Abstract
Background
Recent studies suggest that polymorphisms in genes encoding enzymes involved in drug detoxification and metabolism may influence disease outcome in pediatric acute lymphoblastic leukemia (ALL). We sought to extend current knowledge by using standard and novel statistical methodology to examine polymorphic variants of genes and relapse risk, toxicity, and drug dose delivery in standard risk ALL.
Procedure
We genotyped and abstracted chemotherapy drug dose data from treatment roadmaps on 557 patients on the Children’s Cancer Group ALL study, CCG-1891. Fourteen common polymorphisms in genes involved in folate metabolism and/or phase I and II drug detoxification were evaluated individually and clique-finding methodology was employed for detection of significant gene-gene interactions.
Results
After controlling for known risk factors, polymorphisms in four genes: GSTP1*B (HR=1.94, p=0.047), MTHFR (HR=1.61, p=0.034), MTRR (HR=1.95, p=0.01), and TS (3R/4R, HR=3.69, p=0.007), were found to significantly increase relapse risk. One gene-gene pair, MTRR A/G and GSTM1 null genotype, significantly increased the risk of relapse after correction for multiple comparisons (p=0.012). Multiple polymorphisms were associated with various toxicities and there was no significant difference in dose of chemotherapy delivered by genotypes.
Conclusions
These data suggest that various polymorphisms play a role in relapse risk and toxicity during childhood ALL therapy and that genotype does not play a role in adjustment of drug dose administered. Additionally, gene-gene interactions may increase the risk of relapse in childhood ALL and the clique method may have utility in further exploring these interactions. childhood ALL therapy.
Keywords: genotype, acute lymphoblastic leukemia, prognosis, toxicity, gene-gene interactions
Introduction
Although the majority of children treated for acute lymphoblastic leukemia (ALL) are cured, disease recurrence and treatment related toxicities remain important, and often unpredictable, clinical issues. Recently, evaluation of multiple biologic characteristics of both the patient and the blast cells has improved prediction of treatment outcomes.[1] A number of studies have shown that response to chemotherapeutic agents, as well as drug-related toxicity, can vary with polymorphism frequency.[2–4] Most of the work in this area has centered on single-nucleotide polymorphisms (SNPs) on the genes encoding thiopurine methyltransferase (TPMT), thymidylate synthase (TS), and methylenetetrahydrofolate reductase (MTHFR).
We sought to validate and extend these previous pharmacogenetic analyses by examining the relationship between germline genetic polymorphisms, chemotherapy dose delivered, disease relapse, infection, and toxicity risks in the Children’s Cancer Group trial CCG-1891, a phase III trial for standard risk ALL. We have previously shown in this sample set that the MTHFR C677T allele was associated with a statistically significantly increased risk of relapse.[5] This result, along with the work of other investigators, led us to undertake a more comprehensive genotyping and statistical analysis to determine the role of host genetic polymorphisms in determining treatment response.
While prior studies, including our own, have evaluated odds ratios, relative risks, and hazard ratios associated with genotype status, these metrics have well described limitations in the clinical prediction of treatment outcomes.[6] Specifically, clinicians treating patients with ALL need to know the probability of relapse for a given genotype rather than an odds ratio which describes the relative increase in risk. To address this gap in research knowledge, we estimated the positive predictive value (PPV) for relapse for all genotypes under consideration.
Furthermore, prior investigations have evaluated gene-gene interactions that would determine relapse and toxicity risk. However, these searches have not employed an exhaustive method that would evaluate all possible interactions while appropriately controlling for multiple comparisons. We sought to address this gap by applying the clique methodology with appropriate control for multiple comparisons to comprehensively evaluate all two-way gene-gene interactions in our data.[7] While multiple methods exist for searching for gene-gene interactions including classification and regression trees (CART)[8], multi-factor dimensionality reduction[9], random forests[10], support vector machine[11], neural networks[12] and others, these approaches have important limitations. The clique methodology has been shown to be superior to CART in simulation studies[7], and is less sensitive to assumptions of underlying biologic model and hierarchy. Moreover, the clique methodology accommodates heterogeneity in the etiology of the outcome of interest.
For these analyses, we obtained DNA samples from children with standard-risk ALL in a well-defined cohort in a Children’s Cancer Group study and abstracted 90,000 drug administration doses from patient roadmaps. We analyzed 14 candidate genes that were selected based on their known involvement in either folate metabolism or Phase I and II drug detoxification. We hypothesized that germline genetic variation would be associated with relapse risk and that we would be able to determine the PPV for genotypes of interest and determine relevant gene-gene interactions using the clique methodology.
