Abstract
The mosquito midgut represents the first barrier that the Plasmodium parasite encounters after blood ingestion from an infected vertebrate. Previous studies identified the Aedes aegypti (L.) mucin-like (AeIMUC1) and short-chain dehydrogenase/reductase (SDR) genes as midgut-expressed candidate genes influencing susceptibility to infection by Plasmodium gallinaceum (Brumpt). Here we used RNA inference (RNAi) by double-stranded RNA injections (dsRNA) to examine ookinete survival to the oocyst stage following individual gene knockdowns. dsRNA gene knockdowns were performed three days prior to P. gallinaceum infections and oocyst development was evaluated at seven days post infection. Mean numbers of parasites developing to the oocyst stage were significantly reduced by 52.3% among dsAeIMUC1 injected females and by 36.5% among dsSDR infected females compared to females injected with a dsβ-gal control. Prevalence of infection was significantly reduced among dsAeIMUC1 and dsSDR injected females compared to females injected with a dsβ-gal control; this represented a two and three fold increase in the number of uninfected individuals, respectively. Overall, these results suggest that both AeIMUC1 and SDR play a role in Ae. aegypti vector competence to P. gallinaceum.
Keywords: Aedes aegypti, Plasmodium gallinaceum, vector competence, innate immunity, malaria, mosquito midgut
Malaria remains one of the most devastating infectious diseases with a global burden for 2002 estimated at 300 to 600 million episodes of clinical Plasmodium falciparum (Welch) malaria (Snow et al., 2005). In previous studies we have investigated molecular interactions between the avian malaria parasite Plasmodium gallinaceum (Brumpt) and the mosquito Aedes aegypti (L.). Our investigations and others have demonstrated that the mucin-like protein (AeIMUC1) is a component of the PM, and is expressed in the mosquito midgut (Morlais & Severson, 2001; Rayms-Keller et al., 2000). The AeIMUC1 gene showed differential allelic polymorphism between a P. gallinaceum susceptible and refractory strain of Ae. aegypti, and it has been suggested that it might be involved in the interaction of ookinetes with the PM and epithelium surface (Morlais & Severson, 2001). Moreover, this gene is upregulated in response to heavy metal exposure in Ae. aegypti (Rayms-Keller et al., 2000) and has heme-binding activity (Devenport et al., 2006). Another gene differentially expressed in the female mosquito midgut is a short-chain dehydrogenase/reductase enzyme (SDR). We have shown that SDR expression is up-regulated within 12–24 hrs post feeding in response to an uninfected or infected blood meal in a P. gallinaceum susceptible strain compared with a refractory strain (Morlais et al., 2003). SDR is a member of a family of enzymes involved in enzymatic metabolism and oxidative/reductive reactions (Persson et al., 2003). Both the AeIMUC1 and SDR genes map to chromosome 2 in Ae. aegypti and are located within previously identified quantitative trait loci (QTL) for P. gallinaceum susceptibility (Morlais & Severson, 2001; Morlais et al., 2003). In this study, we used RNAi techniques to knock down the expression of these genes and investigated the effect on the number of oocysts as a measure of infection prevalence and intensity.
The Aedes aegypti RED strain, which is highly susceptible to P. gallinaceum (Thathy et al., 1994), was reared and maintained in an environmental chamber following our standard procedures (Clemons et al., 2010). Infection with P. gallinaceum was performed generally as previously described (Morlais & Severson, 2001). For each independent replicate, both control and test mosquitoes were allowed to feed on the same chicken within a less than one hour interval. Only completely engorged females were selected and maintained for further studies. Midgut dissections were performed seven days after blood feeding to assess prevalence (frequency of infection) and intensity (numbers of developing oocysts) of infection, respectively.
