Abstract
Prism adaptation may alleviate some symptoms of spatial neglect. However, the mechanism through which this technique works is still unclear. The current study investigated whether prism adaptation differentially affects dysfunction in perceptual-attentional “where” versus motor-intentional “aiming” bias. Five neglect patients performed a line bisection task in which lines were viewed under both normal and right-left reversed viewing conditions, allowing for the fractionation of “where” and “aiming” spatial bias components. Following two consecutive days of prism adaptation, participants demonstrated a significant improvement in “aiming” spatial bias, with no effect on “where” spatial bias. These findings suggest that prism adaptation may primarily affect motor-intentional “aiming” bias in post-stroke spatial neglect patients.
Keywords: Spatial neglect, Prism adaptation, Rehabilitation, Sensory neglect, Motor neglect
Introduction
Spatial neglect is a common disorder following right-hemisphere stroke, causing functional disability [1,2]. Neglect patients typically fail to orient and execute movements toward left-sided stimuli. Among various rehabilitation techniques, prism adaptation may be particularly promising [3]. This procedure consists of training pointing movements toward visual targets while patients wear prism goggles that displace viewed objects rightward. Initially, patients err to the right of the target, in the direction of the visual shift. This initial error is gradually reduced during the exposure phase. Once prisms are removed, patients err leftward, in the direction opposite the visual displacement (aftereffect), and toward the “neglected” hemispace. Although studies have shown that prism adaptation can improve a range of neglect symptoms, not all symptoms improve, nor do all neglect patients improve [2]. Critically, the mechanisms through which prism adaptation affects spatial neglect remains unclear. Knowing more about brain systems responsive to prism adaptation may help our understanding of which symptoms, or which patients, improve optimally after prism adaptation training.
Recent research suggests that the ameliorative effect of prism adaptation may be at least partly due to an influence on motor-intentional “aiming” errors (i.e., planning and executing actions towards the contralesional hemispace), rather than on perceptual-attentional “where” errors [4,5]. In a recent study of healthy participants, we investigated whether prism adaptation differentially affects perceptual-attentional and motor-intentional spatial performance components [4]. Participants performed a computerized line bisection task either under Natural (right-left congruent with reality) or Reversed viewing conditions, in which the visual feedback was horizontally left-right reversed with respect to actual movements in the workspace where participants bisected lines. This paradigm, modified from that of Na et al. [6], allows for the separation of perceptual-attentional and motor-intentional contributions to visually guided spatial performance, as the procedure dissociates the direction of visually-viewed hand movement from the direction of actual hand movements in the workspace. In that study, we observed that prism adaptation selectively improved a motor-intentional “aiming” spatial error component of the line bisection task [4].
In the present study, using the same paradigm, we investigated whether prism adaptation differentially affects dysfunction in perceptual-attentional “where” versus motor-intentional “aiming” bias in neglect patients. If adaptation primarily affects the motor-intentional “aiming” bias, as we observed in healthy individuals, then we would expect a significant reduction in the “aiming” component of the line bisection error after prism adaptation, whereas perceptual-attentional “where” errors would remain unchanged.
