Abstract
Background
To analyse the overall clinical outcome and benefits by applying protocol based image guided adaptive brachytherapy combined with 3D conformal external beam radiotherapy (EBRT) ± chemotherapy (ChT).
Methods
Treatment schedule was EBRT with 45–50.4 Gy ± concomitant cisplatin chemotherapy plus 4 × 7 Gy High Dose Rate (HDR) brachytherapy. Patients were treated in the “protocol period” (2001–2008) with the prospective application of the High Risk CTV concept (D90) and dose volume constraints for organs at risk including biological modelling. Dose volume adaptation was performed with the aim of dose escalation in large tumours (prescribed D90 > 85 Gy), often with inserting additional interstitial needles. Dose volume constraints (D2cc) were 70–75 Gy for rectum and sigmoid and 90 Gy for bladder.
Late morbidity was prospectively scored, using LENT/SOMA Score. Disease outcome and treatment related late morbidity were evaluated and compared using actuarial analysis.
Findings
One hundred and fifty-six consecutive patients (median age 58 years) with cervix cancer FIGO stages IB–IVA were treated with definitive radiotherapy in curative intent. Histology was squamous cell cancer in 134 patients (86%), tumour size was >5 cm in 103 patients (66%), lymph node involvement in 75 patients (48%). Median follow-up was 42 months for all patients.
Interstitial techniques were used in addition to intracavitary brachytherapy in 69/156 (44%) patients. Total prescribed mean dose (D90) was 93 ± 13 Gy, D2cc 86 ± 17 Gy for bladder, 65 ± 9 Gy for rectum and 64 ± 9 Gy for sigmoid.
Complete remission was achieved in 151/156 patients (97%). Overall local control at 3 years was 95%; 98% for tumours 2–5 cm, and 92% for tumours >5 cm (p = 0.04), 100% for IB, 96% for IIB, 86% for IIIB. Cancer specific survival at 3 years was overall 74%, 83% for tumours 2–5 cm, 70% for tumours >5 cm, 83% for IB, 84% for IIB, 52% for IIIB. Overall survival at 3 years was in total 68%, 72% for tumours 2–5 cm, 65% for tumours >5 cm, 74% for IB, 78% for IIB, 45% for IIIB.
In regard to late morbidity in total 188 grade 1 + 2 and 11 grade 3 + 4 late events were observed in 143 patients. G1 + 2/G3 + 4 events for bladder were n = 32/3, for rectum n = 14/5, for bowel (including sigmoid) n = 3/0, for vagina n = 128/2, respectively.
Interpretation
3D conformal radiotherapy ± chemotherapy plus image (MRI) guided adaptive intracavitary brachytherapy including needle insertion in advanced disease results in local control rates of 95–100% at 3 years in limited/favourable (IB/IIB) and 85–90% in large/poor response (IIB/III/IV) cervix cancer patients associated with a moderate rate of treatment related morbidity. Compared to the historical Vienna series there is relative reduction in pelvic recurrence by 65–70% and reduction in major morbidity. The local control improvement seems to have impact on CSS and OS. Prospective clinical multi-centre studies are mandatory to evaluate these challenging mono-institutional findings.
Keywords: Cervical cancer, Image guided adaptive brachytherapy, Clinical outcome, GEC-ESTRO recommendations
Progress in radiation technology has been significant during the last decades in regard to imaging, treatment planning, and various forms of advanced dose delivery, e.g. Intensity Modulated and Image Guided Radiotherapy [1–3]. The impact of these developments on the daily process of planning and performance of external beam radiotherapy has been dramatic [4,5]. However, the clinical evidence for an improvement of outcome has been rather limited, due to various reasons [3].
Similar developments have taken place in brachytherapy. In gynaecology this has been mainly related to cervix cancer with the integration of (repetitive) 3D imaging (MRI) into the treatment planning process which had to be changed completely with the introduction of an adaptive target concept and a dose volume assessment balancing constraints for CTV and OAR [6,7]. The imaging modality of choice has become MRI with also some place for CT and maybe in future also for US [8,9]. Best technical improvements so far have been mainly the adaptation of classical applicators to become CT and MRI compatible and more recently the combination of intracavitary brachytherapy with a guided and focussed interstitial approach (Vienna ring applicator, [10–12], Utrecht ovoid applicator [12]).
