Abstract
Factors contributing to infection risk following cord blood transplantation (CBT) include the use of anti-thymocyte globulin (ATG), prolonged neutropenia, and failure to transfer immunity. To potentially reduce the infection risk we have investigated double unit CBT without ATG, and have evaluated the nature of serious infections in the first year after CBT using this approach. Seventy-two predominantly adult patients were transplanted for hematologic malignancies; 52 patients received myeloablative and 20 had non-myeloablative conditioning. The peak incidence of bacterial infections, fungal infections, or bacterial/ fungal pneumonias was in the first 30 days post-transplant and affected 32%, 14%, and 10% of patients, respectively. Three such infections contributed to early mortality. The peak incidence of viral infections was 31-60 days post-transplant affecting 30% of patients. Cytomegalovirus (CMV) was the most common viral infection. CMV infections prior to day 120 (n = 23) had no relationship with graft-versus-host disease (GVHD), whereas CMV infections after day 120 (n = 5), and all Epstein-Barr virus viremia (EBV, n = 5) and adeno-viral enteritis (n = 2) occurred exclusively in the context of GVHD therapy or corticosteroid use for another indication. Viral infections had the highest lethality: 2 were a direct cause of death and 3 contributed to death. Patients exhibited steady immune recovery achieving a median CD3+4+ T-cell count > 200 cells/microL by day 120, and after day 120 there were no infection-related deaths. Our results suggest that double unit CBT without ATG is associated with prompt T-cell recovery, and unlike CBT incorporating ATG, infection is rarely a primary cause of death. However, CBT without ATG is associated with a significant risk of GVHD and serious infections remain a burden, especially in the setting of GVHD. New strategies are required to further reduce infectious complications after CBT and will require earlier neutrophil recovery and more effective prevention of GVHD, ideally without the profound T-cell depletion associated with ATG.
Introduction
As single unit cord blood (CB) transplantation (CBT) has been associated with a high risk of delayed or failed engraftment, the risk of bacterial and fungal infections in the pre-engraftment period has been substantial and has received much emphasis. In recent years, however, strategies to ensure a sustained donor engraftment incidence of over 90% after CBT1,2, along with improved methods to prevent, detect, and treat infectious complications in allograft recipients, have been effective in decreasing the incidence and lethality of bacterial and fungal3 infections. Viral infections are a particular challenge following CBT due to failure to transfer memory immunity4, and the inadequacy and/or toxicity of currently available anti-viral therapies. Further, viral infection risk can be exacerbated by inclusion of anti-thymocyte globulin (ATG) in the preparative regimen5-9. We, therefore, are investigating double unit CBT without ATG in all CBT recipients with hematologic malignancies at Memorial Sloan-Kettering Cancer Center (MSKCC) in an effort to both augment engraftment and reduce the risk of lethal infections. This study reports the frequency and nature of serious infections in the first year after double unit CBT without ATG, as well as the immune recovery as measured by absolute lymphocyte counts, CD3+4+ and CD3+8+ T-cell counts, and PHA responses.
Methods
Patient and Graft Characteristics
This analysis included all consecutive recipients of first allograft transplanted at MSKCC for the treatment of hematologic malignancies between October 1, 2005 and May 31, 2009 using CB as a stem cell source (n = 72). Patients provided Institutional Review Board approved informed consent for analysis of transplant outcomes. The median follow-up among survivors was 20 months (range 4-48 months). Patient and graft characteristics are summarized in Table 1. Patients had diagnoses of high-risk or advanced hematologic malignancies with a median age of 36 years (range 1-66). They received fludarabine-based conditioning as previously described2 (with the exception of 4 patients in whom the fludarabine was substituted with clofarabine). Thirty-four patients received high-dose myeloablative conditioning that was either hyperfractionated total body irradiation (TBI)-based (n = 30), or used clofarabine, melphalan, and thiotepa (n = 4). Eighteen patients received reduced intensity but myeloablative conditioning with either cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 10 mg/kg, and TBI 400 cGy (n = 11), or melphalan 140 mg/m2, fludarabine 150 mg/m2 (n = 7). Twenty patients received non-myeloablative conditioning with cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, and 200 cGy TBI. No patient received ATG. All patients received calcineurin inhibitor (CNI)- based graft-versus-host disease (GVHD) prophylaxis (predominantly with cyclosporine-A aiming for a therapeutic level of 200-400 ng/ml) with mycophenolate mofetil beginning on day −3. Mycophenolate mofetil was stopped after day 45 or tapered over approximately 2 months according to protocol in the absence of GVHD. CNI was continued at least 3 months post-CBT with subsequent taper in the absence of GVHD. All patients received granulocyte-colony stimulating factor support post-CBT. Double unit grafts were used to enhance engraftment in all patients.
Table 1.
Patient and graft characteristics.
| Characteristics | |
|---|---|
| N | 72 |
| N (%) Male | 41 (56%) |
| Median (range) Age | 36 years (1-66) |
| Median (range) Weight | 68 kg (7-109) |
| N (%) Diagnosis | |
| Acute leukemia | 37 (51%) |
| MDS/ CML | 3 (4%) |
| NHL/ CLL | 22 (31%) |
| HL | 10 (14%) |
| N (%) Conditioning | |
| Myeloablative | 52 (72%) |
| Non-myeloablative | 20 (28%) |
| N (%) Prior Autologous Transplant | 14 (19%) |
| N (%) Recipient CMV+ | 39 (54%) |
| N (%) Recipient EBV+ | 63 (88%) |
| N (%) HLA-A,-B Antigen, -DRB1 Allele Match | |
| 6/6 | 5 (4%) |
| 5/6 | 75 (52%) |
| 4/6 | 64 (44%) |
| Median (range) Infused TNC × 107/kg | |
| Larger unit | 2.56 (1.42-11.33) |
| Smaller unit | 1.94 (0.91-7.09) |
Abbreviations: MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HLA, human leukocyte antigen; TNC, total nucleated cell.
Prevention and Treatment of Infection
Patients were nursed in single high-efficiency particulate air filtered rooms. Patients received quinolone-based prophylaxis for neutropenia. Febrile neutropenia was treated with vancomycin, piperacillin/ tazobactam, and either a quinolone or an aminoglycoside, unless colonized with vancomycin-resistant enterococcus (VRE) in which case vancomycin was substituted with linezolid until the results of blood cultures were known10. During the study period, prophylaxis with vancomycin from day −2 to at least day 7 was instituted in recipients of myeloablative conditioning to prevent Streptococcus viridans sepsis11. All patients received micafungin for fungal prophylaxis during conditioning. Following transplantation, this was switched to a mold active azole (voriconazole or posaconazole) in adults, whereas in pediatric patients micafungin was continued. Patients who developed a fever unresponsive to antibiotics, or had symptoms or signs suggestive of a fungal infection, were treated with a mold active azole or liposomal amphotericin. Mold active fungal prophylaxis was given to all patients with active GVHD requiring systemic corticosteroids.
