM Tris-HCl pH 7.4, 150mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1mM EDTA, 1mM EGTA, 1mM Na3VO4) supplemented with protease and phosphatase inhibitors (Roche). Cells were incubated on ice for 15 minutes and the lysates were clarified by centrifugation. Equal amounts of lysates (30-50μg) were subjected to SDS-PAGE, transferred onto a nitrocellulose membrane, blocked for 1 hour at room temperature in tris buffered saline with 0.05% Tween-20 and 5% non-fat milk and incubated with the indicated antibodies overnight. Blots were incubated with the appropriate secondary antibody for 45 minutes at room temperature and developed using ECL detection reagent (GE).
Quantitative Real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen), digested with DNase I (Promega), and used for reverse transcription (SuperScript II kit, Invitrogen). All Taqman primers were obtained from Applied Biosystems. Expression levels of GusB were used to normalize the amount of the investigated transcripts.
Viral Production and Transduction
Virus was produced by transient transfection of 293T cells with pCL-10A1 (Imgenex) and a retroviral vector using Fugene at a 1:1 ratio. Viral supernatant was collected 24 and 48 hours post-transfection and concentrated using centrifugal filter units (Amicon Ultra, Millipore). Target cells were resuspended at 0.5×106 cells/ml in RPMI with viral supernatant in 6-well plates and spun at 2500 rpm for 1 hour at room temperature. Cells were incubated with viral supernatant for an additional 3 hours at 37°C and then plated in RPMI for an additional 24-48 hours before harvest for experiments.
Results
Inhibition of IKKβ results in apoptosis of BCR-ABL-expressing cells
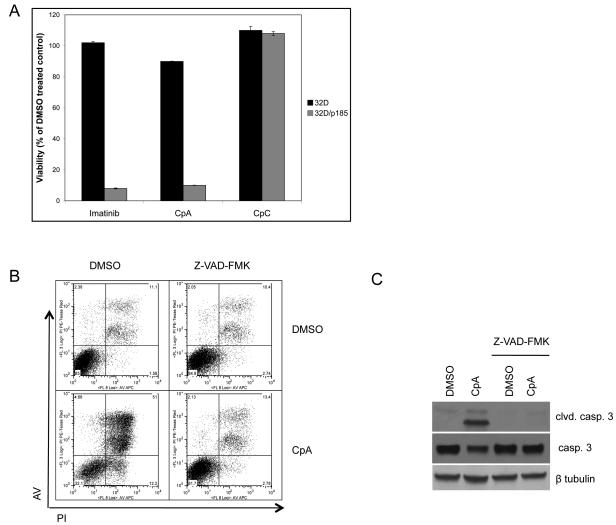
Recently, we and others have shown that IKKβ activity is required for survival of BCR-ABL-expressing myeloid cells, including cells with mutations resistant to the commonly used BCR-ABL inhibitors Imatinib and Dasatinib (Duncan et al., 2008, Cilloni et al., 2006). That data showed the importance of IKKβ in BCR-ABL-induced oncogenesis. However a mechanism mediating IKK inhibitor-induced cell death and involvement of NF-κB in cell survival was not shown. As analyzed before, cell viability was measured to determine the effect of IKKβ inhibition using Compound A (a well validated IKKβ inhibitor, Ziegelbauer et al., 2005) in parental 32D cells and in 32D cells stably expressing BCR-ABL p185 (32D/p185). Compound A treatment resulted in decreased cell viability similar to treatment with Imatinib, while Compound C, an inactive analog of Compound A, did not affect the viability of 32D/p185 cells (Fig. 1A and see Duncan et al., 2008). The decrease in cell viability with Compound A treatment corresponds with cleavage of caspase 3, a marker of apoptosis (Fig. 1B). Similar results were seen in parental BaF3 pro-B cells and BaF3 cells expressing BCR-ABL (Fig. S1). Co-incubation with ZVAD-FMK, an inhibitor of caspase activation, potently blocks Compound A-induced cell death. These results show that IKKβ activity is required to block apoptosis in cells expressing BCR-ABL (Fig. 1B and Duncan et al, 2008).
Figure 1. Inhibition of IKKβ causes apoptosis in BCR-ABL-expressing myeloid cells.