Fragments of the AeIMUC1 and SDR genes for double-stranded RNA (dsRNA) production were selected based on conserved sequences represented in GenBank. Sequence alignments and consensus sequences were determined using the BioEdit Sequence Alignment Editor 4.7.3 (North Caroline State University, 1999). Individual fragments were amplified from the previously cloned Ae. aegypti RED strain cDNAs (GenBank accession nos. AF308864, AY033626) of each gene (Morlais & Severson, 2001; Morlais et al., 2003). A 478 bp fragment (gene positions 406 to 884) was selected for AeIMUC1 and a 605 bp fragment (gene positions 262 to 867) for SDR; as a control, a 357 bp fragment of the β-galactosidase gene (β-gal) was amplified from pBluescriptIIKS (Info S1). Sense and antisense RNAs were synthesized using the MegaScript kit and purified according to the manufacturer’s instructions. CO2-anesthetized females (2–7 days old) were injected in the thorax with 0.1–0.2 μl of dsRNA solution (~1.5 μg/μl) using a Narishige IM300 Microinjector.
Quantitative real-time PCR (qRT-PCR) was performed using an ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Total RNA was extracted from 5–10 mosquito midguts per replicate with Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was prepared by reverse transcription in a 20 μl reaction with Superscript II (Invitrogen) following the manufacturer’s instructions. All qRT-PCR reactions were performed in triplicate according to the manufacturer’s instructions with 0.4 μl of cDNA in a total volume of 25 μl containing 12.5 μl of SYBR Green PCR Master Mix, 300 nmol of each primer and 0.5 μl of Reverse Transcriptase reaction. Standard curves were prepared for each gene using 10-fold serial dilutions of Ae. aegypti RED genomic DNA and the amplification efficiency for each primer pair was calculated (Info S2). Expression levels were normalized to the ribosomal gene RpS17 (GenBank accession no. AF164153) as an endogenous control (Morlais et al., 2003).
We used qRT-PCR to assess gene expression following dsAeIMUC1 or dsSDR induced knockdown. We injected adult RED strain females ~3 days prior to a P. gallinaceum infected blood meal. Our aim was to reduce target gene expression after the first 24 hours following an infected blood meal, when Plasmodium ookinetes are moving from the gut lumen through the midgut epithelium. RNAi mediated reductions in transcript levels of AeIMUC1 and SDR, compared with transcript levels of the control β-gal, at four days post-injection with dsRNA are shown in Fig. 1A. For dsAeIMUC1, mean transcription levels were reduced 82% compared to control levels. For dsSDR, mean transcriptional levels were reduced 91% compared to control levels. Additionally, we analyzed the transcription levels for AeIMUC at several earlier time points, in light of the evidence provided by Devenport et al. (2006) that mRNAs encoding AeIMUC1 protein are stored in the midgut cells previous to blood feeding. Our results confirm reductions in the transcript levels of AeIMUC1 compared to the control β-gal at four hours before blood feeding (76%) as well as four hours (88%) and 20 hours (69%) after blood feeding (Fig. 1B).
Figure 1.
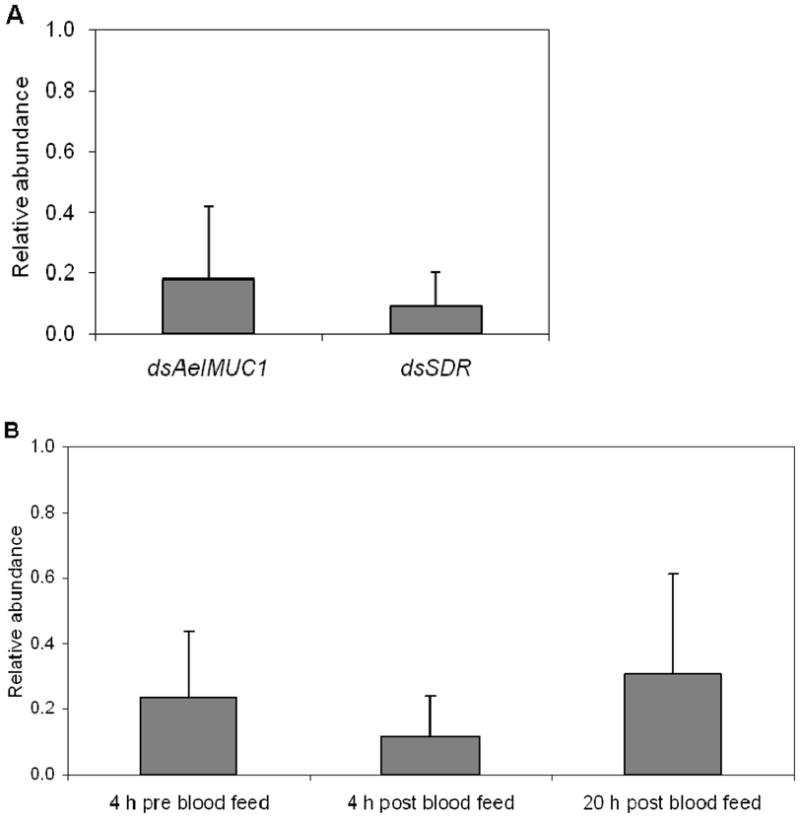
Relative abundance in transcript levels of AeIMUC1 and SDR genes in female midguts of Aedes aegypti injected with the corresponding dsRNAs compared with control mosquitoes injected with dsβ-gal RNA. Transcript data for control and test genes were normalized using RpS17. (A) Results represent means ± SD for real-time PCR analysis of four replicates in each assay. Quantifications were performed at 24 hours after blood feeding. (B) Results represent means ± SD for AeIMUC1 gene real-time PCR analysis of three replicates. Quantifications were performed at 4 hours before and 4 hours and 20 hours after blood feeding.