These changes in brachytherapy technology seem to have a major direct impact on clinical outcome [13]. For cervix cancer, in the past major improvements in regard to loco-regional, distant control and survival has been achieved through simultaneous chemo-radiotherapy which became standard of care in 1999, when five randomized trials were published [14–18]. However, according to a recent joint and balanced meta-analysis of these trial groups the absolute benefit is pronounced, but limited which is in particular true for advanced disease [19]. The absolute benefit for overall survival, disease free survival and local control at five years has been estimated as 6%, 8%, and 9%, respectively, which is well in line with what has been shown for other cancer sites, e.g. head and neck [20]. The absolute overall survival benefit is largest for limited disease (I/IIA) with 10% and decreases in more advanced stage with 7% for IIB and 3% for III/IVA. These findings imply a significant need for further improvement, which may become possible through introduction of more intensive (adjuvant) chemotherapy [21], but also through improvements of radiotherapy, in particular for more advanced disease.
The significant improvements of brachytherapy and EBRT technology during the last decades have not resulted in obvious major overall improvements in clinical outcome for cervical cancer according to what has been published, e.g. in the annual treatment reports, in regard to overall survival [22] and according to published mono-institutional series [23–25]. Nevertheless, major differences have been reported between different brachytherapy traditions for certain clinical scenarios, e.g. stage III, [23,25–29].
Favourable outcome after IGABT combined with 3D conformal external beam radiotherapy ± chemotherapy has been recently reported within the frame of some limited mono-institutional series applying different treatment schedules [13,30–33]. IGABT seems to play a major role in the improvement of clinical outcome, in particular of local control in advanced cervical cancer with no increase in treatment related morbidity based on this mono-institutional experience. The large Vienna clinical series on a consecutive number of 145 patients including the learning period published in 2007 has played a major role in elucidating the issue of improvement of dose volume adaptation, dose escalation and clinical outcome.
In order to further clarify the present and future clinical impact of IGABT in cervical cancer, an analysis was performed to evaluate the dosimetric parameters and overall clinical outcome in the Vienna protocol period with applying the HR CTV concept and dose volume constraints for OAR according to the later GEC ESTRO Recommendations in 156 consecutive patients with mature 3-year follow-up data and to define the major clinical benefits to be expected from this new treatment method.
Material and methods
Patient and tumour characteristics
A total of 218 consecutive patients with cervical cancer were referred for definitive radiotherapy to the Radiation Oncology Department at the Vienna General Hospital between January 2001 and August 2008. For the current analysis, the following eligibility criteria were applied: patients with stage IB1 to IVA disease, who underwent the complete definitive radiotherapy and were followed in our department and who did not have a previous history of malignancy. Sixty-two patients were ineligible for this analysis: 30 with stage IVB disease, 5 as treated with palliative intent, 7 as referred to our department only for BT, 7 due to prior or simultaneous second malignancy (5 breast, 1 colon, 1 ovarian cancer); 13 patients due to incomplete radiotherapy for the following reasons: 2 refused brachytherapy, 3 did not finish brachytherapy because of missing compliance, 1 had progressive metastatic disease during EBRT, 4 interrupted treatment due to worsening medical conditions, and 3 died during treatment (1 from sepsis and pneumonia (no chemotherapy), 2 from cardiovascular disease).
One hundred and 56 patients with locally advanced cervical cancer met the eligibility criteria and were thus available for analysis. All patients were treated with curative intent in the Department of Radiotherapy applying a prospective in-house protocol with High Risk CTV and dose volume constraints for organs at risk (according to the Gyn GEC ESTRO Recommendations), with definitive radiotherapy consisting of 3D conformal external beam radiotherapy (EBRT) ± concomitant chemotherapy and High-Dose-Rate (HDR)-Brachytherapy. Median age was 58 years.
Patients were clinically staged according to the International Federation of Gynaecology and Obstetrics (FIGO) criteria [34]: 12 stage IB1 (8%), 9 stage IB2 (6%), 4 stage IIA (3%), 88 stage IIB (56%), 5 stage IIIA (3%), 32 stage IIIB (21%) and 6 stage IVA (4%). Abdominal CT and pelvic MRI scans were performed in all patients, in order to determine local, regional and abdominal tumour spread, beside chest CT to rule out distant metastases. Tumour size as defined by clinical and MRI examination (maximum width) was 2–5 cm in 53 patients (34%) and more than 5 cm in 103 patients (66%).