Materials and Methods
Patient Population and Study Design
CCG-1891 enrolled 1204 NCI standard risk ALL patients in a three-armed randomized trial that has been previously reported.[13] Patients were treated with modified Berlin-Frankfurt-Munster (BFM) therapy and were randomized at diagnosis to a single delayed intensification, two delayed intensifications, or a single delayed intensification with compressed vincristine/prednisone pulses in maintenance. The doses of mercaptopurine (6-MP) and methotrexate (MTX) prescribed were identical among treatment arms. At time of study enrollment, patient guardians gave permission for the use of archived samples in future studies. The Children’s Oncology Group (COG) scientific council, CTEP, the Children’s Hospital of Philadelphia Institutional Review Board and the University of Pennsylvania Institutional Review Board approved this research.
A nested case-control design was employed as the study design. The primary outcomes of interest were disease relapse, occurrence of toxicity, and infectious complications. For relapse risk analyses, patients were considered cases if they experienced disease relapse at any site and controls if they remained in continuous remission as defined by < 5% blasts on bone marrow aspirate or absence of documented extramedullary disease. For toxicity and infection analyses, cases were defined as patients who experienced the toxicity or infection under analysis and controls were patients who did not experience the toxicity or infection under analysis. All patients with available genotype and toxicity data were included in the analyses.
Sample and Data Collection
Data on patient and leukemia characteristics, toxicities, and infectious complications were collected prospectively by local institutions using CCG case report forms. Data on disease relapse are reported in an ongoing manner. Toxicity and infection data were graded according to the CCG toxicity criteria which are similar to CTC v 2.0. These included, but were not limited to, grade III and IV central nervous system (CNS), diarrhea, hyperbilirubinemia, peripheral neuropathy, and transaminitis. Infectious complications included fever with neutropenia, sepsis, interstitial pneumonia, and other infections. Data on 6-MP and MTX doses were abstracted from treatment roadmaps and entered into a relational database in MS Access. The relational database was programmed with data validation rules and all recorded drug doses were checked against the paper records by a second data entry operator.
Laboratory Analyses
The COG Biopathology Center provided marrow and peripheral blood slides on patients. DNA extraction was performed in a dedicated DNA extraction area, as previously reported. Each extraction was performed with a separate set of gloves and consumable items and the extraction area was cleaned with 70% ethanol between extractions. Genotyping was performed with different methodologies: DHFR, TS-6 bp insertion, MTRR, MTHFR, RFC, TPMT, C3A4, C3A5, GSTP, and UGT genotypes were assayed on the Pyrosequencing platform, SHMT genotypes were assayed by direct sequencing, and TS-28 bp insertion, GSTT1, and GSTM1 were assayed by standard RFLP techniques.[3,14] All primer sequences are available from the corresponding author. The technician performing the assays was blind to the case/control status of the sample. All PCR reactions were set up in a dedicated PCR area with dedicated pipettes and reagents. All RFLP digest and Pyrograms were reviewed by 2 individuals who were blind to the case/control status of the sample. All genotype calls were checked again prior to statistical analysis.
Statistical Analysis
The primary outcomes of interest were disease relapse, occurrence of toxicity, and infectious complications. All patients with genotype data were analyzed. Univariate analyses were performed with standard summary statistics and a chi-squared test. A t-test was used to compare drug dose delivered between groups of interest. Relapse free (RFS) survival was estimated by the Kaplan-Meier method and the log-rank test was used to compare time to relapse. Cox proportional hazards modeling accounting for clinically important covariates (age, gender, ethnicity, and day 7 bone marrow response) was used to model relapse risk.
Time dependent positive and negative predictive values (PPV, NPV) for genotypes were estimated as described by Moskowitz and Pepe.[15,16] Statistical significance for PPV and NPV comparisons was determined by bootstrapping simulations performed in STATA version 10 (STATA Corporation, College Park, TX).
Clique analysis was performed as previously described.[7] In brief, the analysis evaluated the effect of all two-way and three-way genotyping combinations among the 14 SNPs studied. For each set, an odds ratio was computed that estimated the risk associated with that particular SNP combination. The 10 combinations with the largest protective and adverse effects were ranked, and the pathways contained in these patterns were examined. Correction for multiple comparisons was performed as described by Benjamini and Hochberg.[17]
Results
Table I presents the characteristics of all patients on CCG-1891. There were 557 genotyped patients and 647 patients who did not have DNA available for genotyping. The 557 patients who were genotyped were representative of the entire study population. Specifically, there were no differences in age, gender, treatment arm, and day 7 bone marrow status between genotyped and non-genotyped patients. African-American patients were over-represented in the patients experiencing relapse. However, the frequency of other ethnic groups was similar between patients with and without relapse.