(A) 32D cells expressing BCR-ABL (32D/p185) were incubated with 1μM Imatinib, IKK inhibitor (Compound A) or inactive analog of Compound A (Compound C) for 20 hours. Cell viability was measured by MTS reduction. (B) 32D/p185 cells were coincubated with Z-VAD-FMK (100μM) and DMSO (vehicle control) or Compound A for 20 hours. Cells were stained with propidium iodide and annexin V and cell death was measured by FACS analysis or (C) lysed and analyzed by immunoblot.
NF-κB activity is required for the survival of BCR-ABL-expressing cells
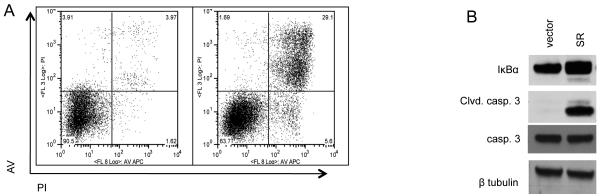
Although IKKβ is known to activate NF-κB through the phosphorylation-mediated ubiquitination and degradation of IκBα, it also has other targets (Hu et al., 2004; Lee et al., 2007). Therefore, to determine if NF-κB is necessary for the survival of BCR-ABL-expressing cells downstream of IKKβ, and to rule out off target effects of Compound A, NF-κB activity was blocked by expressing IκBα super-repressor (IκBα-SR), a form of IκBα containing serine to alanine mutations at residues 32 and 36 that prevent its phosphorylation and degradation, thereby sequestering NF-κB in the cytoplasm of the cell. Expression of IκBα-SR led to apoptosis in BCR-ABL-expressing 32D cells over time as measured by Annexin V/PI staining (Fig. 2A) and expression of cleaved caspase 3 (Fig. 2B) while the viability of cells transduced with empty vector were not affected. Taken together, these results show a requirement for NF-κB activity downstream of IKKβ in hematopoietic cells expressing BCR-ABL to prevent apoptosis.
Figure 2. NF-κB activity is required for survival of BCR-ABL-expressing cells.
(A) 32D/p185 cells were transduced with retrovirus expressing vector control or IκBα superrepressor (IκBα-SR). Cells were harvested 36 hours after retroviral transduction and stained with propidium iodide and Annexin V. Cell death was analysed by FACS, or (B) analyzed by immunoblot.
IKKβ inhibition in BCR-ABL-expressing cells results in the accumulation of intracellular oxygen species
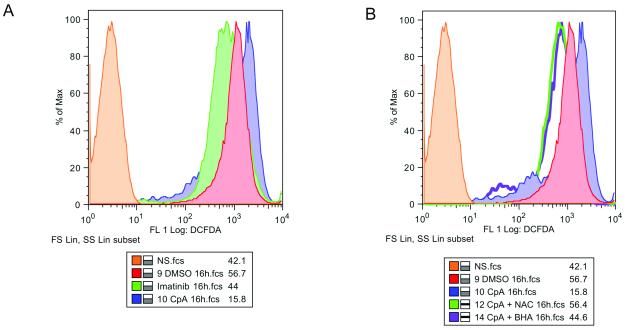
While the inhibition of both IKKβ and NF-κB in BCR-ABL-expressing cells results in apoptosis, the mechanism that precedes cell death remains unclear. Cells that have undergone oncogenic transformation, including those overexpressing Ras, c-myc and BCR-ABL, have increased levels of intracellular ROS (Irani et al., 1997, Vafa et al. 2002, Sattler et al., 2000). Transformed cells utilize increased ROS as secondary signaling molecules to enhance proliferation and tumor development. However, because transformed cells harbor higher levels of ROS, a further increase in free radicals can result in apoptosis or necrosis (Pelicano et al, 2004). As BCR-ABL expression is known to enhance reactive oxygen species production in hematopoietic cells (Sattler et al, 2000; Kim et al, 2005; Naughton et al, 2009) and NF-κB can regulate antioxidant gene expression (Tang et al, 2002; Pham et al, 2004; Kamata et al, 2005), we asked if IKKβ inhibition with Compound A results in altered ROS levels leading to cell death. Relative ROS levels were measured in 32D/p185 cells treated with Imatinib or Compound A over time. Treatment with the BCR-ABL inhibitor Imatinib decreased intracellular ROS levels (Fig. 3A) as previously reported (Sattler et al., 2000), while IKKβ inhibition using Compound A caused an increase in intracellular ROS as measured by DCF-DA staining. Cells treated for 12 to 16 hours showed an accumulation of ROS (Fig. 3) while cells treated for 1 hour did not (data not shown), suggesting that an indirect mechanism leads to the accumulation of ROS in these cells. The accumulation of ROS upon treatment with Compound A is reversed through the addition of antioxidants n-acetyl-cysteine (NAC) or butylated hydroxyanisole (BHA) (Fig. 3B). These data indicate that IKKβ inhibition leads to significantly enhanced levels of ROS, over those induced by BCR-ABL.