Method
Participants
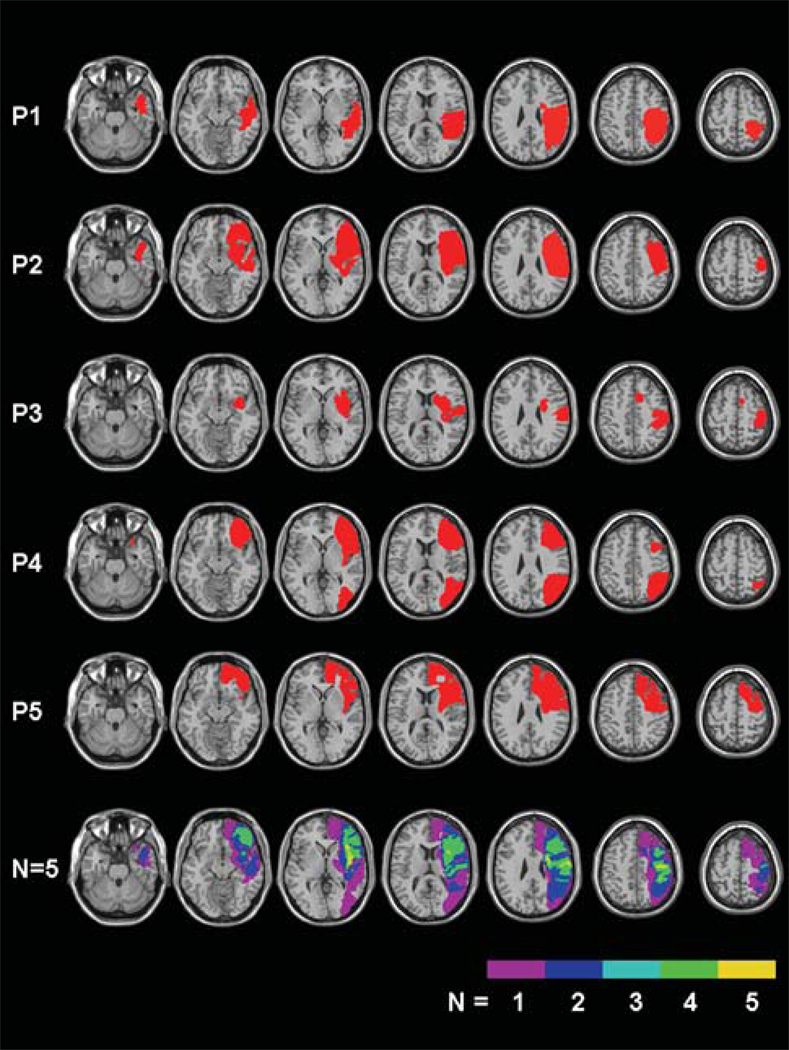
Five consecutive neglect patients with right hemisphere strokes were enrolled from an inpatient rehabilitation hospital after providing written consent. See Table 1 for patient characteristics. Participants were right-handed, with normal or corrected-to-normal vision and no history of other neurological or psychiatric disorders. All participants showed neglect symptoms either on the Behavioural Inattention Test [7] or the Catherine Bergego Scale [8]. The presence of deficits in vision, somato-sensation, and audition was evaluated by a double-stimuli confrontation test [9]. All participants had ischemic (N=4) or hemorrhagic (N=1) stroke, confirmed by CT (N=3) or MR images (N=2). We visualized lesion locations using MRIcro software [10], drawing manually on an MRI template, and using the closest matching transverse slice for each patient. Figure 1 shows the regions of interest (ROIs) for each patient. The areas of greatest lesion overlap were in the frontal-parietal, and frontal-subcortical regions.
Table 1.
Patients’ demographic and clinical data.
| Patient | Age (Years) |
Gender | Education (Years) |
Duration of Disease (Weeks) |
Visual Field |
Tactile Perception |
Auditory Sensation |
BIT | CBS | Etiology, Lesion Site |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 67 | M | 12 | 2 | + | e | e | 109 | 24 | H, P-T |
| P2 | 52 | F | 13 | 5 | e | e | e | 31 | 26 | I, F-P-T |
| P3 | 78 | F | 12 | 2 | + | + | + | 57 | 28 | I, F-P-Bg |
| P4 | 68 | M | 12 | 2 | + | e | e | 14 | 27 | I, F-P-T-O |
| P5 | 51 | M | 12 | 5 | − | + | − | 128 | 25 | I, F-P-Bg |
+/− = presence or absence of deficits. For Visual Field: + = left homonymous hemianopia; for Tactile Perception + = hemianestesia at the left hand; for Auditory Sensation + = auditory loss at the left ear; e: presence of extinction at double stimuli. The BIT (Behavioural Inattentional Test), range 0–146, is rated from 0 (maximum deficit) to 146 (no impairment); cut-off 129.The CBS (Catherine Bergego Scale), range 0–30; each item is rated from 0 (no deficit) to 3 (severe neglect impairment); the cumulative score is rated as “mild” (score 0–10), “moderate” (score 11–20), and “severe (21–30) neglect. I/H: Ischemic/Hemorraegic lesion; F: Frontal, P: Parietal, T: Temporal, O: Occipital, Bg: Basal ganglia.
Figure 1.
