Abstract
Successful treatment of diffuse large B-cell lymphoma (DLBCL) is frequently hindered by development of resistance to conventional chemotherapy resulting in disease relapse and high mortality. High expression of anti-apoptotic and/or drug transporter proteins induced by oncogenic signaling pathways has been implicated in the development of chemoresistance in cancer. Previously, our studies showed high expression of ATP-binding cassette drug transporter ABCG2 in DLBCL correlated inversely with disease-free and failure-free survival. In this study, we have implicated activated hedgehog (Hh) signaling pathway as a key factor behind high ABCG2 expression in DLBCL through direct upregulation of ABCG2 gene transcription. We have identified a single binding site for GLI transcription factors in the ABCG2 promoter and established its functionality using luciferase reporter, site-directed mutagenesis and chromatin-immunoprecipitation assays. Furthermore, in DLBCL tumor samples, significantly high ABCG2 and GLI1 levels were found in DLBCL tumors with lymph node involvement in comparison to DLBCL tumor cells collected from pleural and/or peritoneal effusions. This suggests a role for the stromal microenvironment in maintaining high levels of ABCG2 and GLI1. Accordingly, in vitro co-culture of DLBCL cells with HS-5 stromal cells increased ABCG2 mRNA and protein levels by paracrine activation of Hh signaling. In addition to ABCG2, co-culture of DLBCL cells with HS-5 cells also resulted in increase expression of the antiapoptotic proteins BCL2, BCL-xL and BCL2A1 and in induced chemotolerance to doxorubicin and methotrexate, drugs routinely used for the treatment of DLBCL. Similarly, activation of Hh signaling in DLBCL cell lines with recombinant Shh N-terminal peptide resulted in increased expression of BCL2 and ABCG2 associated with increased chemotolerance. Finally, functional inhibition of ABCG2 drug efflux activity with fumitremorgin (FTC) or inhibition of Hh signaling with cyclopamine-KAAD abrogated the stroma-induced chemotolerance suggesting that targeting ABCG2 and Hh signaling may have therapeutic value in overcoming chemoresistance in DLBCL.
Keywords: ABCG2, ATP binding cassettes, hedgehog pathway, GLI, diffuse large B-cell lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of B-cell non-Hodgkin lymphoma (30-40%) (Alexander et al., 2007). Although DLBCL is potentially curable with conventional anthracycline-based therapy, variability in response is often observed. Approximately, 50% patients relapse and ultimately die of their disease (Fisher et al., 1993). The cellular mechanisms responsible for drug resistance in DLBCL are poorly understood and their elucidation could improve current therapeutic approaches.
The development of multidrug resistance (MDR), a situation in which cancer cells become resistant to a variety of structurally and mechanistically unrelated drugs, remains a major challenge in the treatment of cancer (Gottesman, 2002). One mechanism of MDR is the increased expression of adenosine triphosphate binding cassette (ABC) drug transporters that mediate energy-dependent transport of drugs out of the cells against a concentration gradient, resulting in low intracellular drug concentrations (Gottesman et al., 2002). Drugs affected by classical MDR include the vinca alkaloids (vincristine and vinblastine), anthracyclines (doxorubicin and daunorubicin) the RNA transcription inhibitor actinomycin-D and the microtubule-stabilizing drug paclitaxel (Ambudkar et al., 1999).
The ABC transporter proteins are a large superfamily of transmembrane glycoproteins, with 48 members divided in 7 different families based on their sequence homology and domain organization (Dean and Allikmets, 2001). One of the major MDR pumps is ABCG2, originally named Breast Cancer Resistant Protein (BCRP), first discovered in doxorubicin-resistant breast cancer cells (Doyle et al., 1998) and also called ABCP (Allikmets et al., 1998) or MXR1 (Miyake et al., 1999). ABCG2 encodes a half-molecule ABC transporter of 72 kDa protein which predominantly localizes to the plasma membrane (Rocchi et al., 2000) and forms homodimers to become functional (Kage et al., 2002). ABCG2 transports a wide variety of positively and negatively charged hydrophobic substrates, including natural compounds like flavonoids, porphyrins, sulfated estrogens along with several drugs like mitoxantrone, methotrexate, topotecan, SN38, flavopiridol, tyrosine kinase inhibitors and doxorubicin (Krishnamurthy and Schuetz, 2006; Sarkadi et al., 2004).
Elevated expression of ABCG2 has been reported in several epithelial and hematological cancers (Bleau et al., 2009; Candeil et al., 2004; Diestra et al., 2002; Kawabata et al., 2003; Tsunoda et al., 2006; Yoh et al., 2004). In hematological cancers, the expression and clinical significance of ABCG2 have been frequently investigated in acute leukemias and have produced controversial results, in part due to the overlapping role of the ABC transporters (Abbott et al., 2002; Andreadis et al., 2007; Benderra et al., 2004; Damiani et al., 2010; Jerkeman et al., 2004; Kim et al., 2009; Ohsawa et al., 2005; Rodriguez et al., 1993; Sauerbrey et al., 2002; Stam et al., 2004; Steinbach et al., 2002; Uggla et al., 2005; Yuen and Sikic, 1994). Studies of ABC transporter expression in lymphomas are more limited and have focused more in MDR1 than in ABCG2. One study identified two polymorphisms in ABCG2 gene, G34A (Val12Met) and C421A (Gln141Lys), and associated these polymorphisms with increased risk and poor overall survival of DLBCL (Hu et al., 2007). We previously reported that DLBCL patients with high ABCG2 protein expression showed significantly shorter overall survival and failure free-survival compared with patients with tumors with low or no expression of ABCG2 (Kim et al., 2009). However, Andreadis and colleagues (Andreadis et al., 2007) found no association between ABCG2 mRNA levels with outcome in a series of 130 DLBCL patients.
There is little data available regarding the molecular mechanisms controlling ABCG2 expression. Recent studies suggest ABCG2 expression may be controlled at the transcriptional level by sex hormones and hypoxia (Krishnamurthy et al., 2004; Yasuda et al., 2009). It has also been shown that Hedgehog (Hh) signaling activation upregulates the expression of ABCG2 and MDR1 in several epithelial cancers (Sims-Mourtada et al., 2007; Sims-Mourtada et al., 2006). However, whether this phenomenon occurs through direct transcriptional regulation of transporter genes by GLI1, the transcriptional activator of the Hh pathway (Eichberger et al., 2006), remains to be determined.
Hh signaling is known as a major oncogenic pathway and therefore is a potential target for therapy in several cancers (Taipale and Beachy, 2001). This pathway is composed of 3 ligands (Sonic Hh, Indian Hh and Dessert Hh), 2 receptors, a 12 transmembrane protein, PTC (ligand binding subunit), and a 7 transmembrane protein, smoothened (SMO) (signal transducing component) and 3 five-zinc finger transcription factors, GLI1, GLI2 and GLI3 (Murone et al., 1999). Binding of Hh ligands to PTC releases PTC-mediated inhibition of SMO allowing SMO to activate the pathway. While both GLI2 and GLI3, have transcriptional activation and repression properties, GLI1 is a strong positive regulator of Hh transcriptional targets and is, itself, a transcriptional target of Hh signaling (Eichberger et al., 2006).
Recently, we provided evidence that Hh signaling is activated and involved in the biology of DLBCL (Singh et al., 2010). Here, we investigated if ABCG2 is a direct downstream target of Hh signaling and the role of stromal microenvironment in modulating the expression levels of ABCG2 in DLBCL. We have also investigated if inhibition of ABCG2 transporter activity or inhibition of Hh signaling improves chemotherapy responses in DLBCL cells.