One hundred thirty-four patients had squamous cell cancer (86%), 14 had adenocarcinoma (9%), 5 had adenosquamous carcinoma (3%) and 3 had other entities (2%). Ninety-six patients had a pelvic laparoscopic lymph node assessment (62%) including the removal of involved nodes. If this invasive procedure could not be performed (60 patients, medical reasons: age, Karnofsky status), CT and MRI were used to assess lymph node disease (size >1 cm, loss of oval shape). In total, involved lymph nodes were detected in 75 patients (48%). One hundred and fourteen patients (73%) had concomitant chemotherapy, with 40 mg/m2 weekly cisplatin (selection due to medical reasons).
Treatment characteristics
For the detailed description of the general treatment and the treatment planning characteristics we refer in particular to the preceding report as published before recording the patient population treated from 1998–2003 [13], but also to other publications from the Vienna group describing these procedures in detail [6,7,10,11,35–40]. In the following, specific issues are highlighted which are of importance for the update of some treatment and treatment planning parameters. In limited disease the treatment schedule was changed during the protocol period to pelvic 3D EBRT and 4 × 7 Gy HDR intracavitary brachytherapy. In patients with common iliac lymph node involvement, para-aortic radiotherapy was performed with a 3D conformal box technique (n = 37, 24%). In 69 patients with unfavourable spread of residual disease at the time of brachytherapy, a combined interstitial/intracavitary approach was used (44%) with the Vienna ring applicator in the majority of cases, and some modification in far advanced disease.
In 3 out of 156 patients, the MRI dataset was not sufficient to report meaningful DVH parameter. Dose volume constraints were 75 Gy in D2cc for rectum and sigmoid and 100 Gy for the bladder in the beginning which were then gradually reduced to 70 Gy for rectum and sigmoid, if feasible and 90 Gy for the bladder [38–40]. Dose and target coverage were adapted (D90), considering the institutional dose volume constraints for OAR. If appropriate and feasible, the dose was escalated, especially in advanced disease to arrive at a D90 of at least 85 Gy. For limited and good response disease, the high dose in the HR CTV (>95 Gy D90) was not significantly reduced, only in case of violating dose volume constraints for OAR. The median treatment time was 48 days.
Endpoints
Patients were followed for disease related parameters and adverse side effects every 3 months in the first 2 years, in 6 month intervals for the next 3 years and then annually. MRI was done every 6 months in the first 5 years.
Complete remission (CR) was defined as no evidence of disease 3 months after end of treatment, evaluated by clinical examination and MRI.
Pelvic failure was split in failure in true pelvis (cervix, uterine corpus, vagina and parametria) and pelvis (additional pelvic nodes).
Continuous complete remission (CCR) was defined as no evidence of disease after complete remission. It is subdivided in CCR true pelvis (CCRtp) and CCR pelvis (CCRp).
Progression free survival (PFS) was defined as no progression of disease after tumour biopsy. PFS was subdivided in PFS true pelvis (PFStp) defined as local control, PFS pelvis (PFSp), defined as local and regional control, and PFS for distant metastases (PFSdm), defined as distant control, overall PFS is the freedom of local, regional and distant failure.
Overall survival (OS) and cancer specific survival (CSS) were defined as the period from the date of biopsy until the date of death and death by cervical cancer, respectively.
Late side effects are defined as any side effect appearing earliest 91 days after the end of treatment and are prospectively scored using the Late Effects in Normal Tissues-Subjective, Objective, Management and Analytic score (LENT SOMA, G0: no side effects, G4: worst side effect, LENT SOMA tables 1995). Side effects are reported as absolute number of events for G1–G4 morbidity. In addition, actuarial rates are given for G3 and G4 morbidity.
Statistical analysis and groups
Patients were analysed who underwent the complete definitive radiotherapy treatment (see eligibility criteria). All parameters were calculated for tumours 2–5 cm and tumours >5 cm and for the different stages. For the calculation, the SPSS-program was used (SPSS 15 for windows), applying the Kaplan–Meier method and log rank test. Outcome parameters were calculated at 3 years. Median follow up was 42 months for all patients and 58 months for patients alive. Ten patients were lost to follow up.