Prophylaxis against Herpes simplex virus (HSV) consisted of intravenous acyclovir 250 mg/m2 every 8 hours, which was continued orally at discharge. Patients did not receive routine prophylaxis against cytomegalovirus (CMV) but were monitored for CMV viremia by either antigenemia assay or polymerase chain reaction (PCR) and treated preemptively. Pneumocystis jirovecii prophylaxis was with sulfamethoxazole/ trimethoprim or intravenous pentamidine during conditioning, and aerosolized pentamidine or atovaquone was restarted approximately one month post-transplant. Patients routinely received intravenous immunoglobulin monthly for approximately 3 month starting at around day 30. Thereafter, replacement was guided by IgG levels and was triggered by a total IgG < 500 mg/dl. Acyclovir and Pneumocystis prophylaxis were continued for at least 9 months in children, and one year in adults.
Definitions of Infection
Serious infections were collected prospectively for each patient during the first year post-transplant. They were classified as previously described12 with the exception that probable and possible fungal pneumonias without clinical compromise were not automatically life-threatening by definition given the improved prognosis of these infections with extended spectrum azole therapy. Thus, severe infections were those requiring intravenous therapy and/or hospitalization. Life-threatening infections required vasopressors or mechanical ventilation, and also included any viral pneumonias, central nervous system human herpes virus 6 (HHV6), or Toxoplasmosis gondii infections. Lethal infections either caused or contributed to death, whereas the primary cause of death was defined according to the algorithm of Copelan et al13. Mild or moderate infections, and positive blood cultures with coagulase negative Staphylococcus thought to be a contaminant were excluded. Clostridium difficile infections were also excluded unless they were life-threatening or contributed to death. Bacterial infections were either proven or were presumed based on the combination of clinical presentation plus treatment response to anti-bacterial antibiotics. Fungal infections were defined as proven, probable, or possible according to previously reported criteria14. An additional category of “Pneumonia” was included in order to capture patients with fever and pulmonary infiltrates on chest CT scan consistent with either a bacterial or fungal infection. These patients were treated with combined anti-bacterial and anti-fungal therapy and had no evidence of a viral infection but a specific etiology could not be determined. HHV6 viremia was only counted if the patient received specific anti-viral therapy for HHV6. Recurrence intervals were defined for viruses and molds as previously described12. Patients were censored from analysis of infections after day 30 if they had graft failure, and at the time of relapse or disease progression, whereas surviving patients were only included in the analysis of infections in a particular time-period if they had follow-up for the entire time-period.
Measurements of Immune Recovery
Absolute lymphocyte counts (ALC) were measured from day 30 (±7) post-transplant, and ALC, circulating lymphocyte subsets, T-cell phytohemagglutinin-P (PHA) proliferative responses, and CMV CF antigen responses (measured by thymidine incorporation using standard methodology) were measured at 60 (±7), 120 (±15), 180 (±21) days, and 1 year (±45 days) post-transplant as previously described15. PHA responses were analyzed as the percentage of the lower limit of normal (LLN).
Statistical Analysis
The probability of neutrophil and platelet engraftment, acute GVHD, and transplant-related mortality (TRM) were computed using the cumulative incidence function. For neutrophil engraftment and acute GVHD, early death was the competing event, whereas for TRM death from relapse or disease progression was the competing event. Overall survival and progression-free survival were calculated using Kaplan-Meier methodology. The times to first bacterial/ fungal infection or viral infection were calculated separately using a competing risk analysis with early death as the competing event. A univariate cause-specific hazard Cox model was employed to analyze the association between potential risk factors and infection risk. Exposure to systemic corticosteroids was analyzed as a potential risk factor for infection, and was defined as treatment with at least 0.5 mg/kg methylprednisolone or prednisone, or 9 mg/day budesonide, for greater than or equal to 7 consecutive days in the first post-transplant year. The infection risk factors of neutrophil recovery, acute GVHD, corticosteroid exposure and ALC/ CD3+4+ T-cell recovery were assessed using a time-dependent covariate cause-specific hazard Cox model. Due to the small number of patients having more than one infection, a multivariate analysis to assess the total infection burden was not performed. The Cochran-Armitage trend test using the exact method was utilized to assess whether CMV reactivation was associated with a higher T-cell response to CMV.
Results
Transplant Outcomes
The cumulative incidence of sustained donor engraftment by day 45 was 94% (95%CI: 85-100) with 4 patients having graft failure. The donor-recipient HLA-A, -B antigen and -DRB1 allele match of the unit dominating in engraftment in the 68 patients with sustained donor engraftment was 6/6 (n = 1), 5/6 (n = 38), and 4/6 (n = 29), and it had a median infused TNC dose of 2.14 × 107/kg (range 0.91-11.33). The median time to neutrophil recovery was 23 days (range 11-43) in myeloablative, and 11 days (range 7-36) in non-myeloablative CBT recipients. The cumulative incidence of grade II-IV acute GVHD at day 100 was 43% (95%CI: 33-56), with 12 patients having grade II, 15 patients having grade III, and 4 patients having grade IV acute GVHD. After day 100, 20 of 60 (33%) evaluable patients had ongoing active late acute GVHD requiring therapy, chronic GVHD, or overlap syndromes. The day 180 TRM was 21% (95%CI: 13-33). The Kaplan-Meier estimate of overall survival and progression-free survival at one-year were 68% (95%CI: 57-79) and 59% (95%CI: 48-71), respectively. The primary causes of death13 were relapse (n = 7), graft failure (n = 3), GVHD (n = 6), organ failure (n = 7), and infection (n = 2, both viral).