Results
Inhibition of Hh signaling decreased ABCG2 mRNA and protein levels in DLBCL cell lines
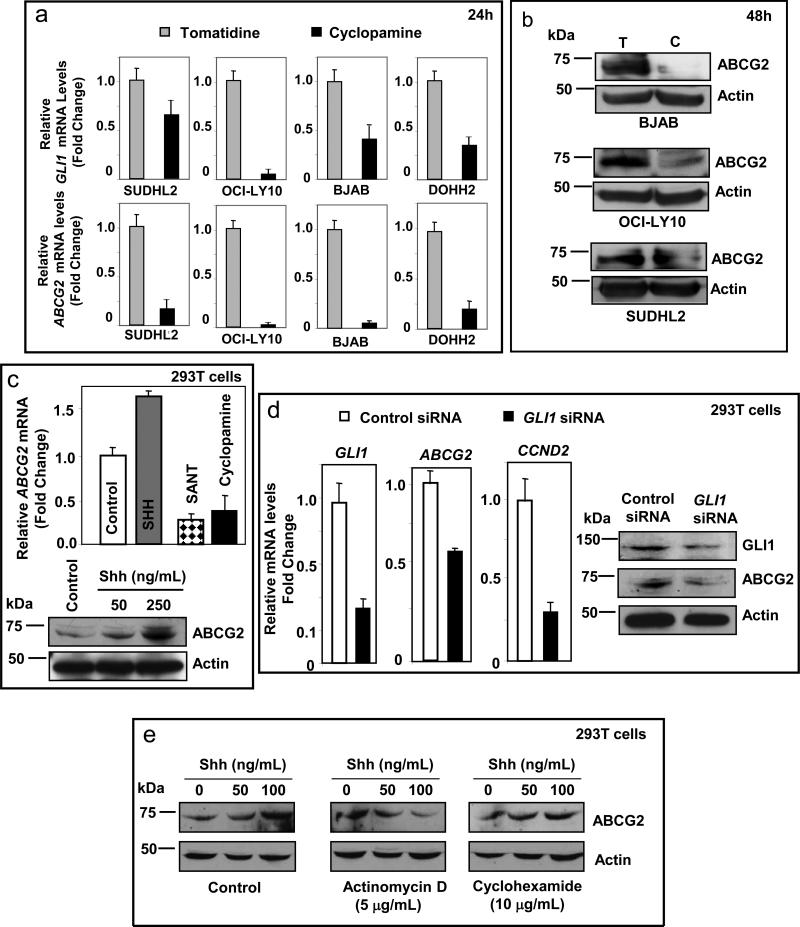
We first studied the effect of Hh signaling inhibition on the expression of ABCG2 in 4 DLBCL cell lines. Treating DLBCL cell lines with the SMO inhibitor cyclopamine-KAAD for 24h resulted in marked decrease of ABCG2 mRNA levels in comparison with tomatidine, alkaloid similar to cyclopamine but lacking the capacity to inhibit SMO. Concomitant decrease of GLI1 mRNA levels confirmed inhibition of the Hh signaling pathway (Figure 1a). Similarly, cyclopamine-KAAD also decreased ABCG2 protein levels in DLBCL cell lines (Figure 1b).
Figure 1.
Modulation of ABCG2 expression by Hh signaling. (a) Inhibition of Hh signaling by treating DLBCL cell lines with cyclopamine-KAAD (5μM for 24h) resulted in decreased ABCG2 mRNA levels in comparison to tomatidine controls (lower panels). The decrease of ABCG2 levels was associated with decrease of GLI1 mRNA levels confirming the suppression of Hh signaling (upper panels). (b) Treatment with cyclopamine-KAAD (5μM for 48h) also decreased ABCG2 protein levels in DLBCL cell lines. (c) Treatment of 293T cells with recombinant Shh N-terminal peptide increased ABCG2 mRNA and protein expression (in a concentration dependent manner), whereas blocking of Hh signaling by the SMO inhibitors, SANT and cyclopamine-KAAD (5μM for 24h), resulted in suppression of ABCG2 mRNA levels. (d) Silencing of GLI1 expression by siRNA in 293T cells decreased ABCG2 mRNA levels. Effective silencing of GLI1 was evident by decrease of GLI1 and CCND2 mRNA levels, direct downstream targets of GLI1. Silencing of GLI1 expression also resulted in decreased ABCG2 protein levels in 293T cells. (e) The dose-dependent increase in the levels of ABCG2 protein induced by Shh ligand was abolished by the transcriptional inhibitor actinomycin D but not by the translational inhibitor cyclohexamide.
ABCG2 gene expresion is transcriptionally modulated by Hh signaling
To uncover if ABCG2 is a transcriptional target of Hh signaling, studies involving modulation (activation or inhibition) of Hh signaling and the effects on ABCG2 levels were conducted in 293T cells. 293T cells have functional Hh signaling (supplementary Figure 1) and have been previously used for mechanistic studies (Barnes et al., 2005; Bhatia et al., 2006). Activation of Hh signaling with a recombinant Shh N-terminal peptide resulted in increased expression of ABCG2 whereas inhibition of Hh signaling using SANT (Smoothened ANTagonist) or cyclopamine-KAAD resulted in decrease of ABCG2 mRNA levels (Figure 1c). Silencing of GLI1 resulted in decrease of ABCG2 expression associated with decrease levels of GLI1 and cyclin D2 (CCND2), a known target of Hh signaling (Figure 1d). Furthermore, the dose-dependent increase in ABCG2 protein levels on treatment with recombinant Shh N-terminal peptide could be abrogated by concomitant treatment with actinomycin D (transcriptional inhibitor) but not with cyclohexamide (translational inhibitor) supporting that the increase of ABCG2 are mainly due to the increase of its transcriptional activity but not due to increased translational activity or posttranslational modifications (Figure 1e).
Hh signaling-mediated regulation of ABCG2 is via a functional GLI-binding site in its promoter
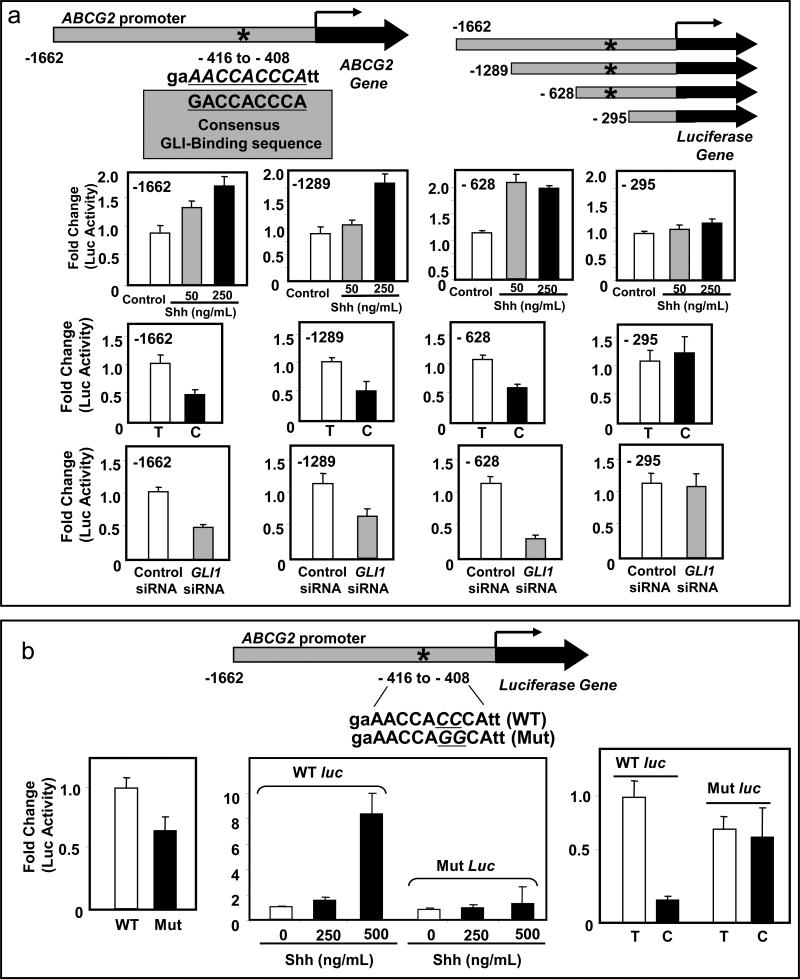
The organization of human ABCG2 gene and its promoter sequence has been previously characterized (Bailey-Dell et al., 2001). Using the program MATINSPECTOR professional version 7.2 (Quandt et al., 1995) we identified a potential GLI binding site on the ABCG2 promoter, 408 base-pair upstream of the transcription start site (Figure 2a). To validate its functionality, we performed luciferase assays in 293T cells transfected with several ABCG2 promoter luciferase constructs; three with the potential GLI binding site and one without the binding site (depicted schematically in Figure 2a). Treatment with recombinant Shh-N-terminal peptide resulted in increase of luciferase activity in those cells transfected with the constructs that included the potential GLI-binding site, whereas no increase of luciferase activity was observed in those cells transfected with the construct that lacked the GLI-binding site (Figure 2a, top panels). Cyclopamine-KAAD or siRNA-mediated silencing of GLI1 expression resulted in a decrease of the luciferase activity in those cells transfected with the constructs with the potential GLI-binding site but not in those transfected with the construct without the GLI-binding site (Figure 2a, middle and lower panels).