Results
Dose volume adaptation and dose escalation
The mean D90 ± 1SD for the whole patient cohort was 93 ± 13 Gy, it was 96 Gy ± 15 Gy for tumours 2–5 cm and 91 Gy ± 11 Gy for tumours > 5 cm. In the period from 2001–2003 the mean D90 was 90 ± 15 Gy in total, with 94 ± 16 Gy for tumours 2–5 cm and 87 ± 14 Gy for tumours >5 cm. The mean D90 increased to 94 ± 10 Gy in the period from 2004–2008. The tumours 2–5 cm had a mean D90 of 100 ± 10 Gy, and the large tumours > 5 cm a mean D90 of 93 ± 9 Gy, respectively. For both tumour size groups there was a mean 6 Gy increase in dose. Mean D2cc for bladder was 86 ± 17 Gy, 65 ± 9 Gy for rectum and 64 ± 9 Gy sigmoid.
Disease control
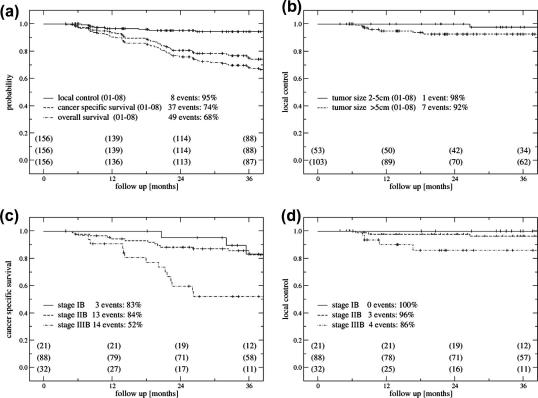
A detailed overview on the different outcome parameters and comparisons in regard to tumour size and stage of disease is given in Tables 1–4 and in Fig. 1a–d with calculated values at a follow-up time of 3 years.
Table 1.
Treatment response and site of relapse according to FIGO stage in 156 patients for the period of interest (3 years); numbers for the whole observation period are given in parentheses ().
| Stage | Total no. | Incomplete response | True pelvic recurrence |
Pelvic lymph node recurrence | Distant relapse alone | Overall failure | No evidence of disease | |
|---|---|---|---|---|---|---|---|---|
| Central | Non-central | |||||||
| IA | 1 | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| IB | 21 | 0 | 0 (0) | 0 (0) | 1 (1) | 2 (3) | 3 (4) | 18 (17) |
| IIA | 3 | 0 | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 2 (2) |
| IIB | 88 | 2 | 0 (0) | 1 (2) | 1 (2) | 12 (15) | 16 (21) | 72 (67) |
| IIIA | 5 | 0 | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 2 (2) | 3 (3) |
| IIIB | 32 | 2 | 0 (2) | 2 (3) | 2 (2) | 8 (11) | 14 (20) | 18 (12) |
| IVA | 6 | 1 | 0 (0) | 0 (1) | 0 (0) | 2 (2) | 3 (4) | 3 (2) |
| Total | 156 | 5 | 0 (2) | 3 (6) | 4 (5) | 27 (34) | 39 (52) | 117 (104) |
Table 2.
Outcome for 156 patients treated with radiotherapy ± chemotherapy and image guided adaptive brachytherapy. Events (n) at 3 years and after whole observation period, patients per subgroup (n), actuarial estimates (%).
| Protocol period image guided adaptive BT 2001–2008 |
||||
|---|---|---|---|---|
| Total | n Events overall period | n Events 3 year period | % 3 years | |
| PFStp (local control) | 156 | 13 | 8 | 95 |
| 2–5 cm | 53 | 1 | 1 | 98 |
| >5 cm | 103 | 12 | 7 | 92 |
| PFSp (pelvic control) | 156 | 18 | 12 | 91 |
| 2–5 cm | 53 | 3 | 3 | 95 |
| >5 cm | 103 | 15 | 9 | 90 |
| PFSdm (distant failure free) | 156 | 34 | 27 | 82 |
| 2–5 cm | 53 | 9 | 6 | 87 |
| >5 cm | 103 | 25 | 21 | 78 |
| PFSoverall (overall failure free) | 156 | 52 | 39 | 75 |
| 2–5 cm | 53 | 12 | 8 | 83 |
| >5 cm | 103 | 40 | 31 | 70 |
| Cancer specific survival | 156 | 49 | 37 | 74 |
| 2–5 cm | 53 | 12 | 8 | 83 |
| >5 cm | 103 | 37 | 29 | 70 |
| Overall survival | 156 | 66 | 49 | 68 |
| 2–5 cm | 53 | 22 | 16 | 72 |
| >5 cm | 103 | 44 | 33 | 65 |
PFS: progression free survival.