Lesion mapping in five right-hemisphere-damaged patients, and lesion overlay plots (bottom row: frequency of overlapping lesions, from violet, N= 1, to yellow, N=5).
Procedure
Assessment of Spatial ”Where” versus “Aiming” Bias
We assessed participants’ “where” and “aiming” biases and prism adaptation aftereffects before and after the two consecutive days of prism adaptation. Participants marked the center of 16 horizontal lines (240 mm length, 3 mm thick), each printed alone on a 278 × 216 mm sheet and presented centrally on a table in front of the participants. Similar to the paradigm of Na et al. [5], participants’ ability to view the line and their arm’s movement directly was prevented by a black cloth. A camera (Sanyo, VCC-5884) positioned 37 cm above the table transferred the image of the line onto a video screen centered 80 cm in front of the participant. Therefore, to bisect lines, participants monitored their hands and the line indirectly via the video screen. Participants first bisected 8 lines in the Natural condition, in which visual information displayed on the video screen was congruent with actual arm movements: rightward movements appeared rightward and leftward movements, leftward. Participants then bisected 8 lines in the Reversed condition, in which a video mixer right-left reversed video feedback such that rightward movements appeared leftward on the video screen, and vice versa. In both conditions, we recorded deviation from the objective midpoint of the line in millimetres (mm), with positive values denoting rightward errors and negative values denoting leftward errors.
We derived participants’ “where” and “aiming” biases by separating Natural and Reversed errors using Equations 1 and 2 [11].
| [Equation 1] |
| [Equation 2] |
Both perceptual-attentional “where” and motor-intentional “aiming” biases contribute to line bisection errors in the Natural and Reversed viewing conditions. However, in the Natural condition these biases are aligned and oriented in the same direction, and thus may contribute additively to performance (Equation 1). In the Reversed condition, however, the “where” bias acts in the direction opposite the “aiming” bias, since the visual feedback is 180-degrees reversed (Equation 2). Algebraically solving these two equations allows quantification of both “where” and “aiming” bias components for each participant. Previous work supported the validity of “where” and “aiming” spatial error fractionation in stroke survivors and controls (see review and data, reference 11).
Prism Adaptation
During prism exposure, participants wore wedge prisms (Bernell™ Deluxe Prism Training Glasses, 20-diopter), displacing the visual field horizontally rightward 12.4°. They performed 60 pointing movements to a visual target located at 0° or 21° to the right or left distal side of a board aligned with the participant’s midsagittal plane. The three target positions (center, right, and left) were presented in a pseudorandom order. During target pointing, a shelf blocked the view of most of the arm’s path, allowing participants to see only the distal part of the movement - i.e. the finger emerging to point to the target. The distal side of the board was marked with a ruler visible only from the experimenter’s side, and pointing error was recorded (in degrees).
We assessed prism adaptation aftereffects with two tests. The visual-proprioceptive test consisted of 6 pointing movements to a visual target presented two times in each of the three positions (0°, 21° right, 21° left) in a pseudorandom order. Although the target was in view, participants could not see their pointing movement, hidden under an occluding shelf. For the proprioceptive test, blindfolded participants pointed 10 times to the position they felt was straight ahead of their body’s center. A transparent panel marked with a ruler and aligned with the participants’ body center allowed the experimenter to measure the distance (in degrees) between indicated and actual target/body center position to determine error in the two tasks, respectively. Rightward errors were recorded as positive, and leftward errors as negative.
Results
Given the small sample size, we used nonparametric statistical analyses to account for anticipated non-normal data distribution.
Error reduction
The presence of error reduction during prism exposure was assessed by comparing pointing errors in the initial and last six trials of prism exposure. Participants made a rightward error in the first six trials (day 1: M = 8.43°, SD = 2.88; day 2: M = 4.67°, SD = 2.34), which was reduced in the last six trials of exposure, on both days (day 1: M = 0.80°, SD = 0.55; day 2: M = 0.65°, SD = 0.43; z = 2.02, p =.043 for both days).