Figure 2.
The ABCG2 promoter has a functional GLI-binding site. (a) A potential GLI-binding site was identified in the ABCG2 promoter by in silico analysis. The 9 base pairs sequence of the GLI-binding site is shown along with the consensus GLI-binding sequence below for comparison. A schematic diagram of the luciferase constructs with the luciferase gene under control of varying lengths of ABCG2 promoter (three constructs with the GLI-binding site, represented by an asterisk, and one without it) is shown in the upper right side. Activation of Hh signaling with recombinant Shh N-terminal peptide in 293T cells transfected with the ABCG2 constructs carrying the potential GLI-binding site stimulated the luciferase activity but not in the cells transfected with the construct without the GLI-binding site (-295). Similarly, inhibiting Hh signaling either by cyclopamine-KAAD (C) or by silencing of GLI1 inhibited the luciferase activity of constructs with the GLI-binding site but not in the one without the GLI binding site (-295). T, tomatidine (control for cyclopamine treatment). (b) Luciferase assays in 293T cells transfected with the mutated ABCG2 construct showed a decreased baseline luciferase activity when compared to the WT construct (left panel). In the presence of a WT construct, treatments with 250ng/mL and 500ng/mL of recombinant Shh N-terminal ligand increased luciferase activity by 2- and 8-fold, respectively, but resulted in no effect (with 250ng/mL of Shh ligand), or in minimal increases (less than 2–fold; with 500ng/mL of Shh ligand) of luciferase activity in the presence of the mutated ABCG2 luciferase construct (middle panel). Similarly, treatment with cyclopamine-KAAD (C) in comparison with tomatidine (T) resulted in decrease of luciferase activity in the presence of the WT construct but not in the presence of the mutated one (right panel). The two cytosines mutated to guanines in the GLI-binding motif are underlined.
Mutation of GLI-binding site nullifies responsiveness of ABCG2 gene promoter to Hh signaling
To further confirm the functionality of this binding site, a luciferase construct with the full length ABCG2 promoter but with two cytosines (at positions 410 and 411) in the GLI-binding motif mutated to guanines was generated by site directed mutagenesis. Mutation of these cytosines at these positions results in loss of binding affinity of GLI to DNA (Winklmayr et al., 2010). Luciferase assay in 293T cells transfected with the ABCG2 construct with the mutated GLI-binding site showed a decrease of baseline luciferase activity when compared to the non-mutated construct indicating a decrease of activity by the mutated promoter (Figure 2b, left panel). Furthermore, in comparison to the non-mutated ABCG2 promoter, the mutated construct lost the ability to respond to modulations of the activation status of Hh signaling (Figure 2b, middle and lower panels).
GLI1 directly binds to the promoter of ABCG2
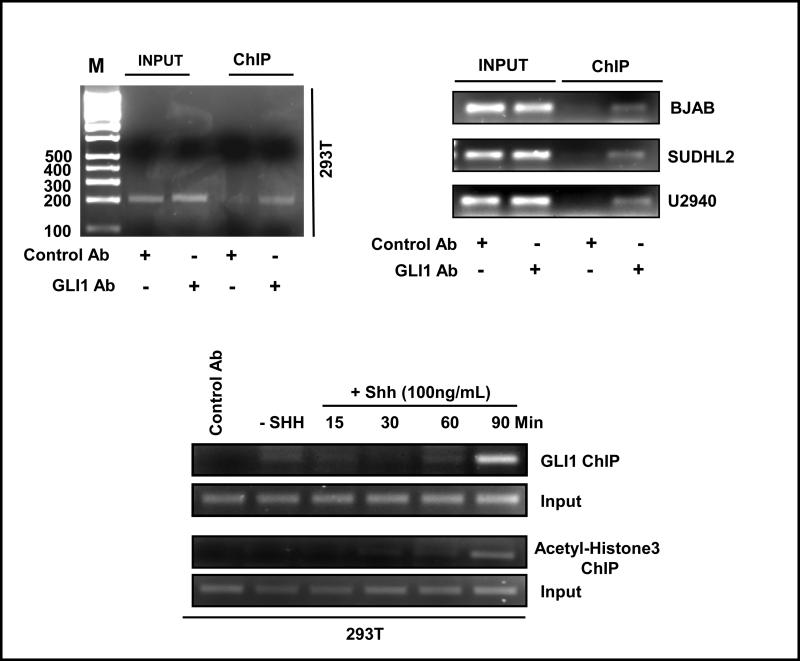
Chromatin immunoprecipitation studies (ChIP) were performed to confirm the direct binding of GLI to the promoter region of ABCG2. In 293T cells, ChIP studies using a GLI1-specific antibody resulted in the precipitation of ABCG2 promoter region encompassing the GLI-binding site (Figure 3, top left panel). ChIP analyses performed with GLI1 antibody in three different DLBCL cell lines also demonstrated the direct binding of GLI1 with the ABCG2 promoter (Figure 3, top right panel). ChIP experiments were also performed with chromatin isolated from 293T cells at different time intervals after Hh stimulation. As shown in Figure 3, enhanced GLI1 binding to the ABCG2 promoter was detected at 90 min after activation of Hh signaling (Figure 3, lower panel). Accordingly, ChIP studies using an antibody specific for acetylated–Histone3 (Lysine18) showed an increase of acetylation detected 90 min after treatment with recombinant Shh N-terminal peptide (Figure 3, lower panel) supporting enhanced transcriptional activity at this time, coinciding with a high association of GLI1.
Figure 3.
ABCG2 is a direct downstream target of Hh signaling. Chromatin immunoprecipitation (ChIP) assay with GLI1-specific antibody resulted in the precipitation of ABCG2 promoter chromatin containing the GLI-binding site in 293T cells (left upper panel) and DLBCL cell lines (right upper panel). ChIP experiments were also performed with chromatin isolated from 293T cells at different time intervals after Hh stimulation (using recombinant Shh N-terminal peptide) to see if activation of Hh signaling results in increased association of GLI1 to the ABCG2 promoter. Enhanced GLI1 binding to the ABCG2 promoter was detected at 90 min after activation of Hh signaling (lower panel). Accordingly, ChIP studies using an antibody specific for acetylated–Histone3 (Lysine18) showed an increase of histone acetylation detected 90 min after treatment with recombinant Shh ligand in the ABCG2 promoter region supporting enhanced transcriptional activity at this time, coinciding with a higher association of GLI1 to the ABCG2 promoter.