Nature and Incidence of Serious Infections
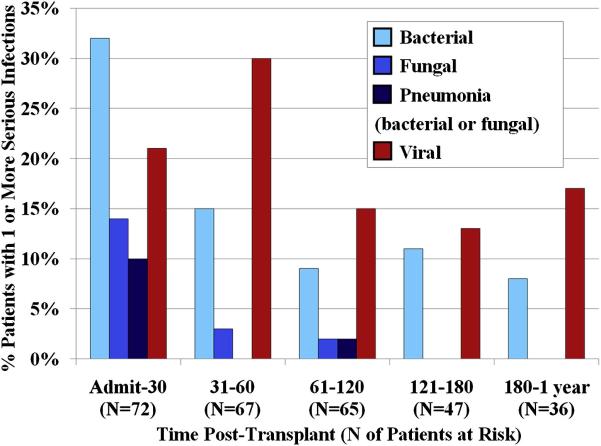
The incidences of serious infections by type and time period are summarized in Figure 1. There were no infections due to Pneumocystis or Toxoplasmosis. Nearly three-quarters (72%) of infections occurred in the first 60 days. Sixty-one percent of patients had one or more infections in the first 30 days, 43% had one or more infections between days 31-60, and 25% had one or more infections between days 61-120 post-transplant. Only 2 infections (both viral) directly caused death according to the criteria of Copelan et al13, but 6 contributed to death with the primary causes of death being subsequent graft failure (n = 2), GVHD (n = 3), and organ failure (n = 1). Thus, 3 of the 6 patients who died of GVHD had bacterial (n = 1) or viral (n = 2) infections contributing to their death. All lethal infections were limited to the first four months post-transplant. After day 120, serious infections were uncommon with 75% of patients being free of serious infections after this time.
Figure 1. Incidence of serious infections by type and time period in the first year post-transplant: there was a marked decrease after day 60 although a low but persistent viral infection risk persisted during the first post-transplant year.
Pneumonias due to a specific organism, or diagnosed as due to a specific infection type (based on the clinical presentation, course, and response to therapy) are included under the bacterial, fungal and viral categories. The category of “Pneumonias” had no organism identified, responded to combined anti-bacterial and anti-fungal therapy, but a specific infection category could not be determined. The fall in patient number is due to lack of complete follow-up or patient death.
Bacterial and Fungal Infections
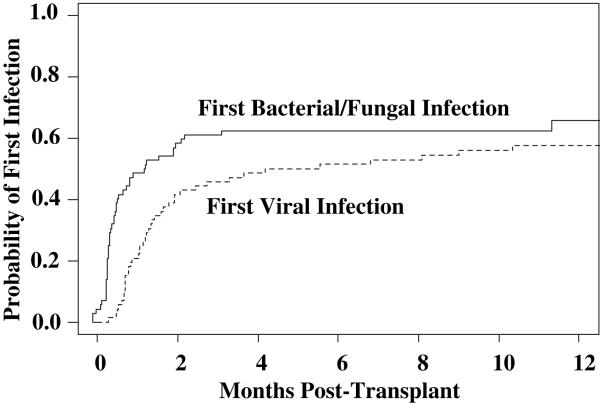
Forty-seven patients had 75 bacterial or fungal infections, or bacterial/ fungal pneumonias. Of these 47 patients, 28 patients had one infection (60%), 13 (28%) had 2 infections, and 6 (13%) had greater than 2 infections. The time to onset of the first bacterial or fungal infection is shown in Figure 2.
Figure 2.
Cumulative incidence of first infection by etiology: bacterial and fungal infections had an earlier onset than viral infections.
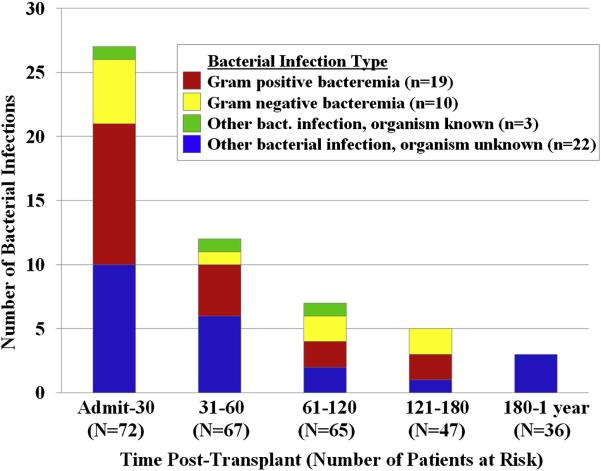
The peak incidence of bacterial infections was in the first 30 days post-transplant (Figure 1). Specific details of bacterial infections are summarized in Table 2 and Figure 3. Only 32 (59%) of infections classified as bacterial had a documented organism. These consisted predominantly of bacteremias (19 gram positive and 10 gram negative) with the most frequent being due to VRE. Of 8 patients with VRE bacteremia, 6 were known to be colonized with VRE whereas 1 was not and 1 was not tested. The majority (n = 11, 92%) of presumed bacterial pneumonias had no organism identified, but all were treated successfully with anti-bacterial antibiotics. Most bacterial infections were classified as severe, and only 2 bacterial infections contributed to death: one patient with Staphylococcus aureus septicemia (onset day 7) with subsequent graft failure and veno-occlusive disease, and one patient with grade IV acute GVHD of the gut who died in the setting of fulminant Clostridium difficile colitis.
Table 2.
Number, type and incidence of serious bacterial infections by post-transplant time period: 72% occurred in the first 60 days, the peak incidence was in the first 30 days, VRE bacteremia was the most common, and only two infections contributed to death.
| Time Period ( N Patients at Risk) |
N Infections// N (%) Patients with One or More Infection |
Type | Severity |
|---|---|---|---|
|
Admit-30
(N=72) |
27 infections// 23/72 (32%) of patients. |
11 Gram+ bacteremia: 5 VRE; 3 Strep. viridans; 2 Staph. aureus; 1 Bacillus species. |
10 severe; 1 lethal*. |
| 5 Gram- bacteremia: 2 E. coli; 1 Klebsiella species; 1 Pseudomonas aeruginosa; 1 Stenotrophomonas maltophilia. |
5 severe. | ||
| 1 Gardnerella vaginalis UTI. | 1 severe. | ||
| 10 Organisms unknown: 6 pneumonia; 3 sinusitis; 1 typhlitis. |
10 severe. | ||
|
31-60
(N=67) |
12 infections// 10/67 (15%) of patients. |
4 Gram+ bacteremia: 1 Enterococcus species (not VRE); 1 VRE; 1 Bacillus species; 1 Micrococcus species. |
4 severe. |
| 1 Gram- bacteremia: 1 Stenotrophomonas maltophilia. | 1 severe. | ||
| 1 Mycobacterium (non-TB) pneumonia. | 1 life-threatening. | ||
| 6 Organisms unknown: 3 pneumonia; 2 sinusitis; 1 typhlitis. |
6 severe. | ||
|
61-120
(N=65) |
7 infections// 7/65 (11%) of patients. |
2 Gram+ bacteremia: 1 VRE, 1 Staph. aureus. | 2 severe. |
| 2 Gram- bacteremia: 2 Stenotrophomonas maltophilia. | 2 severe. | ||
| 1 C. Difficile colitis. | 1 lethal*. | ||
| 2 Organisms unknown: 1 tonsillitis; 1 pharyngitis. | 2 severe. | ||
|
121-180
(N=47) |
5 infections// 5/47 (11%) of patients. |
2 Gram+ bacteremia: 1VRE; 1 Coagulase negative Staphylococcus. |
2 severe. |
| 2 Gram- bacteremia: 1 E. coli; 1 Haemophilus species. | 2 severe. | ||
| 1 Organisms unknown: 1 sinusitis. | 1 severe. | ||
|
180-1
year (N=36) |
3 infections// 3/36 (8%) of patients. |
3 Organisms unknown: 2 pneumonia; 1 sinusitis. | 3 severe. |
Infection contributed to death in 1 patient with Staphylococcus aureus septicemia (onset day 7) with subsequent graft failure and veno-occlusive disease, and one patient with grade IV acute GVHD of the gut who died in the setting of fulminant Clostridium difficile colitis.