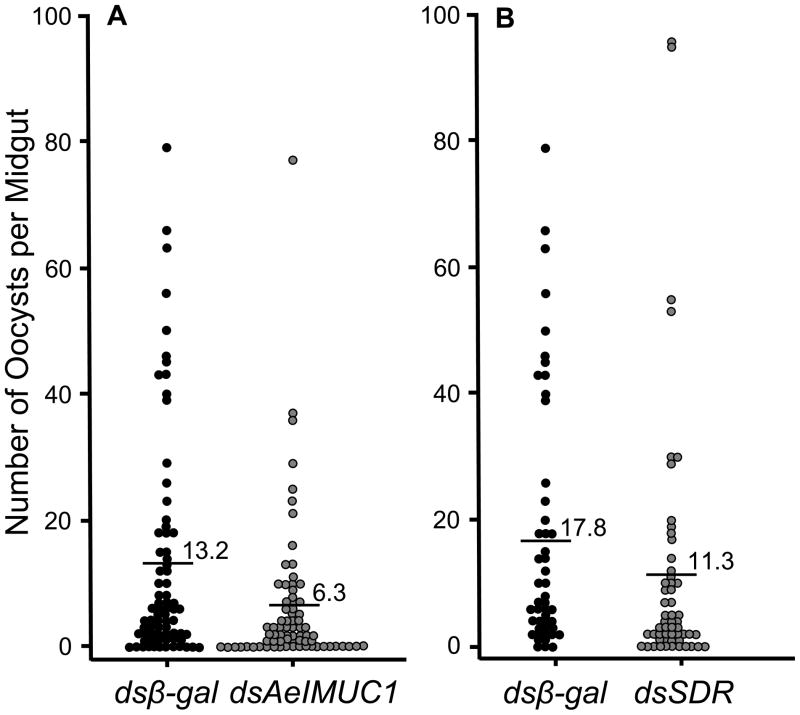
We observed that dsAeIMUC1 induced knockdown had significant effects on Plasmodium development in Ae. aegypti females (Figure 2A, Table 1). The number of parasites developing to the oocyst stage (intensity of infection) was significantly reduced among dsAeIMUC1 injected females compared to females injected with dsβ-gal (P=0.0032, U-test), wherein mean parasite load was reduced by 52.3% among the dsAeIMUC1 injected females. In addition, we observed a significant reduction in prevalence of infection among dsAeIMUC1 injected females compared to females injected with dsβ-gal (P=0.0261, Fisher’s exact test), which represented an ~2-fold increase in the number of uninfected individuals among the dsAeIMUC1 injected females.
Figure 2.
Number of oocysts on midguts of female Aedes aegypti at seven days following a Plasmodium gallinaceum infected blood meal among test gene knockdowns compared to a control (dsβ-gal). Mean numbers of oocysts per midgut are indicated for each dsRNA injected group. (A) dsβ-gal vs. dsAeIMUC1 injected females. (B) dsβ-gal vs. dsSDR injected females. The nonparametric Mann-Whitney U-test was used to compare numbers of oocysts per midgut (intensity of infection) and Fisher’s exact test to compare frequencies of infected vs. uninfected (prevalence of infection) individuals between control and test mosquitoes.