Table I.
Patient characteristics
| Age | Not Genotyped | Genotyped | ||
|---|---|---|---|---|
| 4 years | 4 years | |||
| Gender | ||||
| Male | 437 | 67.5% | 381 | 68.4% |
| Female | 210 | 32.5% | 176 | 31.6% |
| Ethnicity | ||||
| White | 494 | 76.4% | 420 | 75.4% |
| Black | 16 | 2.5% | 31 | 5.6% |
| Hispanic | 99 | 15.3% | 67 | 12.0% |
| Asian | 14 | 2.1% | 13 | 2.3% |
| Other | 24 | 4.9% | 26 | 6.2% |
| Regimen | ||||
| A | 226 | 34.9% | 180 | 32.3% |
| B | 216 | 33.4% | 186 | 33.4% |
| C | 205 | 31.7% | 191 | 34.3% |
| Day 7 BM | ||||
| M1 | 295 | 45.7% | 253 | 45.4% |
| M2 | 133 | 20.6% | 130 | 21.9% |
| M3 | 140 | 21.9% | 122 | 10.8% |
| Unknown | 78 | 11.8% | 52 | 10.8% |
| Relapse | ||||
| Bone marrow | 44 | 47.8% | 77 | 58.8% |
| CNS | 36 | 39.1% | 43 | 32.8% |
| Testicular | 9 | 9.8% | 10 | 7.6% |
| Other | 3 | 3.3% | 1 | 0.8% |
Supplemental Table I summarizes overall and ethnic group genotype frequencies. Observed genotype frequencies were consistent with previously reported frequencies and were in Hardy-Weinberg equilibrium.
Genotype, relapse free survival estimates, and Cox proportional hazards for the most significant polymorphisms are presented in Table II. The complete list of relapse free survival estimates by genotype is found in Supplemental Table II. After adjustment for known prognostic factors, one gene (GSTP1) responsible for Phase II drug metabolism was associated with a statistically significantly increased hazard rate of leukemia relapse. Patients who were homozygous for the GSTP variant allele showed a significant decrease in RFS as compared to patients with the wild- type allele (HR=1.94; 95% CI, 1.010–3.732; P= .047). Three genes (MTHFR, MTRR, TS) involved in folate metabolism were also associated with a decreased RFS: MTHFR C677T (HR=1.61; 95% CI, 1.036–2.505; P=.034), MTRR G/A (HR=1.95: 95% CI, 1.175–3.227, P=.01) and TS 3R/4R (HR=3.69; 95% CI, 1.436–9.481; P=.007). Furthermore, the association of two genes (GSTT1 and TPMT) approached, but did not reach, statistical significance after correction for multiple testing.
Table II.
Genotype frequencies and relapse free survival*
| Gene | Genotype | Total N | Relapse N | RFS | 95% CI | HR* | 95% CI HR* | P value* |
|---|---|---|---|---|---|---|---|---|
| TS 28 bp insertion | 2R/2R | 83 | 15 | 0.80 | .691–.877 | 1 | reference | -- |
| 2R/3R | 196 | 38 | 0.79 | .726–.845 | 1.68 | 0.863–3.255 | 0.13 | |
| 3R/3R | 103 | 27 | 0.73 | .631–.806 | 1.87 | 0.942–3.721 | 0.074 | |
| 3R/4R | 20 | 7 | 0.60 | .342–.789 | 3.69 | 1.436–9.481 | 0.007 | |
| MTHFR C677T | C/C | 224 | 41 | 0.81 | .747–.854 | 1 | reference | -- |
| C/T | 187 | 46 | 0.74 | .667–.798 | 1.61 | 1.036–2.505 | 0.034 | |
| T/T | 72 | 20 | 0.70 | .578–.798 | 1.36 | 0.763–2.411 | 0.3 | |
| MTRR | A/A | 144 | 26 | 0.80 | .720–.860 | 1 | reference | -- |
| G/A | 225 | 62 | 0.72 | .650–.770 | 1.95 | 1.175–3.227 | 0.01 | |
| G/G | 128 | 23 | 0.81 | .724–.867 | 1.23 | 0.672–2.241 | 0.51 | |
| GSTP1*B | A/A | 147 | 26 | 0.81 | .738–.869 | 1 | reference | -- |
| G/A | 176 | 42 | 0.75 | .678–.809 | 1.47 | 0.889–2.442 | 0.13 | |