Abbreviations: N, number; VRE; vancomycin resistant Enterococcus faecium; Strep, streptococcus; Staph, staphylococcus; UTI, urinary tract infection; TB, tuberculosis.
Figure 3.
Type and number of bacterial infections: serious bacterial infections markedly decreased after day 30.
The majority (10/13, 77%) of fungal infections occurred in the first 30 days post-transplant for an incidence of 14% of patients during this time period (Figure 1). All fungal infections were pneumonias and categorized as severe. In only one patient was there a proven organism (Saccharomyces cerevisiae pneumonia and fungemia). The remaining infections classified as fungal based on clinical presentation and response to therapy were probable in 2 patients, and possible in 10 patients as per published criteria14. The Saccharomyces cerevisiae infection resolved with micafungin and voriconazole, and all remaining patients were treated successfully with mold-active therapy.
Pneumonias that Were Either Bacterial or Fungal
Eight additional patients had pneumonias that responded to combined anti-bacterial and anti-fungal therapy. Seven of the 8 (88%) infections occurred in the first 30 days post-transplant for an incidence of 10% (Figure 1). Seven were severe, and one contributed to death in a patient who subsequently died of organ failure. When combining these 8 cases of bacterial/ fungal pneumonia, with those pneumonias that were classified specifically as either bacterial (n = 12) or fungal (n = 13) in nature, there was a total of 33 bacterial/ fungal pneumonias. Seven cases (21%) underwent diagnostic bronchoscopy, but an organism was identified in only 2 cases. However, all cases but one were successfully treated with anti-bacterial and/or anti-fungal therapy.
Viral Infections
Forty-one patients had 62 viral infections (Table 3 and Figure 4). Twenty-four of these 41 patients (59%) had one infection, 15 (37%) had 2 infections, and 2 (17%) patients had 4 separate viral infection episodes. In contrast to bacterial/ fungal infections that diminished after day 30, the incidence of viral infections increased in the early post-engraftment period (Table 3, Figures 1, 2 and 4) with 30% of patients having one or more serious viral infections between days 31-60. Viral infections had the highest lethality with 2 viral infections (1 CMV pneumonia and 1 HHV6 encephalitis) directly causing death, and 3 contributing to death (Table 3). However, the 5 lethal viral infections occurred exclusively in the first 120 days after transplant. After this time the incidence of viral infections decreased, although non-lethal infections remained a burden during the first year post-transplant affecting 13% of patients between days 121-180 and 17% between days 180-1 year post-transplant. The infectious agent was demonstrated in all viral infections with the exception of 2 clinical diagnoses of shingles due to varicella-zoster virus (VZV).
Table 3.
Number, type and incidence of serious viral infections by time period: the peak incidence was during 31-60 days post-transplant, CMV was the most common infection, and lethal infections were limited to the first 120 days post-transplant.
| Time Period (N Patients at Risk) |
N Infections// N (%) Patients with One or More Infection |
Type | Severity |
|---|---|---|---|
|
Admit-30
(N=72) |
19 infections// 15/72 (21%) of patients. |
4 CMV: 2 viremia, 1 pneumonia*, 1 enteritis/ colitis*. |
2 severe, 1 life-threatening, 1 lethal**. |
| 10 HHV6: 9 viremia, 1 encephalitis. | 9 severe; 1 lethal**. | ||
| 4 BK hemorrhagic cystitis. | 4 severe. | ||
| 1 Adenovirus UTI. | 1 severe. | ||
|
31-60
(N=67) |
21 infections// 20/67 (30%) of patients. |
15 CMV: 12 viremia, 1 pneumonia*, 1 enteritis/ colitis*, 1 URI. |
13 severe, 1 life-threatening, 1 lethal**. |
| 1 HHV6 viremia. | 1 severe. | ||
| 4 BK hemorrhagic cystitis. | 4 severe. | ||
| 1 Adenovirus enteritis/ colitis*. | 1 lethal**. | ||
|
61-120
(N=65) |
10 infections// 10/65 (15%) of patients. |
4 CMV viremia. | 4 severe. |
| 1 Adenovirus enteritis/ colitis*. | 1 lethal**. | ||
| 1 RSV URI. | 1 severe. | ||
| 1 Influenza URI. | 1 severe. | ||
| 2 Herpes Zoster (single dermatome). | 2 severe. | ||
| 1 HSV mucositis. | 1 severe. | ||
|
121-180
(N=47) |
6 infections// 6/47 (13%) of patients. |
3 CMV viremia. | 3 severe. |
| 1 EBV viremia. | 1 severe. | ||
| 1 RSV pneumonia. | 1 life-threatening. | ||
| 1 Influenza URI. | 1 severe. | ||
|
180-1 year
(N=36) |
6 infections// 6/36 (17%) of patients. |
2 CMV viremia. | 2 severe. |
| 4 EBV: 3 viremia, 1 NHL. | 4 severe. | ||
Patients with pneumonia or gut disease also had viremia.
Five patients had lethal viral infections: 1 CMV pneumonia and 1 HHV6 encephalitis directly contributed to death whereas in 3 patients viral infections contributed to death (1 patient with early onset CMV infection who subsequently had graft failure and 2 with adenovirus whose primary cause of death was GVHD).
Abbreviations: CMV, cytomegalovirus; HHV6, human herpes virus 6; UTI, urinary tract infection; URI, upper respiratory tract infection; RSV, respiratory syncytial virus; HSV, herpes simplex virus; EBV, Epstein-Barr virus; NHL, Non-Hodgkin lymphoma.