Figure 3. Inhibition of NF-κB causes an increase in intracellular ROS in cells expressing BCR-ABL.
(A) 32D/p185 cell were incubated with DMSO (vehicle control) or 1μM Imatinib or Compound A for 16 hours without or (B) with antioxidants. Cells were then incubated with DCF-DA and fluorescence was measured my FACS analysis.
NF-κB inhibits the activation of JNK downstream of BCR-ABL to promote cell survival
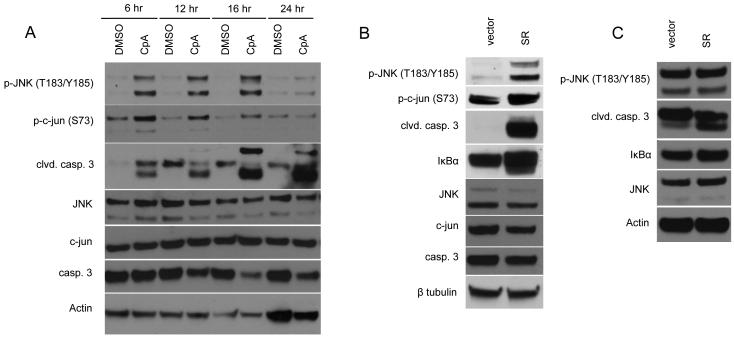
At high levels, ROS have been shown to activate AP-1, resulting in cell death (Shen and Liu, 2006). Interestingly, NF-κB is important for the regulation of JNK, an upstream effector of AP-1, to block death under cell stress conditions (Tang et al., 2002; Pham et al, 2004; Kamata et al, 2005). Given the correlation between increased intracellular ROS and apoptosis in BCR-ABL-expressing cells after Compound A treatment, we asked if NF-κB activation is important for the regulation of intracellular ROS and inhibition of JNK downstream of BCR-ABL. A time course in which 32D/p185 cells were treated with Compound A shows that both the phosphorylation of JNK, its downstream target c-jun, and caspase-3 cleavage occur 6 hours after treatment (Fig. 4A). 32D/p185 cells were transduced with empty vector or IκBα-SR to examine the effect of NF-κB inhibition on JNK activation and apoptosis downstream of BCR-ABL. Cells harvested 36 hours post-transduction showed increased phosphorylation of JNK, c-jun and the cleavage of caspase 3 (Fig. 4B). Parental 32D cells expressing IκBα-SR were not affected to the same extent as 32D/p185 cells, although some apoptosis is apparent as measured by cleavage of caspase 3 (Fig. 4C). This low level of cell death can be attributed to moderate activation of NF-κB in these cells due to their dependence on IL-3 for survival (Reuther et al., 1998). While IL-3 is also known to activate JNK (Yu et al., 2004), expression of IκBα-SR did affect JNK phosphorylation in these cells. Together, these data show that NF-κB actively regulates the level of intracellular ROS and also inhibits the activation of JNK downstream of BCR-ABL to inhibit cells from undergoing apoptosis.
Figure 4. IKKβ inhibition downstream of BCR-ABL induces JNK activation and apoptosis.
(A) 32D/p185 cells were treated with DMSO or 1μM Compound A over a time course. Cells were lysed and subjected to immunoblotting. (B) 32D/p185 or (C) parental 32D cells were transduced with retrovirus expressing vector control or IκBα superrepressor (IκBα-SR) and harvested 36 hours after retroviral transduction, lysed and subjected to immunoblotting.