| G/G | 55 | 16 | 0.68 | .529–.789 | 1.94 | 1.010–3.732 | 0.047 | |
| TPMT (460 and 719) | Normal | 465 | 98 | 0.78 | .733–.812 | 1 | reference | |
| Heterozygote | 31 | 9 | 0.69 | .486–.828 | 1.86 | .932–3.725 | 0.078 | |
| Variant | 1 | 1 | -- | -- | -- | -- | -- | |
| GSTT1 | Present | 433 | 100 | 0.76 | .712–.794 | 1 | reference | -- |
| Null | 65 | 9 | 0.85 | .740–.922 | 0.54 | 0.271–1.078 | 0.081 |
Adjusted for age, gender, ethnicity, and day 7 bone marrow response
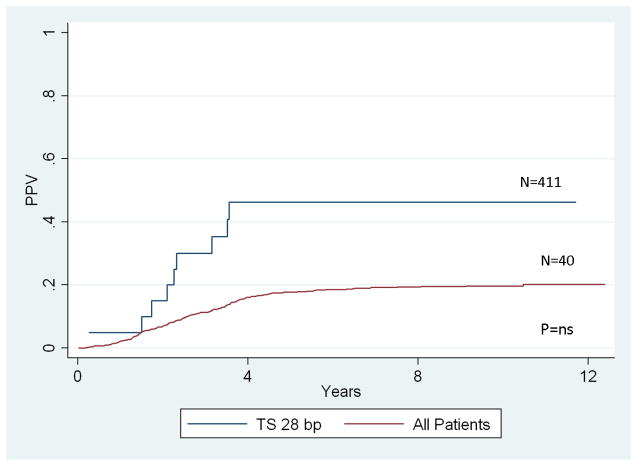
Analysis of genotype positive predictive value was performed for all genotypes that were significantly associated with relapse risk. Figure I illustrates the PPV for the TS 28 bp insertion which had the largest effect size (HR = 3.69) of the significantly associated genotypes. The positive predictive value of the TS 28 bp genotype was not statistically significant (p > 0.5). That is, knowledge of the TS 28 bp genotype did not significantly improve the prediction of relapse risk over the baseline prediction ability based on well-established risk factors for relapse (Figure 1).
Figure 1.
Positive predictive value of TS 28bp insertion and risk of relapse.
The analysis of genotypes associated with altered leukemia-free survival and non-hematologic and infectious toxicities are presented in Table III. All toxicity data that was reported for patients enrolled on CCG1891 was collected. However, analysis was limited to those toxicities believed to be related to drugs whose metabolism may have been affected by those polymorphisms under investigation.
Table III.
Toxicities by genotype
| Gene | Genotype | n | N with toxicity | toxicity | OR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| TPMT (416 and 719) | Normal | 465 | 304 | Fever/Neutropenia | 1 | 0.0051 | |
| Heterozygote | 31 | 27 | Fever/Neutropenia | 3.85 | 1.307–11.320 | ||
| Variant | 1 | 1 | Fever/Neutropenia | ||||
| TPMT (416 and 719) | Normal | 465 | 264 | Infection | 1 | 0.015 | |
| Heterozygote | 31 | 24 | Infection | 2.73 | 1.147–6.515 | ||
| Variant | 1 | 0 | Infection | ||||
| TPMT (416 and 719) | Normal | 465 | 179 | Transaminitis | 1 | 0.0004 | |
| Heterozygote | 31 | 3 | Transaminitis | 0.17 | 0.051–0.568 | ||
| Variant | 1 | 0 | Transaminitis | ||||
| MTHFR C677T | C/C | 224 | 78 | Transaminitis | 1 | 0.019 | |
| C/T | 187 | 83 | Transaminitis | 1.52 | 1.015–2.271 | ||
| T/T | 72 | 21 | Transaminitis | 0.7 | 0.388–1.272 | ||
| TS 28 bp insertion | 2R/2R | 83 | 8 | Neuropathy | 1 | 0.015 | |
| 2R/3R | 196 | 13 | Neuropathy | 0.64 | 0.261–1.591 | ||
| 3R/3R | 103 | 1 | Neuropathy | 0.09 | 0.012–0.768 | ||
| 3R/4R | 20 | 1 | Neuropathy | ||||
| GSTM1 | Present | 185 | 78 | Transaminitis | 1 | 0.021 | |
| Null | 311 | 99 | Transaminitis | 0.64 | 0.439–0.935 | ||
| UGT1A1 TA insertion | 6/6 | 224 | 7 | Hyperbilirubinemia | 1 | 0.045 | |
| 5/6 | 5 | 1 | Hyperbilirubinemia | ||||
| 5/7 | 1 | 0 | Hyperbilirubinemia | ||||
| 6/7 | 206 | 7 | Hyperbilirubinemia | 0.79 | 0.292–2.146 | ||
| 6/8 | 2 | 0 | Hyperbilirubinemia | ||||
| 7/7 | 62 | 7 | Hyperbilirubinemia | 3.27 | 1.154–9.256 | ||