Co-culturing DLBCL cell lines with HS-5 cells enhances expression of ABCG2
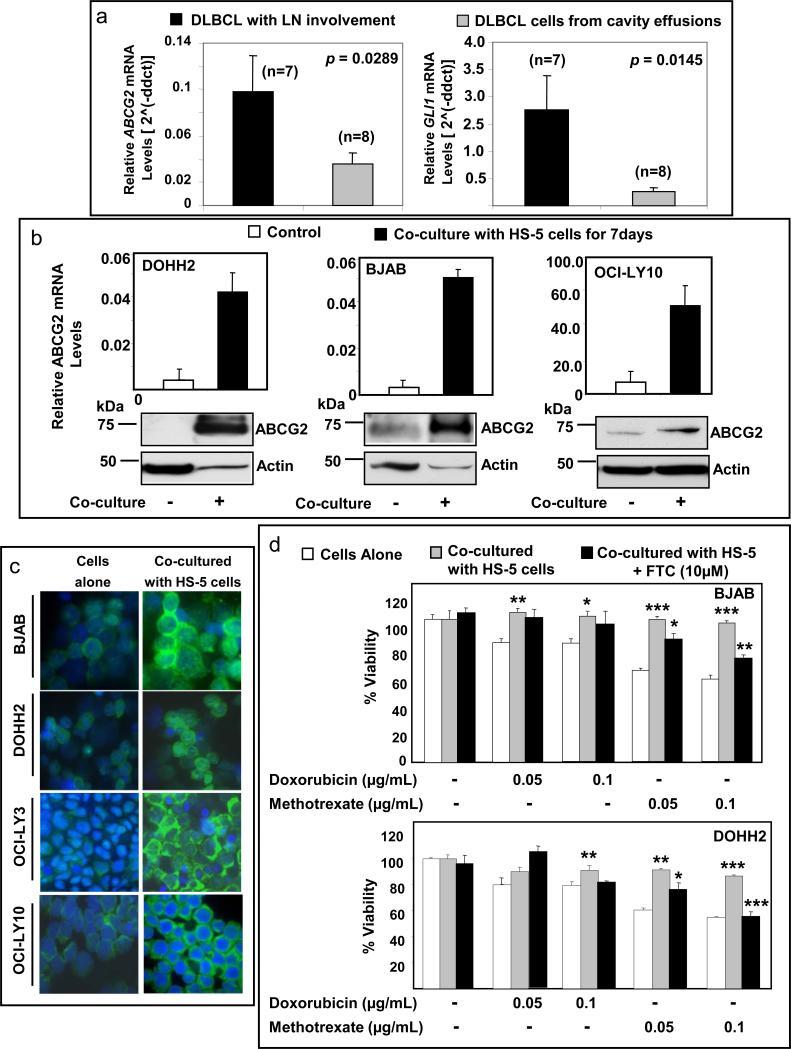
We studied the expression of ABCG2 by real-time q-PCR analysis in DLBCL tumor samples collected from 15 patients; 7 samples were collected from DLBCL involving lymph node and 8 samples were aphaeresis samples collected from pleural and/or peritoneal effusions and thus lacking of a stromal component. Higher expression levels of ABCG2 and GLI1 were found in samples of DLBCL involving lymph nodes than in pleural or peritoneal effusion samples (p=0.0289 and p=0.0145, respectively) (Figure 4a). These findings suggest the possibility of a stimulatory effect by the stromal microenvironment on GLI1 and ABCG2 levels in the tumor cells. To investigate this possibility, we co-cultured DLBCL cell lines with the human bone-marrow stroma cell line, HS-5. Direct contact was not allowed between DLBCL cells and the stroma cells as a nylon membrane separated both cell compartments (trans-well experiments). Co-culturing DLBCL cells (DOHH2, BJAB and OCI-LY10) with HS-5 cells for 5-7 days consistently resulted in increase in ABCG2 mRNA and protein levels in comparison to DLBCL cells alone (Figure 4b, upper and lower panels). Immunofluorescence studies also confirmed the up-regulation of ABCG2 after co-culturing DLBCL cells with stromal cells (Figure 4c).
Figure 4.
Co-culture of DLBCL cells with stromal cells increases ABCG2 expression and induces chemotolerance. (a) Higher levels of ABCG2 mRNA were detected in samples collected from DLBCL involving lymph node (LN) than in samples of DLBCL from cavity effusions. (b) Several-fold increase of ABCG2 mRNA and protein levels was observed in DLBCL cell lines co-cultured with HS-5 cells for 7 days when compared with DLBCL cells alone. The real time q-PCR data is the average of two separate experiments. Similar increase in the protein levels of ABCG2 was obtained after 5 days of co-culture (data not shown). (c) Immunofluorescence staining also showed increase of ABCG2 protein levels in the cytoplasm and membrane of four DLBCL cell lines after 7 days of co-culture with HS-5 cells. (d) Co-culturing BJAB and DOHH2 with HS-5 cells increased chemotolerance to doxorubicin and methotrexate. However, inhibition of ABCG2 activity by fumitremorgin C (FTC) significantly decreased the chemotolerance of DLBCL cells to methotrexate in the presence of stromal cells, but not the tolerance to doxorubicin. *(p < 0.05); ** (p < 0.01); *** (p < 0.005).
DLBCL cell lines and tumors express wild-type ABCG2 (R482) but not the R482G mutant
A point mutation of adenine (A) to guanine (G) at 1443 position of ABCG2 coding sequence results in replacement of an arginine residue at position 482 with glycine or threonine (R482G/T), changing the substrate specificity of ABCG2. Both wild-type (WT) and mutant forms have several common drugs substrates like mitoxantrone, topotecan, tyrosine-kinase inhibitors and Hoechst dye. However, the WT ABCG2 is known to transport methotrexate, whereas the mutant form transports doxorubicin, daunorubicin and epirubicin (Sarkadi et al., 2004). By sequence analysis, ABCG2 was WT (R482) in 9 DLBCL cell lines and 9 tumor samples (data not shown).
Inhibition of ABCG2 drug efflux activity partially abrogates chemotolerance induced by co-culturing DLBCL cells with HS-5 cells
We investigated if the increase of ABCG2 expression induced by stromal cells contributes to chemoresistance of DLBCL cell lines. Treatments with 0.05 and 0.1μg/mL doxorubicin resulted in decreased viability in 16% and 21% for BJAB cells and in 20% and 22% for DOHH2 cells, respectively. Similarly, treatments with 0.05 and 0.1μg/mL methotrexate resulted in decreased viability in 30% and 40% for BJAB cells and in 40% and 45% for DOHH2 cells, respectively. Co-culturing both cell lines with HS-5 cells increased chemotolerance to doxorubicin and methotrexate. (Figure 4d). However, inhibition of ABCG2 activity with fumitremorgin C (FTC), an ABCG2 inhibitor (Rabindran et al., 2000), significantly decreased the chemotolerance of DLBCL cells to methotrexate in the presence of stromal cells (Figure 4d). Decreased chemotolerance induced by FTC in the presence of stroma was not observed with doxorubicin, as doxorubicin is not a substrate for WT ABCG2. Moreover, FTC alone did not induce changes on cell viability of DLBCL cells in the absence of methotrexate or doxorubicin. These findings support that the decrease of chemotolerance induced by FTC does not represent an unspecific effect of FTC. The drugs used in these experiments did not affect cell viability of HS-5 cells (supplementary Figures 2a and b).