Table 1.
Oocyst development following dsAeIMUC1 and dsSDR induced knockdowns.
| dsRNA | Replicates | Totala | Oocysts per midgutb | P-valuec | Prevalence (%)b | P-valued |
|---|---|---|---|---|---|---|
| β-gal | 4 | 74 | 13.2±18.2 | 84.0 | ||
| AeIMUC1 | 74 | 6.3±11.6 | 0.0032 | 69.0 | 0.0261 | |
| β-gal | 3 | 48 | 17.8±20.6 | 93.75 | ||
| SDR | 55 | 11.3±20.3 | 0.0086 | 80.0 | 0.0383 |
Total number of midguts examined across all replicates.
Mean ± SD for all replicates combined.
Mann-Whitney U-test.
Fisher’s exact test, one-tailed.
We observed that dsSDR induced knockdown had significant effects on Plasmodium development in Ae. aegypti females (Figure 2B, Table 1). The number of parasites developing to the oocyst stage was significantly reduced among dsSDR injected females compared to females injected with dsβ-gal (P=0.0086, U-test), wherein mean parasite load was reduced by 36.5% among the dsSDR injected females. In addition, we observed a significant reduction in prevalence of infection among dsSDR injected females compared to females injected with dsβ-gal (P=0.0383, Fisher’s exact test), which represented an ~3-fold increase in the number of uninfected individuals among the dsSDR injected females.
AeIMUC1 protein has been shown in vitro to bind to chitin, the major component of the PM and also has been associated with microvilli of the midgut epithelium (Devenport et al., 2006). In addition, the protein contains multiple potential O-glycosylation sites and the AeIMUC1 glycoprotein has been suggested as a candidate for a PM recognition molecule by the parasite (Morlais & Severson, 2001). It is well-documented that ookinetes recognize and bind to carbohydrate ligands of glycoproteins on the midgut epithelium (Dinglasan et al., 2005; Ramasamy et al., 1997; Rudin & Hecker, 1989; Zieler et al., 1999, 2000). Further, a potential transmission blocking monoclonal antibody (MG96) appears to recognize a mannose α1–6 substitution on an O-linked oligosaccharide (Dinglasan et al., 2005).
SDRs are a complex family of enzymes that includes greater than 3000 primary protein structures (Kallberg et al., 2002; Oppermann et al., 2003; Persson et al., 2003). These display considerable substrate specificity and intracellular localization, and play important roles in steroid metabolism and carbonyl reduction of potentially toxic xenobiotic compounds including aldehydes, ketones and quinones. It has been suggested that SDRs and other metabolic detoxification enzymes may play important roles in longevity assurance in Caenorhabditis elegans (Maupas), yet because these reactions are energetically costly their relative enzyme activities and subsequent longevity would reflect balancing selection that maximized overall fitness (Gems & McElwee, 2005). The Ae. aegypti Moyo-R strain which shows limited up-regulation of SDR following a blood meal (Morlais & Severson, 2001), was selected for high refractoriness to P. gallinacuem and shows reduced fitness relative to several life history traits including longevity when compared to another closely related strain, Moyo-S which was selected for high susceptibility to P. gallinaceum (Yan et al., 1997). In the present study we observed no differences in survival of the dsSDR and control dsβ-gal groups, indicating that dsSDR induced knockdowns did not influence survival for at least ten days post-injection with dsRNA (data not shown).
In summary, in this study we investigated the roles of the AeIMUC1 and SDR genes in determining Ae. aegypti vector competence to P. gallinaceum. Based on results from RNAi induced gene knockdowns, we conclude that both AeIMUC1 and SDR likely play a role during Plasmodium infection in the mosquito midgut. Although neither gene appears to be a major effector in the immune response against the parasite, their individual actions were observed to significantly impact parasite invasion of the midgut epithelium and subsequent development to the oocyst stage.