| 7/8 | 3 | 2 | Hyperbilirubinemia | ||||
| GSTT1 | Present | 433 | 18 | Hyperbilirubinemia | 1 | 0.047 | |
| Null | 65 | 7 | Hyperbilirubinemia | 2.74 | 1.079–6.984 |
The MTHFR C677T variant allele showed an increased risk of transaminitis as compared to the wild-type allele (OR=1.52; 95% CI, 1.015–2.271; P=.019). Patients who were heterozygous for TPMT variants were found to have an increased risk of fever and neutropenia (OR=3.85; 95% CI, 1.307–11.320; P=.0051) and infection (OR=2.73; 95% CI, 1.147–6.515; P=.015). In addition, the UGT1A1 TA insertion polymorphism was associated with an increased risk of hyperbilirubinemia with an OR = 3.27 (95% CI 1.154–9.256, p = 0.045) for the UGT1A1 7/7 TA insertion. Other significant associations were observed for transaminitis with RFC-1 and GTSM1, and infection with the TS-6 bp insertion.
Data on the percent of expected chemotherapy dose actually prescribed throughout maintenance is presented in Table IV. Delivered doses of methotrexate range between 69.3 and 77.8% and those of 6-MP were 71.8–81.2% of prescribed doses. The differences in exposure to 6-MP and MTX are relatively modest for all genotypes studied. In contrast, the delivered doses of prednisone and vincristine were greater than 90% of prescribed doses.
Table IV.
Average chemotherapy exposure by genotype
| Methotrexate | Prednisone | 6-MP | Vincristine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | ||
| TS 28 bp insertion | 2R/2R | 74.4 | 18.6 | 97.5 | 3.5 | 76.3 | 15.4 | 96.4 | 8.0 |
| 2R/3R | 73.2 | 18.3 | 95.6 | 5.9 | 74.4 | 15.9 | 94.8 | 9.8 | |
| 3R/3R | 76.1 | 18.6 | 96.1 | 7.8 | 79.0 | 16.5 | 96.2 | 11.9 | |
| 3R/4R | 77.8 | 17.2 | 96.0 | 5.2 | 81.2 | 11.8 | 96.1 | 4.6 | |
| MTHFR C677T | C/C | 73.4 | 18.8 | 95.9 | 5.2 | 75.3 | 16.1 | 95.4 | 9.7 |
| C/T | 75.4 | 18.9 | 96.5 | 5.1 | 77.2 | 15.9 | 98.5 | 23.2 | |
| T/T | 71.8 | 20.0 | 95.1 | 8.7 | 74.1 | 16.5 | 93.4 | 15.3 | |
| MTRR | A/A | 76.1 | 18.7 | 96.3 | 5.6 | 76.6 | 17.0 | 96.7 | 7.3 |
| G/A | 73.7 | 18.8 | 95.2 | 7.8 | 75.6 | 15.4 | 96.5 | 22.5 | |
| G/G | 72.7 | 18.3 | 96.5 | 5.1 | 75.8 | 15.7 | 94.7 | 12.7 | |
| GSTP1*B | A/A | 73.2 | 17.3 | 95.8 | 7.3 | 76.5 | 14.1 | 96.0 | 7.9 |
| G/A | 74.2 | 18.7 | 95.5 | 7.2 | 74.8 | 16.0 | 96.5 | 25.7 | |
| G/G | 75.8 | 15.8 | 96.5 | 4.9 | 78.0 | 13.7 | 95.5 | 10.8 | |
| TPMT (416 and 719) | Normal | 73.9 | 18.2 | 96.1 | 6.8 | 75.9 | 15.6 | 96.5 | 18.7 |
| Heterozygote | 69.3 | 21.1 | 94.3 | 6.1 | 71.8 | 17.5 | 91.2 | 14.0 | |
| Variant | -- | -- | -- | -- | -- | -- | -- | -- | |
| GSTT1 | Present | 74.3 | 18.3 | 96.0 | 6.7 | 75.9 | 15.7 | 96.2 | 18.1 |
| Null | 71.9 | 21.5 | 95.8 | 5.7 | 75.9 | 16.5 | 96.3 | 5.4 | |
Clique analysis revealed multiple gene combinations that were associated with altered relapse risk. After correction for multiple comparisons and collapsing across minimum support levels, only the combination of the MTRR A/G and GSTM1 null genotype two-way gene-gene interaction was associated with a significantly increased risk of relapse, p = 0.012 at a minimum support level of 30.