IKKβ inhibition leads to downregulation of transcription of antioxidant genes
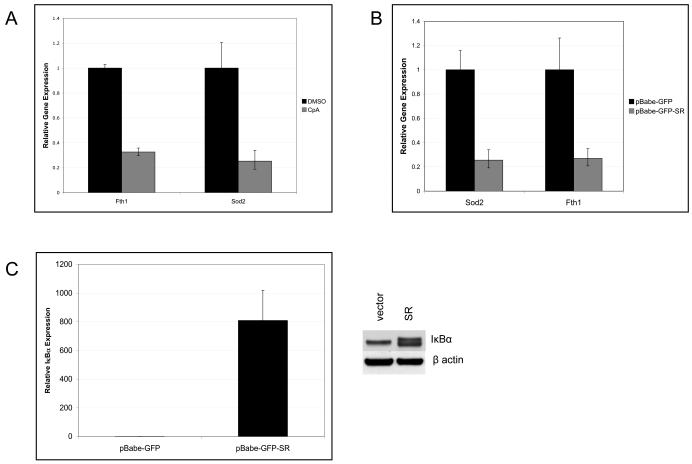
Our results show that NF-κB activity is important for the regulation of intracellular ROS and JNK activity downstream of BCR-ABL to prevent cells from undergoing apoptosis. NF-κB is known to regulate the expression of genes encoding proteins with antioxidant properties (Kamata et al., 2005; Pham et al, 2004). Due to the increase in intracellular ROS upon inhibition of IKKβ, we asked if NF-κB transcriptionally regulates genes known to clear excess ROS from the cell. BCR-ABL-expressing cells were treated with vehicle or Compound A and quantitative real-time PCR was used to screen NF-κB target genes known to have antioxidant properties. 32D/p185 cells treated with Compound A for 12 hours showed decreased levels of both Sod2 and Fth1 mRNAs (Fig. 5A), corresponding with the phosphorylation of JNK and apoptosis (Fig. 4A). This result indicates that blocking IKKβ activity results in decreased production of two known ROS scavengers, possibly resulting in accumulation of intracellular ROS and apoptosis. To rule out potential off target effects of Compound A, IκBα-SR was overexpressed to block NF-κB activity in 32D/p185 cells. Similar to the results obtained using Compound A treatment, cells expressing IκBα-SR (Fig. 5C) also showed decreased mRNA levels of Sod2 and Fth1 (Fig. 5B), correlating with apoptosis as measured by cleavage of caspase 3. Overexpression of Sod2 and Fth1 did not rescue the cell death response induced by IKKβ inhibition (data not shown), suggesting that multiple mechanisms controlled by IKK and NF-κB contribute to the control of ROS levels in oncogenically transformed cells.
Figure 5. NF-κB regulates antioxidant gene expression in BCR-ABL-expressing cells.
(A) 32D/p185 cells were treated for 12 hours with DMSO or 1μM Compound A. RNA was purified using Trizol and then subjected to reverse transcription before analysis using quantitative real-time PCR. (B) 32D/p185 cells transduced with retrovirus expressing empty vector or IκBα-SR. Gene expression was analyzed by quantitative real-time PCR as described. (C) Expression levels of IκBα of cells used in panel B.