Aftereffects
Participants experienced a significant leftward shift in visual-proprioceptive error after 2 days of prism adaptation (before prism adaptation: M = −0.80°, SD = 1.57; after prism adaptation: M = −7.27°, SD = 1.47; z = 2.02, p = 0.043). Although not significant, the group also experienced a leftward proprioceptive error shift after 2 days of prism adaptation (before prism adaptation: M = 5.07°; SD = 3.30; after prism adaptation: M = −0.60°; SD = 6.76; z = 1.75, p = 0.080). Exploration of individual scores revealed that 4 of 5 participants experienced a leftward shift in proprioceptive error post prism adaptation.
”Where” versus “Aiming” Bias
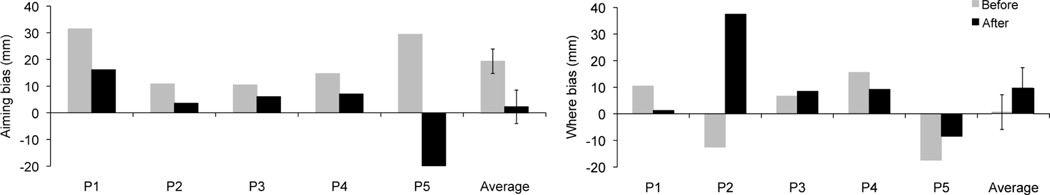
Errors in the Natural and Reversed line bisection conditions appear in Table 2, and fractionated “where” and “aiming” biases are depicted in Figure 2. Critically, the motor-intentional “aiming” bias improved after prism adaptation in all participants. The initial rightward “aiming” spatial error (M = 19.37; SD = 10.27) was reduced after two days of prism adaptation (M = 2.30; SD = 14.03; z = 2.02, p = 0.043). In contrast, no change was detected in pre- (M = 0.53, SD = 14.63) versus post-prism adaptation (M = 9.58, SD = 17.11) perceptual-attentional “where” spatial bias (z = 0.40, p = 0.69).
Table 2.
Patients’ performance on the Natural and Reversed line bisection conditions before and after two days of prism adaptation. Positive value means rightward deviation, and negative value means leftward deviation (mm).
| Natural | Reversed | |||
|---|---|---|---|---|
| Patient | Before | After | Before | After |
| P1 | 41.9 | 17.4 | 21.0 | 14.9 |
| P2 | −1.8 | 41.0 | 23.4 | −33.9 |
| P3 | 17.1 | 14.5 | 3.8 | −2.4 |
| P4 | 30.3 | 16.3 | −0.9 | −2.1 |
| P5 | 12.0 | −29.8 | 46.9 | −12.9 |
| Mean | 19.9 | 11.9 | 18.8 | −7.3 |
Figure 2.
a: Motor-intentional “aiming” bias and b: perceptual-attentional “where” bias. “Where” and “aiming” biases were derived from the fragmentation of the Natural and Reversed line bisection errors (mm, positive/negative scores indicate rightward/leftward errors, error bars indicate SEM). Results refers to the group of five subjects and the average of the group; before (grey column) and after (black column) two days of prism adaptation training.
Discussion
The goal of the current study was to determine whether prism adaptation selectively reduces motor-intentional “aiming” and/or perceptual-attentional “where” spatial bias in neglect patients. In all patients, motor-intentional “aiming” spatial bias improved after two days of prism adaptation. By contrast, perceptual-attentional ”where” spatial bias demonstrated no consistent change after prism adaptation training. These results mirror recent findings from a study in our laboratory of 84 healthy individuals, in whom we observed selective effects of prism adaptation on “aiming”, but not “where” spatial bias [4]. Similarly, others have reported that after prism adaptation neglect patients improved on a standard line bisection task, which has both visual and motor components, but not on the landmark version of this task, which lacks a spatial motor response [5].
Our results demonstrate an important role for the motor-intentional “aiming” spatial systems in response to prism adaptation. These results may help explain the beneficial effect of prism adaptation in neglect patients on motor activation tasks, such as manual tasks under visual guidance (e.g., cancellation or drawing [1]), oculomotor scanning [12], postural imbalance [13], and wheelchair navigation [14]). Our results may also account for previous results in studies recording eye movements in perceptual tasks (e.g., detection of chimeric faces [14,15] and size estimation [16]) revealing a selective effect of prism adaptation on the oculomotor bias, without effect on perceptual-attentional errors.