tp/p = True pelvis/pelvis (uterus, vagina, parametria/lymph nodes).
Table 3.
Late adverse effects (LENT SOMA) after radiotherapy ± chemotherapy and image guided adaptive brachytherapy in 156 patients (absolute numbers).
| Late adverse effects | Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|---|---|---|---|---|---|
| n | n | n | n | n | |
| Vagina | 23 | 84 | 44 | 2 | 1 |
| Bladder | 121 | 20 | 12 | 3 | 0 |
| Rectum | 137 | 8 | 6 | 2 | 3 |
| Bowel/Sigmoid | 152 | 2 | 2 | 0 | 0 |
| Total | 114 | 64 | 7 | 4 | |
| without vagina | 30 | 20 | 5 | 3 |
Table 4.
Three year outcome in 418 patients after definitive radiotherapy ± chemotherapy in cervical cancer patients treated from 1993–1997 [62], 1998–2000 [31] and 2001–2008 at the Medical University of Vienna.
| 3 Year-OS (%) | 3 Year-CSS (%) | 3 Year-PFS pelvis (%) | 3Year-G3/G4 morbidity (%)⁎ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIGO stage | FIGO stage | FIGO stage | All stages | |||||||||||||
| No. of patients n = 418 | IB | IIB | IIIB | IVA | IB | IIB | IIIB | IVA | IB | IIB | IIIB | IVA | Bladder | Bowel/rectum | Vagina | |
| Vienna: 1993–1997a | 189 | 62 | 70 | 46 | 40 | 77 | 78 | 59 | 53 | 100 | 87 | 69 | 60 | 3 | 10 | 31 |
| Vienna: 1998–2000b | 73 | 80 | 61 | 12 | 25 | 80 | 71 | 28 | 25 | 95 | 92 | 67 | 70 | 3 | 5 | 7 |
| Vienna: 2001–2008c | 156 | 74 | 79 | 45 | 33 | 83 | 84 | 52 | 40 | 94 | 96 | 75 | 75 | 2 | 4 | 1 |
Radiotherapy alone; CT assisted brachytherapy treatment planning.
Radiotherapy ± chemotherapy; MRI guided adaptive brachytherapy: learning period.
Radiotherapy ± chemotherapy; MRI guided adaptive brachytherapy: protocol period.
Actuarial rates for G3/G4 morbidity (LENT-SOMA score).
Fig. 1.
Outcome after radiotherapy ± chemotherapy and image-guided adaptive brachytherapy. (a) Local control, cancer specific survival and overall survival for all 156 patients. (b) Local control and tumour size. (c) Cancer specific survival for FIGO stages IB, IIB, IIIB. (d) Local control for FIGO stages IB, IIB, IIIB.
Complete remission was achieved in 151/156 patients (97%). Five patients had locally progressive disease. Recurrence in true pelvis occurred in 8 patients, 3 simultaneously with distant metastases. In addition, 5 patients had recurrence in the pelvic lymph nodes. Thirty-four patients had distant metastases alone. The events are shown in Tables 1 and 2.
Continuous complete remission for the true pelvis (CCRtp) was 97% at three years. For small tumours it was 98%, while for large tumours CCRtp was 95%.
Overall actuarial local control (3y) was 95%; 98% for tumours 2–5 cm, and 92% for tumours >5 cm (Table 2), 100% for IB, 96% for IIB, and 86% for IIIB (Fig. 1b and d). Actuarial progression free survival for distant metastases (3y) was 82% for all tumours, 87% for tumours 2–5 cm, 78% for tumours >5 cm. According to tumour stage it was 88% for IB, 85% for IIB, 69% for IIIB, 60% for IVA.