JNK inhibition rescues BCR-ABL+ cells from apoptosis after Compound A treatment
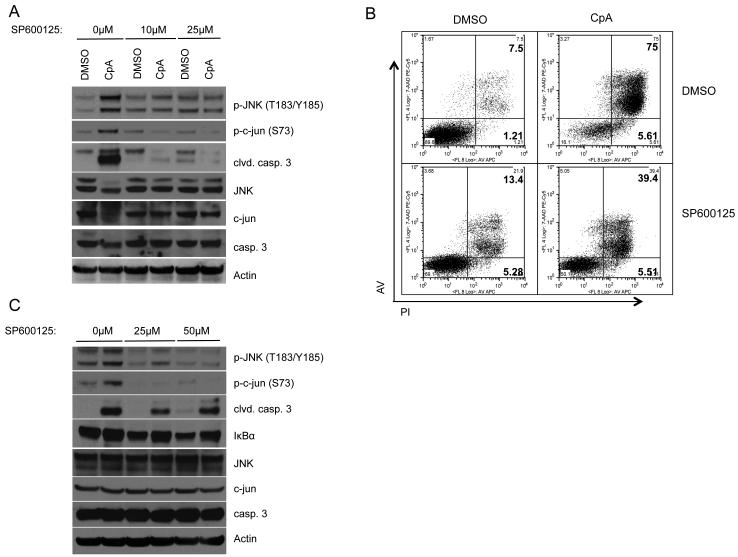
Our results show that NF-κB activity regulates intracellular ROS levels and JNK activation in BCR-ABL-expressing cells. To determine the importance of JNK activity in the death of BCR-ABL-expressing cells after inhibition of NF-κB, we blocked JNK using a specific inhibitor, SP600125, and treated 32D/p185 cells with Compound A. Cells that were treated with SP600125 and Compound A showed decreased apoptosis as indicated by caspase 3 cleavage (Fig. 6A) and FACS analysis (Fig. 6B). However, cells treated with high concentrations of SP600125 (≥25μM) underwent apoptosis without IKKβ inhibition, indicating that BCR-ABL-expressing cells also require low levels of JNK activity for survival as previously shown (Raitano et al, 1995; Hess et al., 2002). Similar results were obtained from 32D/p185 cells that were treated with SP600125 upon expression of IκBα-SR (Fig. 6C). These data show that increased JNK activity is required for cell death in BCR-ABL-expressing cells when NF-κB is inhibited. These data further suggest an important role for JNK regulation and evasion of apoptosis by NF-κB downstream of BCR-ABL.
Figure 6. JNK activation in the absence of NF-κB activity leads to apoptosis in BCR-ABL-expressing cells.
(A) 32D/p185 cells were pretreated with vehicle or indicated concentrations of SP600125 for 1 hour. 1μM Compound or DMSO were then added and cells were incubated for an additional 24 hours. Cells were then harvested, lysed and subjected to immunoblot. (B) 32D/p185 cells were incubated with vehicle or 25 μM SP600125 for 1 hour prior to the addition of DMSO of 1μM Compound A. Cells were stained using Annexin V and propidium iodide. Cell death was measured by FACS. (C) 32D/p185 cell were transduced with IκBα-SR. 20 hours post-transduction, cells were treated with SP600125 additional 8 hours. Cells were then lysed and analyzed by immunoblot.
Inhibition of ROS prevents death due to IKKβ inhibition in BCR-ABL+ cells
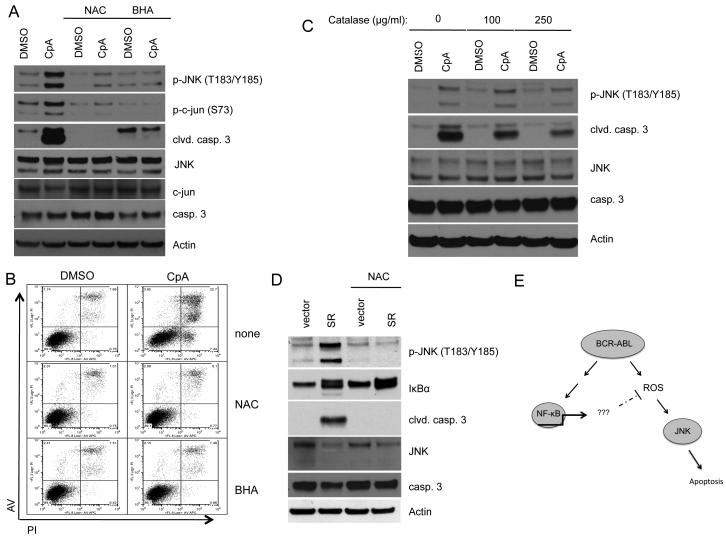
The increase in intracellular ROS in transformed cells enhances proliferation and tumorigenicity. However, these cells are also sensitive to further increases in intracellular ROS, which may lead to apoptosis (Pelicano et al, 2004). Our data show that inhibition of NF-κB leads to a further increase in intracellular ROS, activation of JNK and apoptosis downstream of BCR-ABL. To better understand the role of NF-κB in the regulation of intracellular ROS in cells expressing BCR-ABL, we inhibited ROS and measured cell death after Compound A treatment. Interestingly, 32D/p185 cells incubated with n-acetyl-cysteine (NAC) or butylated hydroxyanisole (BHA) in conjunction with Compound A treatment showed a pronounced decrease in phosphorylated JNK and were resistant to apoptosis (Fig. 7A and 7B). Similar results were obtained in Ba/F3 cells expressing BCR-ABL (Fig. S2). Cells were also coincubated with bovine catalase and Compound A, resulting in decreased JNK phosphorylation and apoptosis (Fig. 7C). Lastly, 32D/p185 cells were incubated with NAC upon expression of IκBα-SR as determined by GFP expression. JNK activation and apoptosis induced by the overexpression of IκBα-SR were also inhibited by NAC treatment (Fig. 7D). These results show that NF-κB activity is required to regulate increased intracellular ROS following transformation with BCR-ABL. Upon inhibition of NF-κB, the accumulation of ROS in the cell leads to the activation of JNK and apoptosis (Fig. 7E).