Inhibition of Hh signaling by cyclopamine-KAAD abrogates chemotolerance induced by co-culturing DLBCL cells with HS-5 cells
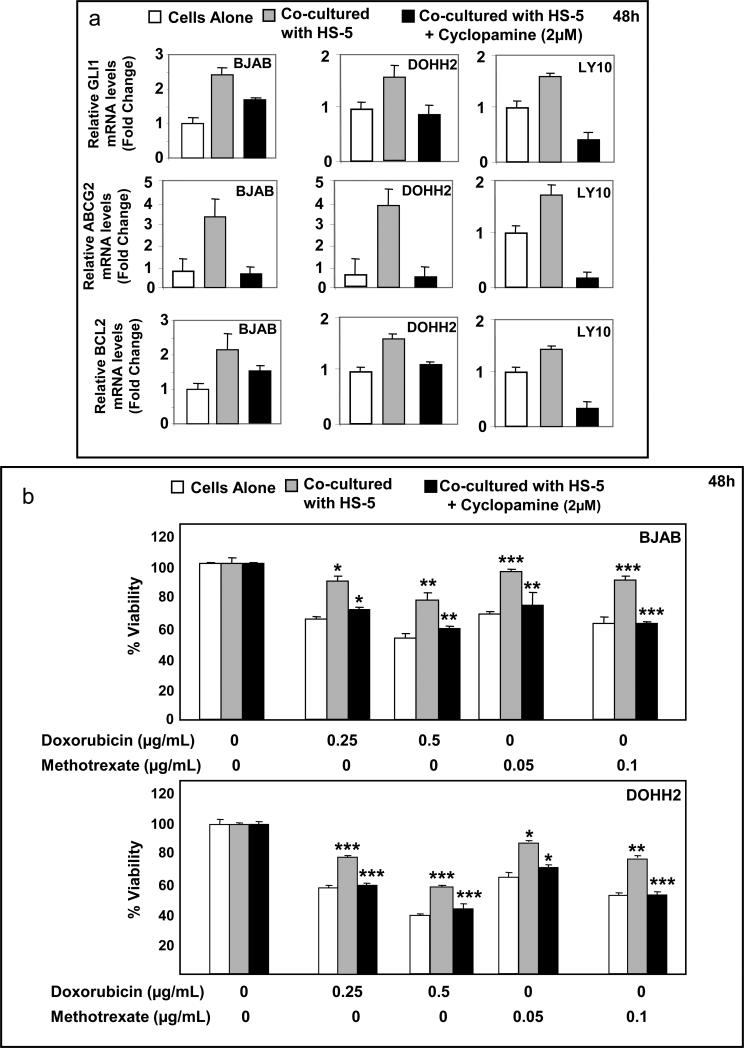
HS-5 cells secrete Hh ligands and are capable of activating Hh signaling (Dierks et al., 2007; Hegde et al., 2008; Singh et al., 2010). So, we investigated if the activation of Hh signaling induced by stromal cells contributes towards upregulation of ABCG2 and chemotolerance of DLBCL cell lines. DLBCL cell lines were co-cultured with HS-5 cells in the presence and absence of cyclopamine-KAAD (2μM) for 48h. Co-culturing DLBCL cells with stroma resulted in activation of Hh signaling in DLBCL cells as indicated by the increase of GLI1, BCL2 and ABCG2 mRNA levels. Cyclopamine-KAAD blocked Hh signaling activation induced by HS-5 cells (Figure 5a), as indicated by the decrease of GLI1, BCL2, and ABCG2 mRNA levels.
Figure 5.
Co-culture of DLBCL cells with HS-5 cells activates Hh signaling in DLBCL cells, which contributes to chemoresistance. (a) Co-culture of DLBCL cell lines with HS-5 increased the mRNA levels of GLI1 indicating activation of Hh signaling that was associated with increased expression of BCL2 and ABCG2. Concomitant treatment with cyclopamine-KAAD (2μM) decreased the expression of GLI1 (evidence of inhibition of Hh signaling) that was associated with decreased expression of BCL2 and ABCG2 mRNA levels. (b) In the presence of HS-5 cells, BJAB and DOHH2 cells were more tolerant to increasing concentrations of doxorubicin and methotrexate than cells alone. This acquired chemotolerance for both drugs could be abrogated by inhibiting Hh signaling using sublethal doses (2μM) of cyclopamine-KAAD. Note that treatment with cyclopamine-KAAD (2uM) alone did not affect cell viability of BJAB and DOHH2 cells in the presence of stroma. *(p < 0.05); ** (p < 0.01); *** (p < 0.005).
Treatments with 0.25 and 0.5μg/mL doxorubicin resulted in decrease of cell viability by 37% to 50% for BJAB and 42.0% to 60.5% for DOHH2 cells. Similarly, treatments with 0.05 and 0.1μg/mL methotrexate decreased cell viability by 30% to 40% for BJAB and 35% to 45% for DOHH2 (Figure 5b). Co-culturing cell lines with HS-5 cells increased chemotolerance to both drugs and inhibition of Hh activity by cyclopamine-KAAD significantly decreased the chemotolerance of DLBCL cells in the presence of stromal cells (Figure 5b). Treatment with cyclopamine-KAAD (2μM) did not affect cell viability of HS-5 cells (supplementary Figure 2c).
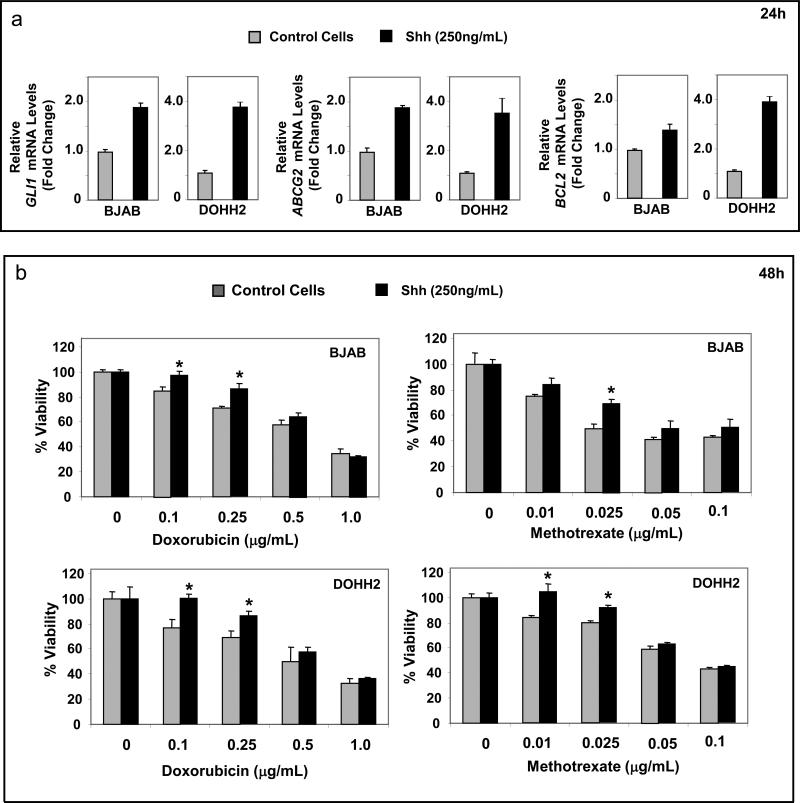
Stimulation of Hh signaling with Shh N-terminal peptide also enhances chemotolerance in DLBCL cell lines in the absence of stromal cells
Treatment with recombinant Shh ligand (250 ng/mL) activates Hh signaling in BJAB and DOHH2 cells as evidenced by the increase of mRNA levels of GLI1, ABCG2 and BCL-2 within 24h (Figure 6a) and induces chemotolerance to doxorubicin and methotrexate. These findings support that activation of Hh signaling is one of the factors that contribute to the chemotolerance induced by stroma. The fact that the increase of chemotolerance was seen for both drugs, suggests that in addition to ABCG2 other Hh signaling downstream targets are involved (supplementary Figure 3c). In fact, we found that the chemotolerance induced by HS-5 cells in DLBCL cells could also be partially reversed by functional inhibition of BCL2 using the BH3-mimetic YC-137 (supplementary Figure 4).
Figure 6.
Treatment with Shh N-terminal peptide induces chemotolerance in DLBCL cells. (a) Treatment with Shh N-terminal peptide (250ng/mL) for 24h significantly increases mRNA levels of GLI1, BCL2 and ABCG2 in BJAB and DOHH2 cell lines indicating activation of Hh signaling. (b) Treatment with Shh N-terminal peptide increases chemotolerance of BJAB and DOHH2 cell lines to both doxorubicin and methotrexate (in particular to low-doses of these agents) in comparison with doxorubicin and methotrexate alone. *(p < 0.05).