Ninety patients of the whole patient cohort were still alive at the time of study (58%), 49 patients died because of cancer and 17 due to other reasons. Actuarial cancer specific survival (3y) was 74% for all patients, 83% for tumours 2–5 cm, 70% for tumours >5 cm, 83% for IB, 84% for IIB, 52% for IIIB (see Tables 2 and 4, Fig. 1a and c). Actuarial overall survival (3y) was in total 68%, 72% for tumours 2–5 cm, 65% for tumours >5 cm, 74% for IB, 79% for IIB, 45% for IIIB (see Tables 2 and 4, Fig. 1a).
Late adverse side effects
In regard to late morbidity altogether 188 grade 1 + 2 and 11 grade 3 + 4 events, respectively, were observed in 140 patients. Sixteen patients did not develop any side effects. For details we refer to Table 3.
Actuarial rate for G3 + G4 morbidity was 2%/3% for the bladder, 4%/4% for the rectum, 0%/0%, for the bowel and 1%/3% for the vagina at 3/5 years, respectively.
Bladder
Twenty patients had grade 1 and 12 patients grade 2 side effects (mainly urinary urgency and frequency). Three patients developed grade 3 late side effects in the bladder (urinary frequency, urge).
Rectum
Eight patients experienced grade 1 and 6 patients grade 2 morbidity (mainly urgency and bleeding). Two patients suffered from massive rectal bleeding and also needed blood transfusions (G3). Three patients underwent stoma surgery (G4): 1 patient had rectal wall ulceration, 3–4 cm in diameter, which resulted in a fistula to the vagina, a second patient developed a recto-vaginal fistula, and a third patient rectal perforation.
Bowel (including sigmoid)
Two patients developed grade 1 side effects suffering from mild diarrhoea with frequency 2–3 times daily (G1) and one patient had moderate diarrhoea up to 8 times daily (G2). No patient developed grade 3 or 4 side effects.
Vagina
Grade 1 side effects were seen in 84 patients and grade 2 in 44 patients (mainly adhesions, teleangiectasia, dyspareunia). Two patients developed irreversible obliteration of more than two-thirds of the entire vaginal length (G3). One patient developed complete obliteration of the vagina (G4).
Discussion
The clinical impact of MRI assisted dose volume adapted brachytherapy combined with 3D conformal EBRT ± cisplatin in locally advanced cervix cancer, based on a large single-institution study (n = 145), was first reported in our previous publication divided into a learning period and a subsequent protocol period following the GEC ESTRO Recommendations prospectively [13]. There has been some additional evidence with favourable outcome provided from mono-institutional experience from IGR, Paris and Tata Memorial, Mumbai with MRI guided definitive adaptive PDR/HDR brachytherapy applying the GEC ESTRO Recommendations, in limited patient numbers, with no local recurrences in 45 patients (82% stage IB2/II) and 3 in 24 patients, respectively [41,42]. The CT experience published so far (Addenbrooke, Pittsburgh, Seoul) has revealed high local control rates (96%; 75%; 97%) in limited patient numbers [31,33,43], with clear and significant improvements compared to historical controls, for example 96% vs. 76% and 97% vs 81% [31,33]. The Ultrasound experience also provides challenging results [32]. The first prospective multicentre trial (STIC, France) comparing 2D to 3D CT image guided PDR brachytherapy in definitive radiotherapy at 2 years revealed a significant improvement in local control (74% vs. 79%) and a significant reduction in treatment related morbidity (G3/G4 14% vs. 1%) [44].