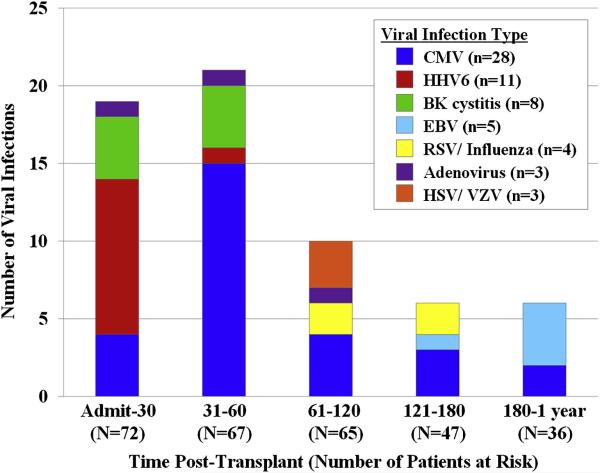
Figure 4. Type and number of viral infections: serious viral infections markedly decreased after day 60.
Notably, however, the incidence remained stable during time periods day 61-1 year (see Figure 1), although mortality from viral infections was limited to the first 120 days.
Twenty-three patients had 28 episodes of CMV infection. CMV accounted for 28 of the 62 (45%) serious viral infections and was therefore the most common viral infection in the study. CMV reactivation occurred at a median of 46 days (range 15-274) post-transplant. It was observed exclusively in CMV sero-positive recipients with 23/39 (59%) of sero-positive CBT recipients reactivating CMV. Eighteen patients had CMV viremia alone (23 infection episodes), 4 patients had CMV disease (2 pneumonias and 2 enteritis/ colitis), and one patient had upper respiratory tract infection. Thus, 4/39 (10%) CMV sero-positive patients developed end-organ CMV disease of the lung or gastro-intestinal tract. Twenty-six CMV infections were treated successfully in 21 patients (91% of patients with CMV infection) with induction therapy of ganciclovir (n = 1), oral valganciclovir (n = 13), or foscarnet (n = 12), whereas CMV disease caused or contributed to death in both cases with pneumonia.
The next most common viral infection (n = 11) was human herpes virus 6 (HHV6). Viremia was treated successfully in 9 patients with foscarnet, whereas one patient had secondary graft failure possibly related to this infection and/ or its therapy, and another had lethal HHV6 encephalitis. Eight patients had BK virus-associated hemorrhagic cystitis; 4 had grade II (macroscopic hematuria) and 4 had grade III (hematuria with clots) disease16. The cystitis spontaneously resolved in all patients. Both HHV6 and BK virus infections were limited to the first 60 days post-transplant.
Five patients (8% of sero-positive patients) reactivated Epstein-Barr virus (EBV) at a median of 215 days (range 104-314) post-transplant. Four had viremia and one had widespread EBV-related post-transplant lymphoproliferative disease. All 4 cases of isolated EBV viremia were successfully treated with rituximab, whereas the patient with lymphoma failed rituximab but was treated successfully with third-party EBV-specific cytotoxic T-lymphocytes17.
Three patients developed adenovirus infections, one with a urinary tract infection and 2 with enteritis/ colitis. Adenovirus contributed to death in both cases of gastro-intestinal disease. The incidences of HSV or VZV infections were low in the first post-transplant year, as was the incidence of lower respiratory tract infections with seasonal viruses.
Immune Recovery
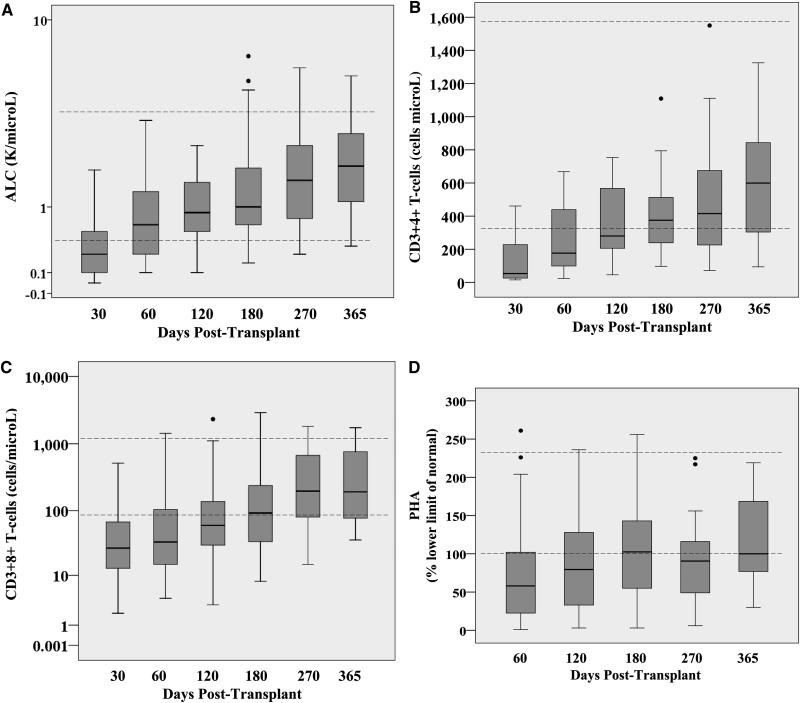
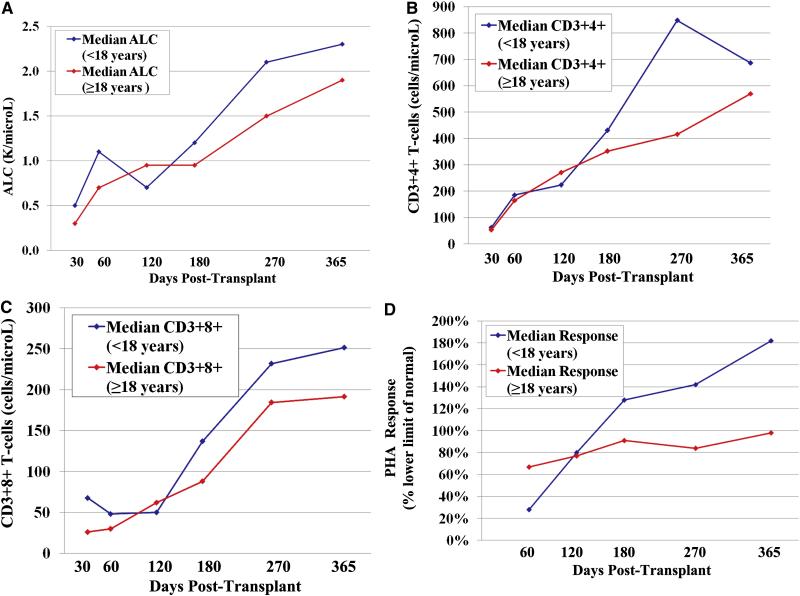
The recovery of the ALC, CD3+4+ and CD3+8+ T-lymphocytes, and PHA responses for all patients are shown in Figure 5. Some patients had sustained low lymphocyte levels and low PHA responses for at least one year. However, the median ALC of 800 cells/microL (range 100-14,200) was within the normal range (500-5,300 cells/microL) by day 60. Moreover, the median CD3+4+ T-cell count of 268 cell/microL (range 20-753) was over 200 by day 120, and was at the lower limit of the normal range (359-1570 cells/microL) by day 180. The median CD3+8+ T-cell count of 92 cells/microL (range 8-2911) was approaching the lower limit of the normal range (98-1217 cells/microL) by day 180. The median PHA response was 80% the LLN (range 3-236) by day 120. Progressive immune recovery was seen in both adult and pediatric patients (Figure 6).