Our findings are, further, consistent with a recent proposal that prism adaptation may primarily influence the visuomotor circuits of the dorsal visual stream, mediating motor-related processes [18]. Since the dorsal visual pathway is also critically involved in processes related to attentional control, this interpretation may explain the beneficial effects of prism adaptation on covert visual attentional tasks that do not require eye movements [19–21].
The present study does not provide evidence that prism adaptation influences perceptual-attentional “where” spatial errors. However, we do not exclude this possibility, since improvement on perceptual tasks following prism adaptation has been reported in neglect patients [22–25]. Indeed, it has been suggested that prism adaptation could influence perceptual processes indirectly, through connections between the ventral and dorsal visual streams located in the inferior parietal cortex [18]. Finally, it should be noted that, on average, the current group of neglect patients showed a stronger “aiming” than “where” spatial bias before the prism adaptation training (Figure 2). It is possible that prism adaptation may also reduce “where” spatial bias in patients in whom this bias is more strongly present than in the current patients, although results in healthy participants do not support a directionally-specific effect of prism adaptation on “where” spatial bias [4].
Our group of patients had common areas of injury in frontal, parietal, and subcortical brain regions. Further research is needed to systematically test the effects of prism adaptation on “aiming” and “where” systems in large groups of patients with diverse brain lesions.
Conclusion
Our results, together with previous findings [4,5], suggest that prism adaptation training may act primarily on “aiming” spatial bias. This translates to the clinical possibility that neglect patients primarily disabled as a result of “aiming” spatial errors may benefit most from prism adaptation training, whereas those with primarily “where” spatial errors may improve less. Further research is needed to confirm this hypothesis in a large and diverse group of neglect patients.
Acknowledgments
The authors thank Jenny Masmela, Milda Woods, Terry Carolan, Monika Eller, Kimberly Hreha, Naureen Zaidi, Franco Amati, Andrew LeBlanc, and Jeffrey Kornitzer for recruiting patients and staff support, and Marius Peelen for helpful comments on an earlier draft of this manuscript.
Support
This work was supported by the Kessler Foundation, the NIH/National Institute of Neurological Disorders and Stroke K02 NS47099; R01 NS 055808 and, K24 HD062647, the College of Arts & Sciences, Seton Hall University and, the IRCSS Italian Auxological Institute.
References
- 1.Barrett AM, Buxbaum LJ, Coslett HB, Edwards E, Heilman KM, Hillis AE, et al. Cognitive rehabilitation interventions for neglect and related disorders: moving from bench to bedside in stroke patients. Journal of cognitive neuroscience. 2006;18:1223–1236. doi: 10.1162/jocn.2006.18.7.1223. [DOI] [PubMed] [Google Scholar]
- 2.Adair JC, Barrett AM. Spatial neglect: clinical and neuroscience review; a wealth of information on the poverty of spatial attention. Annals of the New York Academy of Science. 2008;1142:21–43. doi: 10.1196/annals.1444.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166–169. doi: 10.1038/25988. [DOI] [PubMed] [Google Scholar]
- 4.Fortis P, Goedert KM, Barrett AM. Prism adaptation differentially affects motor-intentional and perceptual-attentional biases in healthy individuals. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2011.05.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Striemer CL, Danckert J. Dissociating perceptual and motor effects of prism adaptation in neglect. Neuroreport. 2010;21:436–441. doi: 10.1097/WNR.0b013e328338592f. [DOI] [PubMed] [Google Scholar]
- 6.Na DL, Adair JC, Williamson DJ, Schwartz RL, Haws B, Heilman KM. Dissociation of sensory-attentional from motor-intentional neglect. Journal of Neurology, Neurosurgery and Psychiatry. 1998;64:331–338. doi: 10.1136/jnnp.64.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson B, Cockburn J, Halligan PW. Behavioural inattention test. Titchfield, Hants: Thames Valley Test Company; 1987. [Google Scholar]