DISCUSSION
The regulation of ABC transporters in the context of cancer is poorly understood. Sims-Mourtada and colleagues found that Hh signaling was responsible in maintaining high expression levels of MDR1 and ABCG2 in esophageal cancer cell lines and that inhibition of Hh signaling increases the response of these cell lines to multiple structurally unrelated drugs (Sims-Mourtada et al., 2007). However, this study did not provide any proof whether the modulation of expression of these ABC transporters by Hh signaling was due to a direct transcriptional regulation by GLI1 or indirectly by modulating levels of other transcriptional regulators.
Here, we demonstrated the presence of a GLI1 transcription factor-binding site in the ABCG2 promoter and established ABCG2 as a direct downstream target of Hh signaling. Direct physical interaction of GLI1 to the ABCG2 promoter chromatin was evident in ChIP assays done in 293T and DLBCL cell lines. We also show that the binding of GLI1 to the ABCG2 promoter was enhanced in the presence of recombinant Shh ligand and resulted in increased histone acetylation, a hallmark for formation of euchromatin and enhanced gene transcription.
We also found that the expression levels of ABCG2 and GLI1 are higher in DLBCL samples collected from lymph node specimens (which included nodal stromal cells) when compared with DLBCL involving pleural or peritoneal effusions (without stromal component). Our previous immunohistochemical studies performed in lymph node specimens showed that ABCG2 and GLI1 were highly expressed by DLBCL cells in tissue samples (Kim et al., 2009).
Interactions between tumor cells and the stromal microenvironment are increasingly implicated as critical components for MDR (Fidler, 2003; Grigorieva et al., 1998; Mueller and Fusenig, 2004). To test if the microenvironment contributes to the activation of Hh signaling we co-cultured DLBCL cells with HS-5 cells and analyzed expression of ABCG2 and GLI1. HS-5 cells are an integral in the bone marrow and lymph node microenvironment and have been used previously to study the effect of microenvironment on tumor cells and vice versa (Baumann et al., 2009; Fernandez et al., 2010; Lwin et al., 2009; McMillin et al., 2010). HS-5 cells are also known to synthesize and secrete Hh ligands and hence are capable of the paracrine activation of the Hh signaling (Desch et al., 2010; Dierks et al., 2007; Singh et al., 2010). We found that co-culturing DLBCL cells with stromal cells increased the expression of GLI1, ABCG2 and BCL2 in the tumor cells and that this activation of Hh signaling is blocked with a SMO inhibitor.
The mode of action of Hh signaling in cancer appears rather complex and may be tumor dependent. Several studies suggest an autocrine action in some tumors and others favor a paracrine model. It has been shown that Hh signaling has a paracrine role as a survival factor for low-grade B-cell neoplasms, including chronic lymphocytic leukemia (CLL) and plasma cell myeloma (Dierks et al., 2007; Hegde et al., 2008). Sacedon and colleagues (Sacedon et al., 2005) have also found that Hh ligands are produced by follicular dendritic cells inside the germinal centers and that inhibition of Hh signaling induces apoptosis in germinal center B-cells, which are rescued by addition of Hh ligand, supporting a paracrine role of Hh signaling in the germinal center compartment. In DLBCL, our study supports that, in the presence of stroma, Hh signaling works in a paracrine manner. However, we also recently showed that in the absence of stroma, DLBCL cells, synthesize, secrete, and respond to endogenous Hh ligands, also providing support of an autocrine signaling loop (Singh et al., 2010).
Hh signaling has been identified as an essential component in MDR in acute myeloid leukemia. Hh signaling induces resistance to radiotherapy and a MDR phenotype in esophageal adenocarcinomas (Queiroz et al., 2010; Sims-Mourtada et al., 2007). Targeting Hh signaling to decrease chemotolerance is of interest because this pathway affects different mechanisms involved in chemoresistance, including expression levels of antiapoptotic molecules such as BCL2, and BCL-xL as well as ATP binding cassettes such as ABCG2 and MDR1. Interfering with pathways like Hh signaling that block multiple molecules involved in chemotolerance could lead to potential more effective therapeutic strategies to enhance the effect of current chemotherapeutic agents. Our data show that the stroma is important inducing chemotolerance in DLBCL and that this effect is, in part, mediated by activation of Hh signaling and by inducing high expression of downstream targets such as ABCG2 and BCL2 (Bigelow et al., 2004).
Inhibiting ABCG2 in DLBCL could be also therapeutically relevant because methotrexate, a substrate of WT ABCG2, is also used to treat primary DLBCL of the central nervous system (CNS) and as prophylaxis for CNS involvement in systemic DLBCL. Methotrexate is also currently used in protocols for aggressive cases of systemic DLBCL (i.e. ACVBP followed by consolidation protocols that include high-doses of methotrexate and CODOX-M that includes cyclophosphamide, vincristine, doxorubicin and methotrexate). Methotrexate is also part of some protocols for relapsed or refractory DLBCL and is included in some protocols for conventional DLBCL (i.e. RMACOP-B)(Armitage, 2007). In addition, mutations in the ABCG2 gene acquired during the course of chemotherapy can change substrate specificity. It has been shown that mutation of amino-acid 482 in the ABCG2 gene confers resistance to doxorubicin and daunorubicin (Honjo et al., 2001; Robey et al., 2003). Moreover, it has been also shown that cell lines selected with doxorubicin frequently show enrichment of cells with the R482G/T ABCG2 mutant form (Robey et al., 2003). A similar scenario is also plausible in DLBCL patients, where initial treatment with doxorubicin may result in selection of cells with a mutant ABCG2 gene carrying the R482G/T mutation that could contribute to chemotolerance.
In summary, we have established ABCG2 as a direct downstream target of Hh signaling pathway. We have also demonstrated the role of stromal microenvironment in inducing chemotolerance in DLBCL cells and that paracrine activation of Hh signaling in lymphoma cells play a role in inducing chemotolerance in DLBCL. Inhibition of Hh signaling reverses stroma-induced chemotolerance suggesting that targeting Hh signaling may be of therapeutic value to decrease chemotolerance in DLBCL. Also, inhibiting ABCG2 results in a partial reversal of stroma-induced chemotolerance highlighting the possibility of ABCG2 being involved in chemoresistance mechanisms in DLBCL and possibly in a wide variety of other cancers with abnormal activation of Hh signaling.
Materials and Methods
Cell lines
Nine DLBCL cell lines were used; 4 germinal center type (BJAB, DOHH2, Pfeiffer and SUDHL4), 4 activated cell type (HBL1, OCI-LY3, OCI-LY10 and SUDHL2) and 1 primary mediastinal B-cell lymphoma (PMBL) cell line (U2940). DOHH2, SUDHL4, and U2940 were from DSMZ (Braunschweig, Germany); Pfeiffer and the human bone marrow stromal cell line HS-5 were from ATCC. OCI-LY3 and OCI-LY10 were kindly provided by Dr Michael G Rosenblum and SUDHL2 and BJAB were a gift from Dr. Felipe Samaniego, MD Anderson Cancer Center. All cell lines were maintained at 37°C in RPMI 1640 (ATCC, Manassas, VA, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Sigma, St Louis, MO, USA) in a humidified atmosphere containing 5% CO2. Human embryonic kidney epithelial cell line 293T were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS.
DLBCL patient samples
ABCG2 expression was studied in samples collected from 15 patients with DLBCL; 7 samples were collected from DLBCL involving lymph nodes and 8 samples were collected from pleural and/or peritoneal effusions.
Real-Time quantitative (q) PCR and reverse transcriptase (RT)-PCR
Total RNA was extracted using RNeasy mini RNA extraction kit (Qiagen, Valencia, CA, USA) as per the manufacturer's protocol. cDNA synthesis was done using random primers and SuperScript II™ Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). The Taqman minor groove binder probe and the ABI Prism® 7900 HT Sequence Detection System (PE Applied Biosystems, Carlsbad, CA, USA) were used. The primers and probes for GLI1, GLI2, GLI3, PTC, SMO, ABCG2, CCND2, BCL2, BCL-xL, BCL2A1, GUSB and 18s rRNA were obtained from Applied Biosystems. Each target was amplified individually and in duplicate. These experiments were done at least in duplicate. The relative levels of mRNA were calculated using delta CT (ΔCT) method. For comparison of the levels of ABCG2 in samples the 2-(ΔΔCT) method (Schmittgen, 2001) was used using expression of ABCG2 in Human Reference total RNA (Stratagene, Santaclara, CA, USA) as control.