Figure 5. ALC recovery (5A), CD3+4+ T-cell recovery (5B), CD3+8+ T-cell recovery (5C), and PHA response (5D) after double unit CBT: median ALC counts were within the normal range by day 60, the median CD3+4+ T-lymphocyte counts were over 200, and PHA responses at 80% the LLN by day 120 post-transplant, respectively.
The dotted lines (---) represent the normal ranges. The boxes represent the interquartile range, and the solid lines within the boxes represent the median value. The closed circles (•) represent outlier values > 1.5 the interquartile range.
Figure 6.
The median ALC recovery (6A), CD3+4+ T-cell recovery (6B), CD3+8+ T-cell recovery (6C), and PHA response (6D) after double unit CBT according to age: progressive immune recovery is seen in both adult and pediatric patients.
CMV specific immunity was investigated as a measure of functional lymphocyte responses in 58 patients starting at day 60. T-cell responses to CMV were seen as early as 60 days after transplant. While it was not possible to show that the T-cell responses were protective, there was a highly significant association between CMV reactivation and development of CMV-specific T-cell responses (p < 0.0001, Table 4).
Table 4.
T-cell response to CMV: there was a strong association between recipient CMV reactivation and development of a CMV-specific T-cell response.
| N Tested According to Recipient Serostatus & CMV Reactivation |
Positive T-cell Response to CMV / First Time Detected Post-Transplant |
P value |
|---|---|---|
|
CMV sero-negative
(N = 27) |
2 (7%) / Days 180 & 270. |
P < 0.0001 |
|
CMV sero-positive,
no reactivation (N = 12) |
6 (50%) / Days 60 (n = 2), 120 (n = 2), & 270 (n = 2) |
|
|
CMV sero-positive,
reactivation (N = 19) |
15 (79%) / Days 60 (n = 3), 120 (n = 7), 180 (n = 2), 270 (n = 1), & 1 year (n = 2). |
Abbreviations: CMV, cytomegalovirus.
Risk Factors for Developing the First Infection
Univariate analysis demonstrated no correlation between the time to first bacterial or fungal infection (including bacterial/ fungal pneumonias) and patient age, cell dose (infused TNC/kg or CD34+ cell/kg dose of the unit dominating in engraftment), donor-recipient HLA-match (dominating unit 5-6/6 versus 4/6 HLA-match), time to neutrophil recovery ≥ 0.5 × 109/L, acute GVHD (grade 0-I versus II-IV), time to ALC ≥ 500/microL, time to CD3+4+ T-cell count ≥ 200 cells/microL, or exposure to systemic corticosteroids (Table 5). However, univariate analysis identified a delayed time to developing a bacterial/ fungal infection in recipients of non-myeloablative conditioning [hazard-ratio (HR) 0.25 (95%CI: 0.11-0.57) (p = 0.001)], which was also significant (p = 0.007) in multivariate analysis. Univariate analysis for the first viral infection (predominantly CMV viremia) showed no relationship with conditioning intensity (Table 5). Univariate analyses identified recipient age and CMV sero-positivity as significant risk factors for the first viral infection, but only CMV sero-positivity was confirmed on multivariate analysis [HR 3.97 (95% CI: 1.75-9.02) (p = 0.001)].
Table 5.
Univariate analysis using a cause-specific hazard model of time to first infection according to patient/ graft characteristics and transplant outcome: NMA conditioning was associated with a prolonged time to first bacterial/ fungal infection, whereas recipient CMV sero-positivity was associated with a reduced time to first viral infection.
| Variables | Bacterial/ Fungal Infections | Viral Infections | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Recipient age | 1.00 | (0.98-1.01) | 0.73 | 1.02 | (1.00-1.04) | 0.05 |
| Recipient CMV sero-positive | NA | NA | NA | 3.87 | (1.88-7.95) | 0.0002 |
| NMA versus MA conditioning | 0.25 | (0.11-0.57) | 0.001 | 0.59 | (0.28-1.25) | 0.17 |
| HLA-match 4/6 versus 5-6/6 | 1.59 | (0.88-2.90) | 0.13 | 1.14 | (0.60-2.14) | 0.70 |
| Log_TNC dose (dominating unit) | 0.79 | (0.37-1.70) | 0.54 | 0.47 | (0.20-1.12) | 0.09 |
| Log_CD34+ dose (dominating unit) | 0.70 | (0.45-1.08) | 0.11 | 0.81 | (0.54-1.21) | 0.30 |
| Neutrophil recovery * | 0.39 | (0.13-1.15) | 0.09 | 1.10 | (0.45-2.66) | 0.84 |
| Grade II-IV acute GVHD * | 0.72 | (0.19-2.75) | 0.63 | 0.56 | (0.18-1.74) | 0.32 |
| Corticosteroid exposure * | 0.86 | (0.30-2.49) | 0.78 | 0.59 | (0.23-1.56) | 0.29 |
| ALC 500 cells/microL * | 0.50 | (0.13-1.90) | 0.31 | 0.41 | (0.12-1.39) | 0.15 |
| CD3+4+ T-cells 200 cells/microL * | 0.35 | (0.05-2.50) | 0.30 | 0.29 | (0.05-1.60) | 0.16 |
Time-dependent analysis.
Abbreviations: HR, hazard ratio; CI, confidence interval; CMV, cytomegalovirus; NMA, non- myeloablative; MA, myeloablative; HLA, human leukocyte antigen; TNC, total nucleated cell dose; GVHD, graft-versus-host disease; ALC, absolute lymphocyte count; NA, not applicable.
Relationship Between Infection and GVHD or Corticosteroid Exposure for Another Indication
Before day 120, 37/67 (55%) of serious bacterial/ fungal infections (including pneumonias) occurred in patients with grade II-IV acute GVHD or who received systemic corticosteroids for another indication. From day 120-1 year, a similar number (5/8, 63%) of such infections occurred in this context. For serious viral infections, 29/50 (58%) occurred in patients with GVHD or who received corticosteroids before day 120. However, 11/12 (92%) viral infections occurred in this setting from day 120-1 year. Specifically, while only 11/23 (48%) early CMV infections before day 120 occurred in patients with GVHD or receiving corticosteroids, all (5/5, 100%) late CMV infections after day 120 occurred in this setting. All 5 cases of EBV occurred in the context of treatment for GVHD (n = 3) or corticosteroid therapy for another indication (n = 2). Similarly, both cases of gastro-intestinal infection with adenovirus occurred in patients with severe GVHD.