Figure 7. Antioxidants rescue BCR-ABL-expressing cells from death when NF-κB is inhibited.
32D/p185 cells were pretreated with vehicle, 20mM NAC or 50μM BHA for 1 hour prior to the addition of DMSO or 1μM Compound A. Cells were incubated for an additional 20 hours and (A) lysed and subjected to immunoblot, or (B) stained with Annexin V and propidium iodide and analyzed using flow cytometry. (C) 32D/p185 cells were pretreated with bovine catalase for 1 hour prior to the addition of DMSO or 1μM Compound A. Cells were incubated for an additional 20 hours and then harvested for immunoblot. (D) 32D/p185 cells were transduced with retrovirus expressing empty vector or IκBα-SR. 24 hours after transduction, cells were replated in media with or without 20μM NAC for an additional 12 hours. Cells were harvested, lysed and used for immunoblotting. E. Model showing the role of NF-κB in the regulation of intracellular ROS in BCR-ABL-expressing cells.
Discussion
Increased ROS has been documented in several cell types after oncogenic transformation and in various cancers (Benhar et al., 2002, Pelicano et al., 2004). It was first discovered that human tumor cells produce increased amounts of hydrogen peroxide (Szatrowski and Nathan, 1991), leading to the hypothesis that cancer cells are subject to persistent oxidative stress, possibly explaining characteristics of cancer including genomic instability and increased proliferation (Toyokuni et al., 1995). Indeed, several reports have shown an increase in reactive oxygen species in primary human tumors, including brain (Iida et al., 2001), colorectal carcinoma (Toyokuni et al., 1999), and ovarian cancer (Senthil et al., 2004). Additionally, reports showed that oncogenic transformation by Ras, c-myc and BCR-ABL lead to increased ROS which important for increased proliferation and tumorigenic potential (Irani et al., 1997; Felsher et al., 1999; Vafa et al., 2002, Sattler et al., 2000; Kim et al, 2005; Naughton et al, 2009).
Relative to oncogenic Ras expression, increased ROS levels were shown to be required for cellular transformation (Irani et al., 1997). In this regard, ROS generated from the Qo site of mitochondrial complex III is required for anchorage-independent growth of Ras-transformed cells (Weinberg et al, 2010). Overexpression of Nox1, a superoxide generator, in NIH3T3 results in elevated production of ROS and a transformed phenotype with increased proliferation (Suh et al., 1999). Interestingly, Nox1 knockdown blocks Ras-transformed phenotypes including anchorage independent growth in vitro and in vivo (Mitsushita et al., 2004). Relative to our study, ROS levels are increased downstream of BCR-ABL which leads to increased PI3K/Akt-dependent signaling through inhibition of the phosphatase PP1a (Naughton et al., 2009).