Discussion
Although the current treatment regimens available for pediatric ALL provide a survival of greater than 5 years for the majority of children diagnosed with the disease, approximately 15–20% of patients will relapse.[1] Genetic variations in certain key enzymes in chemotherapy metabolism may play a key role in determining relapse risk and treatment related toxicity.[18] Our results confirm previous reports, particularly the impact of TS on relapse risk, and extend our knowledge of the role of other polymorphisms and gene-gene interactions on childhood ALL.
Our data confirm the report of Krajinovic et al. that the TS 28 bp genotype is associated with altered relapse risk.[3] Despite a hazard ratio of 3.7, the TS 28 bp genotype did not significantly improve risk prediction. Despite the lack of statistical significance, the analysis of positive predictive value is informative in several ways. First, the point estimate for the TS 28 bp 3R/4R PPV for relapse is approximately 40%, which is approximately twice the baseline risk for all patients on study. Moreover, the positive predictive value of relapse is much more informative for discussions of altering treatment than the hazard ratio which does not provide any direct information on the absolute magnitude of risk for a group of patients. In this way, this result exemplifies the limitations of standard analyses using measures of association to identify clinically applicable prognostic factors.[19,20] For these reasons, we suggest that the Moscowitz-Pepe methodology for PPV estimation should be included in genotype analyses that evaluate the clinical applicability of germline genetic variation.
Our data are consistent with prior observations that patients with TPMT variants are at increased risk for hematopoietic and infectious complications from ALL therapy.[21] While a statistically significant association between relapse risk and TPMT heterozygous genotype was not observed, the p-value approached statistical significance, p = 0.078. This result is similar to the report from Stanulla et al.[22], who demonstrated an increased risk of minimal residual disease in TPMT heterozygous patients. The absence of statistical significance in our analysis may be due to the expectedly small number of patients with TPMT variant genotypes.
As reported previously by our group and others, these data support the role of MTHFR polymorphisms in determining treatment related outcomes.[5,23,24] Although other investigators have not demonstrated an association between methionine synthase reductase (MTRR) and ALL treatment response,[25,26] we observed a significant association between the MTRR G66A variant allele and relapse risk. This association may be a false positive, or may be a true positive that was not detected by other groups because of more modest study sample sizes.
Other groups have demonstrated conflicting results for the role of GSPT1 variants and ALL treatment response. Krajinovic and Gatedee found no association between GSTP1 genotype and response, while Stanulla et al. reported a decreased risk of relapse that approached statistical significance in patients with the GSTP1 Val/Val genotype.[27–29] Our data support the hypothesis that GSTP1 variants may alter relapse risk, although the direction of effect observed in our data is opposite in direction from that of Stanulla et al.[28] The reason for this difference is unclear, although the sample size from CCG 1891 was substantially larger than the 64 cases and controls reported by the BFM group. Furthermore, differences in therapy among the various patient populations analyzed across the studies may be a factor in the varied findings.
There are multiple methods that can be employed in order to evaluate for gene-gene interactions. While the optimal method is not yet known, we used the clique method which has been used by other research groups extensively. We evaluated second order gene-gene interactions to facilitate the interpretation of results and minimize computational requirements. These analyses discovered one gene-gene interaction that had a substantial effect size and retained statistical significance after correction for multiple comparisons. The interpretation of the MTRR and GSTM1 interaction is not intuitively clear. Neither showed significant effect on univariate analysis and they involve different drug detoxification and metabolic pathways. Thus, this result may be a false positive despite appropriate correction for multiple comparisons. However, the significance after correction for multiple comparisons indicates that this gene-gene interaction should be tested in other sample sets, and if validated, may lead to a better understanding of gene-gene interactions in determining treatment response in ALL.