Discussion
Despite CBT being practiced for 2 decades, relatively little is known about the infection risk and immune recovery post-transplant. High rates of infection have been reported with single unit CBT18-20, and use of ATG has been linked to a high risk of viral infections5-9. Further, there is little data available concerning the infection risk and immune recovery after double unit CBT. Improved understanding of infection risk and immune recovery is critical to the implementation of more effective prophylactic and therapeutic anti-microbial strategies. Moreover, immune recovery is of particular interest not only from the standpoint of infection risk, but also given Parkman and colleagues have demonstrated an association between decreased leukemic relapse and successful recovery of immune responses against Herpes viruses after CBT21.
Our study is the first detailed analysis of infection risk after double unit CBT without ATG. We demonstrate that serious opportunistic infections remain a major problem. However, while bacterial and fungal infections were frequent, mortality from such infections was rare. This is likely due to aggressive management of febrile neutropenia, prophylaxis against and coverage of mold infections, and possibly by improved engraftment with double unit grafts. The only risk factor we identified for the development of these early infections was conditioning intensity. Non-myeloablative conditioning was associated with a delayed time to bacterial/ fungal infections, similar to transplantation of other hematopoietic stem cell (HSC) sources22. Additionally, there were no cases of Pneumocystis jirovecii pneumonia or Toxoplasmosis gondii infection. While this success is important, bacterial and fungal infections (including pneumonias) remain a significant burden, especially after myeloablative conditioning. More rapid neutrophil recovery after double unit CBT would be beneficial and will require a larger CB inventory, ex vivo expansion, or other strategies23. Currently, the best prophylaxis for bacterial infections is unclear given the emergence of gram negative organisms resistant to quinolones, and the increasing incidence of other antibiotic resistant organisms such as VRE. Clinical care would also be considerably enhanced with the implementation of improved methods to establish the causative organism for clinical pneumonias, and strategies to prevent VRE bacteremia in colonized patients.
Viral infections, by contrast, were more likely to cause or contribute to death. While HSV and VZV infections were effectively prevented by acyclovir as reported with other HSC sources24,25, CMV infections posed a challenge. Consequently, close monitoring for CMV reactivation is imperative in order to rapidly institute pre-emptive therapy. We found a similar incidence of CMV viremia and disease as previously reported after CBT26-29 indicating that CMV represents a significant risk after double unit CBT despite omission of ATG. Our observation that early CMV infection was not prevented by avoidance of ATG, and had no relationship with GVHD, is consistent with the report of Beck et al29. Further, while CMV-specific T-cell responses developed as early as day 60, CMV contributed to early deaths in 2 patients. Our center does not routinely prescribe CMV prophylaxis given the risk of myelosuppression from ganciclovir/ valganciclovir and the nephrotoxicity of foscarnet. Thus, while our data support the findings of Cohen et al that naïve CB lymphocytes can relatively rapidly generate antigen specific responses30, more effective and less toxic CMV prophylaxis and treatment are needed31. In addition, our CMV CF antigen response is only a semi-quantitative proliferation assay primarily evaluating CD4+ T-cell responses without assessing the cytolytic function of cytotoxic T-cell responses. More sophisticated measures of CMV-specific T-cell responses such as intracellular cytokine-based assays32 would be of interest to more accurately assess CD4+ and CD8+ CMV-specific T-cell recovery, especially comparing CBT with different methods of GVHD prophylaxis.
The incidence of HHV6 viremia at our institution33 was higher than we report in this study, but here only symptomatic patients or those with rapidly rising viral load who received HHV6 therapy were included. High rates of HHV6 viremia have been observed after CBT34-36, and the uncertainty of its significance is extremely problematic. While one of our patients developed graft failure possibly related to HHV6 viremia as reported by Chevallier36, and another died of HHV6 encephalitis37,38, some asymptomatic viremia resolved without therapy. A better understanding of the clinical course of HHV6 is required to differentiate patients that may derive benefit from potentially toxic anti-viral therapy, and those who may be safely observed.
Our incidences of infections with adenovirus4,7 and EBV6,9 appear lower than previously reported after CBT, possibly due to our omission of ATG from the conditioning. Notably, in our study both cases of adenovirus enteritis, and all cases of EBV, occurred exclusively in the context of systemic GVHD therapy or corticosteroid administration for another indication. Monitoring for EBV reactivation is thus warranted in any CBT recipient receiving systemic immunosuppressive treatment for GVHD or corticosteroids for another reason to facilitate immediate pre-emptive therapy. While EBV viremia resolved with rituximab in 4 patients, the single patient with EBV lymphoma required third-party EBV-specific cytotoxic T-lymphocytes17. Both patients with adenovirus gut disease died, and, although the primary cause of death was GVHD, these viral infections contributed to death.
While we were unable to identify any significant predisposing factors to development of the first viral infection other than recipient CMV sero-positivity, it is notable that mortality from all infections, including viruses, was limited to the first 4 months post-transplant, suggesting the development of immune reconstitution sufficient to prevent serious infections even in patients with ongoing GVHD. We examined ALC and CD3+4+ T-cell counts as simple measures of immune recovery given CD3+4+ T-cell recovery is protective against opportunistic infections15,39. We observed more rapid CD3+4+ T-cell recovery than two early reports of ATG-based single unit CBT40,41, and two more recent CBT series of single or double unit CBT in 32 patients each8,9. Komanduri et al reported a median 6 month CD3+4+ T-cell recovery of approximately 100 cells/microL after single unit ATG-based CBT8 as compared with 356 cells/microL in our study. Brown et al found a similarly low median 6 month CD3+4+ T-cell count of approximately 100 cells/microL in adult reduced intensity ATG-based double unit CBT recipients9. Further, the PHA responses in our patients (median 80% LLN by day 120) exceed those previously reported in a single unit ATG-based CBT series (median <50% LLN by 6 months)40,41. While such retrospective comparisons to published series is limited by the confounding factors of potentially differing patient populations and conditioning regimens, differences in immune recovery in the absence of ATG are suggested. Interestingly, while omission of ATG did not prevent early CMV, a benefit was manifest in the risk of later infections such as EBV or adenovirus or late CMV given patients not exposed to GVHD therapy or corticosteroids for another indication were not at risk for EBV, adeno-viral gut disease, or late CMV at all. By contrast, patients with GVHD or corticosteroid therapy had a significant infection risk, with 92% of late viral infections after day 120 occurring in this context.