Western blot analysis
Western blotting was done as described previously (Singh et al., 2009). Antibodies used were ABCG2 (H-70) and GLI1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (Sigma). Reactions were visualized with suitable secondary antibodies conjugated with horseradish peroxidase using enhanced chemiluminescence reagents (Amersham Piscataway, NJ, USA). Actinomycin D and cyclohexamide were purchased from Sigma.
Immunofluorescence labeling and fluorescence microscopy
Immunofluorescence labeling was performed on cytospin preparations of DLBCL cell lines as previously described (Singh et al., 2009). Staining for ABCG2 was done using a mouse-monoclonal anti-ABCG2 antibody (Clone BXP-21) (Kamiya Biomedical Company, Seattle, WA, USA).
DNA sequencing
Total RNA was extracted from cell lines and patients samples using RNeasy mini RNA extraction kit (Qiagen). cDNA synthesis was done using random primers and SuperScript II™ Reverse Transcriptase (Invitrogen). Primers used were 5′-CTTCCTGACGACCAACCAGT-3′ and 5′-CATCTGCCTTTGGCTTCAAT-3′. PCR product (221bp) was purified using PCR purification kit (Invitrogen) and sequenced.
Transfection of GLI1-specific siRNA
sMARTpool mix of four GLI1-specific siRNA and a control non-specific scrambled siRNA were purchased from Dharmacon Inc (Lafayette, CO, USA). Transfection of siRNA in 293T cells was done using oligofectamine (Invitrogen) as per manufacturer's instructions. Cells were harvested 48h post siRNA transfection. Adequate inhibition of protein expression and mRNA levels was confirmed by western blot and q-PCR.
Luciferase Reporter Gene Assay
Luciferase reporter plasmids with luciferase gene under transcriptional control of ABCG2 gene regulatory chromatin were kindly provided by Dr. Susan E Bates (National Cancer Institute) (To et al., 2006). 293T cells were transfected with the luciferase plasmids using Fugene HD transfection reagent (Roche Applied Sciences, Florence, SC, USA). Renilla luciferase plasmid was also co-transfected to normalize the luciferase activity. Luciferase activity was measured 48h post-transfection using the Dual-Luciferase assay kit (Promega, Madison, WI, USA).
Site-directed mutagenesis of GLI-binding site in ABCG2 promoter
Site directed mutagenesis to mutate the GLI binding site was done using ABCG2 (-1662 bp) luciferase as template. The primers used were 5’-CATTCACCAGAAACCAGGCATTTAACTTGCTCTGG-3’ and 5’-CCAGAGCAAGTTAAATGCCTGGTTTCTGGTGAATG-3’. PCR for mutagenesis was done using Quikchange site-directed mutagenesis kit (Stratagene).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was conducted using SimpleChIP Enzymatic Chromatin IP kit (Cell Signaling Technology, Boston, MA, USA). Monoclonal antibody for GLI1 (Cell Signaling Technology) and rabbit polyclonal antibody for Histone H3 (Acetyl-K18) (Abcam) were used for ChiP. Primers used to PCR-amplify the ABCG2 gene chromatin were 5’-ATCCCATTCACCAGAAACCA-3’ and 5’–CGAACGGAATGAACCAGAGT-3’ resulting in a product size of 205bp.
Co-culture, drug treatment and cell viability assays
HS-5 bone marrow cells were plated in 24- or 6-well plates as required and allowed to attach and grow for 48h. For co-culture, cell culture inserts (BD Falcon, Bedford, MA, USA) with 0.4μM membrane size were inserted into the wells and DLBCL cells were plated in the inserts. Drug treatments were done using doxorubicin and methotrexate hydrate (Sigma), fumitremorgin C (Enzo Lifesciences, Plymouth Meeting, PA, USA) cyclopamine-KAAD (Toronto Research Chemicals Inc, North York, ON, Canada) and YC-137 (EMD Biosciences, San Diego, CA, USA) at required concentrations in the media inside the wells as well as the media in the insert to prevent any discrepancies due to dilution. Recombinant Shh N-terminal peptide was purchased from R&D Biosystems, MN, USA). Cell viability assay was done using Cell Titer 96 Aqueous One Solution (MTS) (Promega).
Immunohistochemistry
Expression of ABCG2 in tumor samples was assessed using tissue microarrays and a monoclonal antibody from Santa Cruz (clone BXP-21) as previously described.
Statistical analysis
To evaluate the significance of ABCG2 expression in tumors with tissue involvement and tumor cells from cavity effusions the nonparametric Mann-Whitney U test was used. To calculate the statistical significance of the changes in the response of DLBCL cell lines to drugs the paired t-test was used. Both tests were performed using GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego CA, USA).
Supplementary Material
Acknowledgments
We thank Dr Susan E Bates (NIH) for providing the ABCG2 promoter luciferase constructs. We also thank Professor Michael G Rosenblum, department of Experimental Therapeutics, MD Anderson Cancer Center for providing OCI-LY3 and OCI-Ly10 cell lines. The primary tumor samples were provided by the Hematopathology and Lymphoma Tissue Banks of the UT M.D. Anderson Cancer Center (supported by the NCI/NIH Grant CA16672).
This research was supported by funds from The Translational Grant of The Leukemia & Lymphoma Society (to RS and FV), K08 Physician-Scientist Award 1 K08 CA143151-01 (NIH) (to FV), SPORE Lymphoma Grant UT M.D. Anderson Cancer Center Lymphoma SPORE 1P50CA136411-01A1 (to FV) and Lauri Strauss Leukemia Foundation Grant award (to RS and FV).