- 8.Azouvi P, Marchal F, Samuel C. Functional consequences and awareness of unilateral neglect : study of an evaluation scale. Neuropsychological Rehabilitation. 1991;5:135–140. [Google Scholar]
- 9.Bisiach E, Perani D, Vallar G, Berti A. Unilateral neglect: personal and extra-personal. Neuropsychologia. 1986;24:759–767. doi: 10.1016/0028-3932(86)90075-8. [DOI] [PubMed] [Google Scholar]
- 10.Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 11.Garza JP, Eslinger PJ, Barrett AM. Perceptual-attentional and motor-intentional bias in near and far space. Brain and Cognition. 2008;68:9–14. doi: 10.1016/j.bandc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeli V, Benassi MG, Ladavas E. Recovery of oculo-motor bias in neglect patients after prism adaptation. Neuropsychologia. 2004;42:1223–1234. doi: 10.1016/j.neuropsychologia.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Tilikete C, Rode G, Rossetti Y, Pichon J, Li L, Boisson D. Prism adaptation to rightward optical deviation improves postural imbalance in left-hemiparetic patients. Current Biologys. 2001;11:524–528. doi: 10.1016/s0960-9822(01)00151-8. [DOI] [PubMed] [Google Scholar]
- 14.Jacquin-Courtois S, Rode G, Pisella L, Boisson D, Rossetti Y. Wheel-chair driving improvement following visuo-manual prism adaptation. Cortex. 2008;44:90–96. doi: 10.1016/j.cortex.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Ferber S, Danckert J, Joanisse M, Goltz HC, Goodale MA. Eye movements tell only half the story. Neurology. 2003;60:1826–1829. doi: 10.1212/01.wnl.0000061478.16239.5c. [DOI] [PubMed] [Google Scholar]
- 16.Ferber S, Murray LJ. Are perceptual judgments dissociated from motor processes? A prism adaptation study. Cognitive Brain Research. 2005;23:453–456. doi: 10.1016/j.cogbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Dijkerman HC, McIntosh RD, Milner AD, Rossetti Y, Tilikete C, Roberts RC. Ocular scanning and perceptual size distortion in hemispatial neglect: effects of prism adaptation and sequential stimulus presentation. Experimental Brain Research. 2003;153:220–230. doi: 10.1007/s00221-003-1595-1. [DOI] [PubMed] [Google Scholar]
- 18.Striemer CL, Danckert JA. Through a prism darkly: re-evaluating prisms and neglect. Trends in cognitive sciences. 2010;14:308–316. doi: 10.1016/j.tics.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Striemer C, Danckert J. Prism adaptation reduces the disengage deficit in right brain damage patients. Neuroreport. 2007;18:99–103. doi: 10.1097/WNR.0b013e3280125670. [DOI] [PubMed] [Google Scholar]
- 20.Nijboer TC, McIntosh RD, Nys GM, Dijkerman HC, Milner AD. Prism adaptation improves voluntary but not automatic orienting in neglect. Neuroreport. 2008;19:293–298. doi: 10.1097/WNR.0b013e3282f4cb67. [DOI] [PubMed] [Google Scholar]
- 21.Striemer C, Sablatnig J, Danckert J. Differential influences of prism adaptation on reflexive and voluntary covert attention. J Int Neuropsychol Soc. 2006;12:337–349. doi: 10.1017/s1355617706060553. [DOI] [PubMed] [Google Scholar]
- 22.Sarri M, Greenwood R, Kalra L, Driver J. Prism adaptation does not change the rightward spatial preference bias found with ambiguous stimuli in unilateral neglect. Cortex. 2010;47:353–366. doi: 10.1016/j.cortex.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarri M, Kalra L, Greenwood R, Driver J. Prism adaptation changes perceptual awareness for chimeric visual objects but not for chimeric faces in spatial neglect after right-hemisphere stroke. Neurocase. 2006;12:127–135. doi: 10.1080/13554790600598774. [DOI] [PubMed] [Google Scholar]
- 24.Berberovic N, Mattingley JB. Effects of prismatic adaptation on judgements of spatial extent in peripersonal and extrapersonal space. Neuropsychologia. 2003;41:493–503. doi: 10.1016/s0028-3932(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 25.Saevarsson S, Kristjansson A, Hildebrandt H, Halsband U. Prism adaptation improves visual search in hemispatial neglect. Neuropsychologia. 2009;47:717–725. doi: 10.1016/j.neuropsychologia.2008.11.026. [DOI] [PubMed] [Google Scholar]