In summary, the mortality risk from bacterial and fungal infections has been significantly abrogated by anti-microbial agents to prevent and treat infection, and possibly by the use of double unit grafts. While the bacterial/ fungal infection risk of myeloablative conditioning is increased compared with non-myeloablative conditioning, such infections were unlikely to be lethal. Viral infections, especially early CMV infections, remain challenging. However, we show a reduced incidence of EBV and adenovirus infections compared to published literature, possibly due to our omission of ATG. Without ATG we have achieved a high rate of sustained engraftment. Our incidences of GVHD are not prohibitive, although it should be acknowledged that a significant minority of patients will develop severe GVHD. Further, the mortality risk from infection was restricted to the first 4 months post-transplant, with the reduced risk coincident with recovery of ALC, CD3+4+ and CD3+8+ T-cells, and PHA responses. Nonetheless, further improvement is indicated. This will require more rapid neutrophil recovery and more effective prevention of GVHD, ideally without the profoundly T-cell depleting effects of ATG. Improved anti-viral agents and investigation of cellular therapy to augment anti-viral immunity should also be a major priority in CBT. The investigation of more sophisticated measures of immune recovery such as T-cell receptor rearrangement excision circles 8,9, T-cell differentiation8 and T-cell repertoire41 will also be of great interest in ATG-free double unit CBT recipients, although the absence of lethal infections after 4 months post-transplant is the most powerful surrogate marker of immune recovery4. These findings support double unit CBT without ATG, and will act as a baseline for the investigation of new strategies to further prevent infection and augment immune recovery.
Acknowledgements
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research (J.N.B.), the Memorial Sloan-Kettering Cancer Center Society (J.N.B.), the Translational and Integrative Medicine Research Grant (J.N.B.), ASCO Young Investigator Award (C.S.) and P01 CA23766 from the National Institutes of Health (M.A.P., T.N.S., J.N.B.).
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Abboud M, Rice RD, et al. A “no-wash” albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15:1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michallet M, Ito JI. Approaches to the management of invasive fungal infections in hematologic malignancy and hematopoietic cell transplantation. J Clin Oncol. 2009;27:3398–3409. doi: 10.1200/JCO.2008.20.1178. [DOI] [PubMed] [Google Scholar]
- 4.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J, Gandhi M, Naik P, et al. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br J Haematol. 2005;129:229–239. doi: 10.1111/j.1365-2141.2005.05439.x. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Weisdorf DJ, Defor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of anti-thymocyte globulin to a non-myeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robin M, Marque-Juillet S, Scieux C, et al. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007;92:1254–1257. doi: 10.3324/haematol.11279. [DOI] [PubMed] [Google Scholar]
- 8.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JA, Stevenson K, Kim HT, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115:4111–4119. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–621. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe D, Jakubowski A, Sepkowitz K, et al. Prevention of peritransplantation viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clin Infect Dis. 2004;39:1625–1632. doi: 10.1086/425612. [DOI] [PubMed] [Google Scholar]
- 12.van Burik JA, Carter SL, Freifeld AG, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 15.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 16.Droller MJ, Saral R, Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982;20:256–258. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- 17.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of Epstein-Barr virus (EBV)-associated post-transplant lymphoma after cord blood transplant using thrid-party EBV specific cytotoxic T-lymphocytes. Blood. 2010 doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 19.Hamza NS, Lisgaris M, Yadavalli G, et al. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol. 2004;124:488–498. doi: 10.1046/j.1365-2141.2003.04792.x. [DOI] [PubMed] [Google Scholar]
- 20.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 21.Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Junghanss C, Marr KA, Carter RA, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8:512–520. doi: 10.1053/bbmt.2002.v8.pm12374456. [DOI] [PubMed] [Google Scholar]
- 23.Rocha V, Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:S126–132. doi: 10.1016/j.bbmt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Erard V, Wald A, Corey L, Leisenring WM, Boeckh M. Use of Long-Term Suppressive Acyclovir after Hematopoietic Stem-Cell Transplantation: Impact on Herpes Simplex Virus (HSV) Disease and Drug-Resistant HSV Disease. JID. 2007;196:5. doi: 10.1086/518938. [DOI] [PubMed] [Google Scholar]
- 25.Boeckh M, Kim HW, Flowers ME, Meyers JD, Bowden RA. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation--a randomized double-blind placebo-controlled study. Blood. 2006;107:1800–1805. doi: 10.1182/blood-2005-09-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Tomonari A, Takahashi S, Ooi J, et al. Preemptive therapy with ganciclovir 5 mg/kg once daily for cytomegalovirus infection after unrelated cord blood transplantation. Bone Marrow Transplant. 2008;41:371–376. doi: 10.1038/sj.bmt.1705910. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura T, Narimatsu H, Kami M, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant. 2007;13:577–583. doi: 10.1016/j.bbmt.2006.12.454. [DOI] [PubMed] [Google Scholar]
- 29.Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:215–222. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen G, Carter SL, Weinberg KI, et al. Antigen-specific T-lymphocyte function after cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:1335–1342. doi: 10.1016/j.bbmt.2006.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boeckh M, Ljungman P. How I treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:9. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi D, Williams RY, O’Reilly RJ, Koehne G. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood. 2005;105:2793–2801. doi: 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 33.Patel KJ, Rice RD, Hawke R, et al. Pre-engraftment syndrome after double-unit cord blood transplantation: a distinct syndrome not associated with acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;16:435–440. doi: 10.1016/j.bbmt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahu X, Rialland F, Touzeau C, et al. Infectious complications after unrelated umbilical cord blood transplantation in adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2009;15:1531–1537. doi: 10.1016/j.bbmt.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Sashihara J, Tanaka-Taya K, Tanaka S, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood. 2002;100:2005–2011. [PubMed] [Google Scholar]
- 36.Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant. 2009;45:1204–1211. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 37.Drobyski WR, Knox KK, Majewski D, Carrigan DR. Fatal Encephalitis Due to Variant B Human Herpesvirus-6 Infection in a Bone Marrow Transplant Recipient. N Engl J Med. 1994;330:4. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- 38.Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, Kamble R. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:705–709. doi: 10.1038/sj.bmt.1705666. [DOI] [PubMed] [Google Scholar]
- 39.Berger M, Figari O, Bruno B, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41:55–62. doi: 10.1038/sj.bmt.1705870. [DOI] [PubMed] [Google Scholar]
- 40.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- 41.Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7:454–466. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]