We also evaluated the effect of various genotypes on the effect of cumulative chemotherapy dose delivered to the patients. Consistent with clinical experience, prednisone and vincristine doses were not substantially adjusted while methotrexate and 6-MP had 20–25% dose reductions in cumulative exposures. While this result is not unexpected, to our knowledge this is the first comprehensive report of chemotherapy dose adjustments in a large cooperative group clinical trial. Interestingly, dose adjustment was not associated significantly with genotype, although no patients had homozygous variant genotypes for TPMT. These results indicate that dose adjustment and the genotypes assessed in this study are not correlated. This provides further confirmation of the results seen with TS 28 bp positive predictive value analysis, namely that statistically significant genotype-outcome associations can be difficult to translate into clinical practice.
This study has several strengths that support the validity of these findings. This was a national prospective clinical trial of standard-risk ALL conducted through the Children’s Cancer Group. As such, patients were on an identical treatment protocol and received standardized supportive care per uniform care guidelines. Moreover, since all patients were enrolled on a CCG trial, the chance of reporting and detection biases are likely less than other study populations. Furthermore, to the best of our knowledge, this is the first study reporting the effect of genotype on relapse risk and drug toxicity with extensive data on drug dose prescribed.
This study also has limitations. We did not genotype all of the functional polymorphisms in all of the genes in the folate drug metabolism pathways. Thus, the candidate genes studied here do not account for all of the genetic variation in folate metabolism. Unfortunately, the limited amount of available DNA prevented more comprehensive genotyping. Furthermore, while substantive, the sample size was not large enough to detect modest effect sizes or to provide very narrow confidence intervals around relapse-free survival estimates. As in any pharmacogenetic study, false positive findings are a concern. We sought to minimize the risk of false positives by limiting the number of subgroup analyses that were performed (e.g. not evaluating CNS versus testicular relapse) and correcting for multiple comparisons in the gene-gene interaction analysis. Likewise, we addressed the issue of population stratification by including ethnicity in the Cox proportional hazards models.
In summary, this report provides additional data supporting the impact of germline genetic variation in ALL relapse and toxicity risk. Our study confirms several associations seen by other groups, and this confirmation lends support to the overall validity of our findings. Furthermore, our report extends the current state of pharmacogenetic research in pediatric ALL in several ways. First, we have applied novel statistical methods to evaluate the clinical utility of incorporating genotype data in treatment stratification. Such evaluation will be critical in the establishment of the clinical utility of genetic variation data. Second, we have demonstrated that the clique analysis can identify gene-gene interactions that withstand correction for multiple comparisons. Additional studies will be needed to confirm these findings, and to determine how germline genotype data may be more broadly used in pediatric ALL therapy.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (grant R01 CA108862)
Footnotes
Contribution: D.M.S. analyzed results, made the figures, and wrote the paper; T.M. performed experiments; J.C. designed the research, analyzed results, and wrote the paper; A.K. designed the research, performed experiments, analyzed results, and wrote the paper; H.Z. performed experiments, analyzed results, and wrote the paper; M.L. performed experiments, analyzed results, and wrote the paper; M.D. analyzed results, and wrote the paper; B.L. designed the research and wrote the paper; T.R. designed the research and wrote the paper; R.A. designed the research, performed experiments, analyzed results and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6(2):149–165. doi: 10.1038/nrd2240. [DOI] [PubMed] [Google Scholar]
- 2.Yang JJ, Cheng C, Yang W, et al. Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. JAMA. 2009;301(4):393–403. doi: 10.1001/jama.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krajinovic M, Costea I, Chiasson S. Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet. 2002;359(9311):1033–1034. doi: 10.1016/S0140-6736(02)08065-0. [DOI] [PubMed] [Google Scholar]
- 4.Gregers J, Christensen IJ, Dalhoff K, et al. The association of reduced folate carrier 80G>A polymorphism to outcome in childhood acute lymphoblastic leukemia interacts with chromosome 21 copy number. Blood. doi: 10.1182/blood-2010-01-256958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aplenc R, Thompson J, Han P, et al. Methylenetetrahydrofolate reductase polymorphisms and therapy response in pediatric acute lymphoblastic leukemia. Cancer Res. 2005;65(6):2482–2487. doi: 10.1158/0008-5472.CAN-04-2606. [DOI] [PubMed] [Google Scholar]
- 6.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford: Oxford University Press; 2003. [Google Scholar]
- 7.Mushlin RA, Gallagher S, Kershenbaum A, et al. Clique-finding for heterogeneity and multidimensionality in biomarker epidemiology research: the CHAMBER algorithm. PLoS One. 2009;4(3):e4862. doi: 10.1371/journal.pone.0004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H. RTREE. 2000 http://masal.med.yale.edu/rtree/Accessed.