Cells transformed with BCR-ABL have increased ROS (Sattler et al, 2000; Kim et al, 2005; Naughton et al, 2009) (and see Fig. 3A ) thus increasing the sensitivity of these cells to a further increase in ROS. Treatment with agents that cause an increase in ROS in BCR-ABL-expressing cells causes to death (Zhou et al, 2003; Chandra et al., 2006; Trachootham et al., 2006; Zhang et al., 2008; Mao et al, 2010). One such agent, phenethyl isothiocyanate (PEITC) results in increased ROS and subsequent apoptosis in cells expressing both wild-type (Trachootham et al., 2006) and Imatinib- and Dasatinib-resistant (Zhang et al., 2008) forms of BCR-ABL. However, the mechanism by which these compounds cause increased ROS and cell death is largely unknown.
Data described above indicate that the maintenance of moderate levels of ROS are necessary for increased proliferative capacity and tumorigenic potential while avoiding death in response to excessive accumulation of free radicals (Schimmel and Bauer., 2002; Benhar et al., 2002; Pelicano et al., 2004). Due to excessive strain on ROS clearing mechanisms that maintain a moderate balance of ROS, a further increase in ROS in transformed cells may result in cancer cell death (Benhar et al., 2002; Pelicano et al. 2004), offering a novel approach to target cancer cells.
Potential therapeutic targets to increase ROS specifically in cancer cells include transcription factors that control the expression of both antiapoptotic and antioxidant genes. One such transcription factor, NF-κB, has been shown to regulate the transcription of genes with antioxidant properties, such as ferritin heavy chain (Pham et al., 2004) and superoxide dismutates (Kamata et al., 2005). NF-κB also inhibits JNK activation downstream of ROS through transcription of genes such as Gadd45 (De Smaele et al., 2001) and XIAP (Tang et al., 2001) and through the inhibition of MAPK (Kamata et al., 2005) and tyrosine phosphatases (Sattler et al., 2000).
Our results show an important role for NF-κB activity in the maintenance of intracellular ROS and the inhibition of JNK activity downstream of BCR-ABL to prevent cell death after oncogenic transformation. Inhibition of IKKβ using a chemical inhibitor, Compound A, results in apoptosis (Fig. 1), along with the accumulation of intracellular ROS and the activation of JNK in BCR-ABL-expressing cells (Fig. 3). Likewise, expression of IκBα-SR, which blocks NF-κB activity, induces JNK phosphorylation and apoptosis (Fig. 4B). These data correlate with previous reports in which NF-κB plays an important role in JNK inhibition when ROS levels increase (Sakon et al., 2003; Pham et al., 2004; Kamata et al., 2005).
Treatment with Compound A or expression of IκBα-SR also results in decreased expression of two NF-κB target genes with antioxidant properties, Fth1 and Sod2 (Fig. 5). These genes have been documented in response to TNFα stimulation in which TNFα-induced ROS was scavenged thereby protecting cells from TNFα induced death in the absence of NF-κB (Pham et al., 2004, Kamata et al., 2005). While inhibition of NF-κB results in decreased antioxidant gene expression (Fth1 and Sod2), our preliminary data indicates that overexpression of either FTH1 or SOD2 in BCR-ABL-expressing cells is not sufficient to inhibit apoptosis in the absence of NF-κB activity. This is not surprising, as many cellular processes control the levels of ROS, indicating that other NF-κB-dependent genes and buffering systems are likely involved in this process.
Our data also show that JNK activity is involved in the initiation of apoptosis in the absence of NF-κB. Blocking JNK activity with a chemical inhibitor, SP600125, results in a decrease in cell death upon Compound A treatment downstream of BCR-ABL (Fig. 6). However, cells expressing BCR-ABL appear to require JNK activity, as the inhibitor alone results in induction of apoptosis in 32D/p185 cells. Importantly, JNK activation by ROS is required for the initiation of apoptosis in the absence of NF-κB activity (Fig. 7). However, inhibition of ROS with antioxidants offers more complete protection from Compound A-induced apoptosis that inhibition of JNK with SP600125. This could simply be due to the efficiency of inhibition by these compounds, or the differences in survival could indicate a more involved role for increased ROS in apoptosis after inhibition of NF-κB. It is probable that ROS activate JNK as well as other proteins in the cell to initiate apoptosis in response to unfavorable conditions, and that inhibiting JNK only partially blocks the effect of increased ROS on cell survival.