Supplementary Material
Acknowledgments
We thank Akio Mori for advice and assistance in mosquito culture and the staff of Freimann Life Sciences Center for assistance with P. gallinaceum infections. Also we are grateful to Diane Lovin and Becky de Bruyn for their invaluable support during this research. This work was supported by grant RO1-AI33127 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
References
- Clemons A, Mori A, Haugen M, Severson DW, Duman-Scheel M. Culturing and egg collection of Aedes aegypti. Cold Spring Harbor Protocols. 2010 Oct 1;(10) doi: 10.1101/pdb.prot5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport M, Alvarenga P, Shao L, Fujioka H, Bianconi M, Olivera P, Jacobs-Lorena M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as heme-binding protein. Biochemistry. 2006;45:9540–9549. doi: 10.1021/bi0605991. [DOI] [PubMed] [Google Scholar]
- Dinglasan RR, Valenzuela JG, Azad AF. Sugar epitopes as potential universal disease transmission blocking targets. Insect Biochemistry and Molecular Biology. 2005;35:1–10. doi: 10.1016/j.ibmb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Gems D, McElwee JJ. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mechanisms of Ageing and Development. 2005;126:381–387. doi: 10.1016/j.mad.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kallberg Y, Oppermann U, Jörnvall H, Persson B. Short-chain dehydrogenase/reductase (SDR) relationships: a large family with eight clusters common to human, animal, and plant genomes. Protein Science. 2002;11:636–641. doi: 10.1110/ps.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlais I, Severson DW. Identification of a polymorphic mucin-like gene expressed in the midgut of the mosquito, Aedes aegypti, using an integrated bulked segregant and differential display analysis. Genetics. 2001;158:1125–36. doi: 10.1093/genetics/158.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlais I, Mori A, Schneider JR, Severson DW. A targeted approach to the identification of candidate genes determining susceptibility to Plasmodium gallinaceum in Aedes aegypti. Molecular Genetics and Genomics. 2003;269:753–64. doi: 10.1007/s00438-003-0882-7. [DOI] [PubMed] [Google Scholar]
- Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B, Jörnvall H. Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chemico-Biological Interactions. 2003;143–144:247–253. doi: 10.1016/s0009-2797(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Persson B, Kallberg Y, Oppermann U, Jörnvall H. Coenzyme-based functional assignments of short-chain dehydrogenases/reductases (SDRs) Chemico-Biological Interactions. 2003;143–144:271–278. doi: 10.1016/s0009-2797(02)00223-5. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Wanniarachchi IC, Srikrishnaraj KA, Ramasamy MS. Mosquito midgut glycoproteins and recognition sites for malaria parasites. Biochimica et Biophysica Acta. 1997;1361:114–122. doi: 10.1016/s0925-4439(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Rayms-Keller A, McGaw M, Oray C, Carlson JO, Beaty BJ. Molecular cloning and characterization of a metal responsive Aedes aegypti intestinal mucin cDNA. Insect Molecular Biology. 2000;9:419–26. doi: 10.1046/j.1365-2583.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Rudin W, Hecker H. Lectin-binding sites in the midgut of the mosquitoes Anopheles stephensi Liston and Aedes aegypti L. (Diptera: Culicidae) Parasitology Research. 1989;75:268–279. doi: 10.1007/BF00931811. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathy V, Severson DW, Christensen BM. Reinterpretation of the genetics of susceptibility of Aedes aegypti to Plasmodium gallinaceum. Journal of Parasitology. 1994;80:705–712. [PubMed] [Google Scholar]
- Yan G, Severson DW, Christensen BM. Costs and benefits of mosquito refractoriness to malaria parasites: implications for genetic variability of mosquitoes and genetic control of malaria. Evolution. 1997;51:441–450. doi: 10.1111/j.1558-5646.1997.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Zieler H, Nawrocki JP, Shahadbuddin M. Plasmodium gallinaceum ookinetes adhere specifically to the midgut epithelium of Aedes aegypti by interaction with a carbohydrate ligand. Journal of Experimental Biology. 1999;202:485–495. doi: 10.1242/jeb.202.5.485. [DOI] [PubMed] [Google Scholar]
- Zieler H, Garon CF, Fischer ER, Shahabuddin M. A tubular network associated with the brush-border surface of the Aedes aegypti midgut: implications for pathogen transmission by mosquitoes. Journal of Experimental Biology. 2000;203:1599–1611. doi: 10.1242/jeb.203.10.1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.