- 9.Ritchie MD, Hahn LW, Roodi N, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69(1):138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Chen X, Zhang M, et al. Detecting significant single-nucleotide polymorphisms in a rheumatoid arthritis study using random forests. BMC Proc. 2009;3 (Suppl 7):S69. doi: 10.1186/1753-6561-3-s7-s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szymczak S, Biernacka JM, Cordell HJ, et al. Machine learning in genome-wide association studies. Genet Epidemiol. 2009;33 (Suppl 1):S51–57. doi: 10.1002/gepi.20473. [DOI] [PubMed] [Google Scholar]
- 12.Serretti A, Smeraldi E. Neural network analysis in pharmacogenetics of mood disorders. BMC Med Genet. 2004;5:27. doi: 10.1186/1471-2350-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange BJ, Bostrom BC, Cherlow JM, et al. Double-delayed intensification improves event-free survival for children with intermediate-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2002;99(3):825–833. doi: 10.1182/blood.v99.3.825. [DOI] [PubMed] [Google Scholar]
- 14.Davies SM, Robison LL, Buckley JD, et al. Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia. J Clin Oncol. 2001;19(5):1279–1287. doi: 10.1200/JCO.2001.19.5.1279. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz CS, Pepe MS. Quantifying and comparing the accuracy of binary biomarkers when predicting a failure time outcome. Stat Med. 2004;23(10):1555–1570. doi: 10.1002/sim.1747. [DOI] [PubMed] [Google Scholar]
- 16.Moskowitz CS, Pepe MS. Quantifying and comparing the predictive accuracy of continuous prognostic factors for binary outcomes. Biostatistics (Oxford, England) 2004;5(1):113–127. doi: 10.1093/biostatistics/5.1.113. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- 18.Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer. 2001;1(2):99–108. doi: 10.1038/35101056. [DOI] [PubMed] [Google Scholar]
- 19.Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 20.Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95(9):634–635. doi: 10.1093/jnci/95.9.634. [DOI] [PubMed] [Google Scholar]
- 21.Relling MV, Hancock ML, Rivera GK, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91(23):2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 22.Stanulla M, Schaeffeler E, Flohr T, et al. Thiopurine methyltransferase (TPMT) genotype and early treatment response to mercaptopurine in childhood acute lymphoblastic leukemia. JAMA. 2005;293(12):1485–1489. doi: 10.1001/jama.293.12.1485. [DOI] [PubMed] [Google Scholar]
- 23.Krajinovic M, Lemieux-Blanchard E, Chiasson S, et al. Role of polymorphisms in MTHFR and MTHFD1 genes in the outcome of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2004;4(1):66–72. doi: 10.1038/sj.tpj.6500224. [DOI] [PubMed] [Google Scholar]
- 24.Chiusolo P, Reddiconto G, Casorelli I, et al. Preponderance of methylenetetrahydrofolate reductase C677T homozygosity among leukemia patients intolerant to methotrexate. Ann Oncol. 2002;13(12):1915–1918. doi: 10.1093/annonc/mdf322. [DOI] [PubMed] [Google Scholar]
- 25.de Jonge R, Hooijberg JH, van Zelst BD, et al. Effect of polymorphisms in folate-related genes on in vitro methotrexate sensitivity in pediatric acute lymphoblastic leukemia. Blood. 2005;106(2):717–720. doi: 10.1182/blood-2004-12-4941. [DOI] [PubMed] [Google Scholar]
- 26.Krajinovic M, Robaey P, Chiasson S, et al. Polymorphisms of genes controlling homocysteine levels and IQ score following the treatment for childhood ALL. Pharmacogenomics. 2005;6(3):293–302. doi: 10.1517/14622416.6.3.293. [DOI] [PubMed] [Google Scholar]
- 27.Krajinovic M, Labuda D, Sinnett D. Glutathione S-transferase P1 genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2002;12(8):655–658. doi: 10.1097/00008571-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Stanulla M, Schrappe M, Brechlin AM, et al. Polymorphisms within glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) and risk of relapse in childhood B-cell precursor acute lymphoblastic leukemia: a case-control study. Blood. 2000;95(4):1222–1228. [PubMed] [Google Scholar]
- 29.Gatedee J, Pakakassama S, Muangman S, et al. Glutathione S-transferase P1 genotypes, genetic susceptibility and outcome of therapy in thai childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev. 2007;8(2):294–296. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.