These data show that NF-κB is required to maintain moderate levels of ROS and inhibit JNK activation downstream of BCR-ABL-induced ROS to inhibit the induction of apoptosis in a model of chronic myeloid leukemia. As increased ROS is common among transformed cells, it is likely that NF-κB plays an essential role in the regulation of ROS to prevent death, illustrating the potential use for IKKβ inhibitors as a therapeutic in CML and possibly other cancers.
Supplementary Material
Footnotes
Supplementary information is available at the Oncogene website.
References
- Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Carvalho G, Fabre C, Grosjean J, Fenaux P, Kroemer G. Targeting NF-kappaB in hematologic malignancies. Cell Death Differ. 2006;13:748–758. doi: 10.1038/sj.cdd.4401874. [DOI] [PubMed] [Google Scholar]
- Chandra J, Tracy J, Loegering D, Flatten K, Verstovsek S, Beran M, et al. Adaphostin-induced oxidative stress overcomes BCR/ABL mutation-dependent and -independent imatinib resistance. Blood. 2006;107:2501–2506. doi: 10.1182/blood-2005-07-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloni D, Messa F, Arruga F, Defilippi I, Morotti A, Messa E, et al. The NF-kappaB pathway blockade by the IKK inhibitor PS1145 can overcome imatinib resistance. Leukemia. 2006;20:61–67. doi: 10.1038/sj.leu.2403998. [DOI] [PubMed] [Google Scholar]
- Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, et al. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Blanco E, Bruns I, Neumann F, Fischer JC, Graef T, Rosskopf M, et al. Molecular signature of CD34(+) hematopoietic stem and progenitor cells of patients with CML in chronic phase. Leukemia. 2007;21:494–504. doi: 10.1038/sj.leu.2404549. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Goetz CA, Stein SJ, Mayo KJ, Skaggs BJ, Ziegelbauer K, et al. IkappaB kinase beta inhibition induces cell death in Imatinib-resistant and T315I Dasatinib-resistant BCR-ABL+ cells. Mol Cancer Ther. 2008;7:391–397. doi: 10.1158/1535-7163.MCT-07-0305. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci U S A. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Hamdane M, David-Cordonnier MH, D’Halluin JC. Activation of p65 NF-kappaB protein by p210BCR-ABL in a myeloid cell line. Oncogene. 1997;15:2267–2275. doi: 10.1038/sj.onc.1201411. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ. Survival signaling mediated by c-Jun NH(2)-terminal kinase in transformed B lymphoblasts. Nat Genet. 2002;32:201–205. doi: 10.1038/ng946. [DOI] [PubMed] [Google Scholar]
- Iida T, Furuta A, Kawashima M, Nishida J, Nakabeppu Y, Iwaki T. Accumulation of 8-oxo-2′-deoxyguanosine and increased expression of hMTH1 protein in brain tumors. Neuro Oncol. 2001;3:73–81. doi: 10.1093/neuonc/3.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- Mao X, Yu CR, Li WH, Li WX. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18:879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- Naughton R, Quiney C, Turner SD, Cotter TG. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23:1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Raitano AB, Halpern JR, Hambuch TM, Sawyers CL. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc Natl Acad Sci U S A. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS., Jr. A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 1998;12:968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- Schimmel M, Bauer G. Proapoptotic and redox state-related signaling of reactive oxygen species generated by transformed fibroblasts. Oncogene. 2002;21:5886–5896. doi: 10.1038/sj.onc.1205740. [DOI] [PubMed] [Google Scholar]
- Senthil K, Aranganathan S, Nalini N. Evidence of oxidative stress in the circulation of ovarian cancer patients. Clin Chim Acta. 2004;339:27–32. doi: 10.1016/j.cccn.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Nox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Tang F, Tang G, Xiang J, Dai Q, Rosner MR, Lin A. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2002;22:8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr., Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt PK. Jun, the oncoprotein. Oncogene. 2001;20:2365–2377. doi: 10.1038/sj.onc.1204443. [DOI] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell. 2004;13(3):329–40. doi: 10.1016/s1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Trachootham D, Lu W, Carew J, Giles FJ, Keating MJ, et al. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22:1191–1199. doi: 10.1038/leu.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T, et al. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol. 2005;145:178–192. doi: 10.1038/sj.bjp.0706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.