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
REFERENCES
- Abbott BL, Colapietro AM, Barnes Y, Marini F, Andreeff M, Sorrentino BP. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100:4594–4601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, et al. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer 120 Suppl. 2007;12:1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Andreadis C, Gimotty PA, Wahl P, Hammond R, Houldsworth J, Schuster SJ, et al. Members of the glutathione and ABC-transporter families are associated with clinical outcome in patients with diffuse large B-cell lymphoma. Blood. 2007;109:3409–3416. doi: 10.1182/blood-2006-09-047621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110:29–36. doi: 10.1182/blood-2007-01-041871. [DOI] [PubMed] [Google Scholar]
- Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta. 2001;1520:234–241. doi: 10.1016/s0167-4781(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Barnes EA, Heidtman KJ, Donoghue DJ. Constitutive activation of the shh-ptc1 pathway by a patched1 mutation identified in BCC. Oncogene. 2005;24:902–915. doi: 10.1038/sj.onc.1208240. [DOI] [PubMed] [Google Scholar]
- Baumann P, Mandl-Weber S, Volkl A, Adam C, Bumeder I, Oduncu F, et al. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol Cancer Ther. 2009;8:366–375. doi: 10.1158/1535-7163.MCT-08-0664. [DOI] [PubMed] [Google Scholar]
- Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, et al. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- Bhatia N, Thiyagarajan S, Elcheva I, Saleem M, Dlugosz A, Mukhtar H, et al. Gli2 is targeted for ubiquitination and degradation by beta-TrCP ubiquitin ligase. J Biol Chem. 2006;281:19320–19326. doi: 10.1074/jbc.M513203200. [DOI] [PubMed] [Google Scholar]
- Bigelow RL, Chari NS, Unden AB, Spurgers KB, Lee S, Roop DR, et al. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- Bleau AM, Huse JT, Holland EC. The ABCG2 resistance network of glioblastoma. Cell Cycle. 2009;8:2936–2944. [PubMed] [Google Scholar]
- Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004;109:848–854. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- Damiani D, Tiribelli M, Michelutti A, Geromin A, Cavallin M, Fabbro D, et al. Fludarabine-based induction therapy does not overcome the negative effect of ABCG2 (BCRP) over-expression in adult acute myeloid leukemia patients. Leuk Res. 2010;34:942–945. doi: 10.1016/j.leukres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001;33:475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- Desch P, Asslaber D, Kern D, Schnidar H, Mangelberger D, Alinger B, et al. Inhibition of GLI, but not Smoothened, induces apoptosis in chronic lymphocytic leukemia cells. Oncogene. 2010;29:4885–4895. doi: 10.1038/onc.2010.243. [DOI] [PubMed] [Google Scholar]
- Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–951. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- Diestra JE, Scheffer GL, Catala I, Maliepaard M, Schellens JH, Scheper RJ, et al. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberger T, Sander V, Schnidar H, Regl G, Kasper M, Schmid C, et al. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics. 2006;87:616–632. doi: 10.1016/j.ygeno.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Pino AM, Figueroa P, Rodriguez JP. The increased expression of receptor activator of nuclear-kappaB ligand (RANKL) of multiple myeloma bone marrow stromal cells is inhibited by the bisphosphonate ibandronate. J Cell Biochem. 2010;111:130–137. doi: 10.1002/jcb.22676. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp Hematol. 1998;26:597–603. [PubMed] [Google Scholar]
- Hegde GV, Peterson KJ, Emanuel K, Mittal AK, Joshi AD, Dickinson JD, et al. Hedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic target. Mol Cancer Res. 2008;6:1928–1936. doi: 10.1158/1541-7786.MCR-08-0142. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- Hu LL, Wang XX, Chen X, Chang J, Li C, Zhang Y, et al. BCRP gene polymorphisms are associated with susceptibility and survival of diffuse large B-cell lymphoma. Carcinogenesis. 2007;28:1740–1744. doi: 10.1093/carcin/bgm113. [DOI] [PubMed] [Google Scholar]
- Jerkeman M, Anderson H, Dictor M, Kvaloy S, Akerman M, Cavallin-Stahl E. Assessment of biological prognostic factors provides clinically relevant information in patients with diffuse large B-cell lymphoma--a Nordic Lymphoma Group study. Ann Hematol. 2004;83:414–419. doi: 10.1007/s00277-004-0855-x. [DOI] [PubMed] [Google Scholar]
- Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97:626–630. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Oka M, Soda H, Shiozawa K, Nakatomi K, Tsurutani J, et al. Expression and functional analyses of breast cancer resistance protein in lung cancer. Clin Cancer Res. 2003;9:3052–3057. [PubMed] [Google Scholar]
- Kim JE, Singh RR, Cho-Vega JH, Drakos E, Davuluri Y, Khokhar FA, et al. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod Pathol. 2009;22:1312–1320. doi: 10.1038/modpathol.2009.98. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- Lwin T, Crespo LA, Wu A, Dessureault S, Shu HB, Moscinski LC, et al. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non-Hodgkin's B lymphoma cells from apoptosis. Leukemia. 2009;23:170–177. doi: 10.1038/leu.2008.266. [DOI] [PubMed] [Google Scholar]
- McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, de Sauvage FJ. Hedgehog signal transduction: from flies to vertebrates. Exp Cell Res. 1999;253:25–33. doi: 10.1006/excr.1999.4676. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Ikura Y, Fukushima H, Shirai N, Sugama Y, Suekane T, et al. Immunohistochemical expression of multidrug resistance proteins as a predictor of poor response to chemotherapy and prognosis in patients with nodal diffuse large B-cell lymphoma. Oncology. 2005;68:422–431. doi: 10.1159/000086984. [DOI] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz KC, Ruela-de-Sousa RR, Fuhler GM, Aberson HL, Ferreira CV, Peppelenbosch MP, et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene. 2010;29:6314–6322. doi: 10.1038/onc.2010.375. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi E, Khodjakov A, Volk EL, Yang CH, Litman T, Bates SE, et al. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem Biophys Res Commun. 2000;271:42–46. doi: 10.1006/bbrc.2000.2590. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Commes T, Robert J, Rossi JF. Expression of P-glycoprotein and anionic glutathione S-transferase genes in non-Hodgkin's lymphoma. Leuk Res. 1993;17:149–156. doi: 10.1016/0145-2126(93)90060-x. [DOI] [PubMed] [Google Scholar]
- Sacedon R, Diez B, Nunez V, Hernandez-Lopez C, Gutierrez-Frias C, Cejalvo T, et al. Sonic hedgehog is produced by follicular dendritic cells and protects germinal center B cells from apoptosis. J Immunol. 2005;174:1456–1461. doi: 10.4049/jimmunol.174.3.1456. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Ozvegy-Laczka C, Nemet K, Varadi A. ABCG2 -- a transporter for all seasons. FEBS Lett. 2004;567:116–120. doi: 10.1016/j.febslet.2004.03.123. [DOI] [PubMed] [Google Scholar]
- Sauerbrey A, Sell W, Steinbach D, Voigt A, Zintl F. Expression of the BCRP gene (ABCG2/MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br J Haematol. 2002;118:147–150. doi: 10.1046/j.1365-2141.2002.03550.x. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD. Real-time quantitative PCR. Methods. 2001;25:383–385. doi: 10.1006/meth.2001.1260. [DOI] [PubMed] [Google Scholar]
- Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- Sims-Mourtada J, Izzo JG, Apisarnthanarax S, Wu TT, Malhotra U, Luthra R, et al. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res. 2006;12:6565–6572. doi: 10.1158/1078-0432.CCR-06-0176. [DOI] [PubMed] [Google Scholar]
- Singh RR, Cho-Vega JH, Davuluri Y, Ma S, Kasbidi F, Milito C, et al. Sonic hedgehog signaling pathway is activated in ALK-positive anaplastic large cell lymphoma. Cancer Res. 2009;69:2550–2558. doi: 10.1158/0008-5472.CAN-08-1808. [DOI] [PubMed] [Google Scholar]
- Singh RR, Kim JE, Davuluri Y, Drakos E, Cho-Vega JH, Amin HM, et al. Hedgehog signaling pathway is activated in diffuse large B-cell lymphoma and contributes to tumor cell survival and proliferation. Leukemia. 2010;24:1025–1036. doi: 10.1038/leu.2010.35. [DOI] [PubMed] [Google Scholar]
- Stam RW, van den Heuvel-Eibrink MM, den Boer ML, Ebus ME, Janka-Schaub GE, Allen JD, et al. Multidrug resistance genes in infant acute lymphoblastic leukemia: Ara-C is not a substrate for the breast cancer resistance protein. Leukemia. 2004;18:78–83. doi: 10.1038/sj.leu.2403168. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16:1443–1447. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- To KK, Zhan Z, Bates SE. Aberrant promoter methylation of the ABCG2 gene in renal carcinoma. Mol Cell Biol. 2006;26:8572–8585. doi: 10.1128/MCB.00650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Okumura T, Ito T, Kondo K, Ortiz C, Tanaka E, et al. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology. 2006;71:251–258. doi: 10.1159/000106787. [DOI] [PubMed] [Google Scholar]
- Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A, et al. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res. 2005;29:141–146. doi: 10.1016/j.leukres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Winklmayr M, Schmid C, Laner-Plamberger S, Kaser A, Aberger F, Eichberger T, et al. Non-consensus GLI binding sites in Hedgehog target gene regulation. BMC Mol Biol. 2010;11:2. doi: 10.1186/1471-2199-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Kobayashi M, Itagaki S, Hirano T, Iseki K. Response of the ABCG2 promoter in T47D cells and BeWo cells to sex hormone treatment. Mol Biol Rep. 2009;36:1889–1896. doi: 10.1007/s11033-008-9395-0. [DOI] [PubMed] [Google Scholar]
- Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- Yuen AR, Sikic BI. Multidrug resistance in lymphomas. J Clin Oncol. 1994;12:2453–2459. doi: 10.1200/JCO.1994.12.11.2453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.