The present study is an update of the Vienna results (2001–2003) [13] with mature 3-year data on the protocol period (2001–2008) in a considerable number of 156 consecutive patients treated up to the start of the large prospective multicentre EMBRACE study (http://www.embracestudy.dk). The results clearly confirm that excellent local control rates can be achieved through MRI guided adaptive brachytherapy following a protocol according to the concepts of the GEC ESTRO Recommendations, with an overall local control rate of 95% at 3 years including 103/156 (66%) patients with tumour size >5 cm. For the large tumours alone, the 3-year rate was 92%, for the tumours 2–5 cm 98%. This is an outstanding treatment result, which has to our knowledge not been achieved so far, neither in cervix cancer nor at any other tumour sites for advanced solid tumours by definitive radio(-chemo-)therapy alone. Local failures in this IGABT experience compare in absolute numbers very favourably with Vienna historical series for tumours >5 cm (Table 4): 7/103 (2001–2008) versus 12/40 (1998–2000, [13]) and 22/124 (1993–97, [45]).1 This evident improvement was (mainly) in large tumours >5 cm, with actuarial data for pelvic control raising from 67%/64% [13,45] in 124/40 patients to 90% in 103 patients.2 This translates into an absolute local control benefit of 23–26% (actuarial) and a relative reduction in local failure of about 65% compared to the historical Vienna series. The major contribution to this major improvement is attributed to the change to MRI guidance with dose volume adaptation and dose escalation to a mean dose of 93 Gy for High Risk CTV (defined according to treatment response) and even much larger doses inside this HR CTV, during 2001–2008 (D90). There may be also some contribution through the introduction of simultaneous cisplatin chemotherapy in 1999 [46] as the beneficial effect of radiochemotherapy on local control has been well described, however less pronounced in more advanced disease [19]. In the period 1993–1997, radiochemotherapy was not applied, whereas in the recent series it was applied in 73%. The excellent regional control may be partly also attributable to laparoscopic pelvic lymph node dissection with macroscopic removal which was first introduced in 1997 and performed in 62% of patients in the recent series.
Current protocols on dose escalation in EBRT using advanced (functional) imaging, treatment planning and delivery techniques try to go into a similar direction aiming at the improvement of local control through volume adaptation and dose escalation at tumour sites where local failure is still very considerable, e.g. in NSCLC [47] (new FP 7 protocol Amsterdam/Maastricht) and in head and neck cancer [48]. In prostate cancer excellent results seem to be clinically achievable and superior to ultra high dose IMRT when implementing image guided prostate brachytherapy into treatment protocols e.g. for intermediate risk prostate cancer [49], underlining the beneficial effect of very focussed dose escalation.
It has to be outlined that the recent cervix cancer experience reported here as well as in some of the dose escalation techniques briefly mentioned try to define analogue new ways of dose prescription which consist of balancing dose volume constraints for OAR and for target volume aiming at achieving the highest target dose as individually possible respecting the OAR dose volume constraints.
Within the protocol period 2001–2008, there was also an improvement in local control at 3 years for large tumours: 2001–2003: 6 failures out of 38 (Table 2 [13]) versus 2004–2008: 1 failure out of 65 (Table 2). This improvement is likely a result of continuous improvement in dose volume adaptation and dose escalation from mean 87 Gy in the first part of the protocol period to 93 Gy in the second part, also increasing the number of combined intracavitary/interstitial applications.
In regard to stage of disease, the major benefit is in advanced disease. For stage IIB/IIIB there are only 3/4 events out of 88/32 patients with a 96%/86% actuarial rate at 3 years, respectively (Fig. 1d). This compares very favourably with literature data [25,28]. However, there are some late events after 3 years, 1 in IIB and 4 in IIIB, however, mainly from the early protocol period. This implies that the failure rate at 5 years for IIIB will considerably drop (overall 8/32) and that there is obvious need for further improvement of local control, in particular for this subset of IIIB patients. Opposed to concomitant chemotherapy with local control improvement of 7% for IIB and 3% for III/IVA [19], the effect of image guided brachytherapy seems to be in fact most pronounced in very advanced disease (tumours >5 cm) with an absolute local control benefit of 23–26% in the Vienna series.
The mean D90 of 90 Gy during 2001–2003 became mean D90 of 94 Gy during 2004–2008. It has to be kept in mind that the mean D90 for tumours 2–5 cm has been 96 Gy for all patients, 94 Gy for the first period, 100 Gy for the second period, whereas for tumours >5 cm the mean D90 has been 91 Gy for the whole period and 87 Gy for the first period and 93 Gy for the second period. Although this dose increase with excellent local control supports strongly the assumption of a dose effect for local control and although dose volume effects could already be clearly shown for advanced disease in the Vienna series [36,37], the large variation of dose values underlines that there is so far only limited evidence for well defined absolute dose values to be applied for a given clinical scenario (e.g. IB, IIB, IIIB). It also has to be taken into account that the dose variation through the various mono-institutional series with similar clinical outcome has been large so far [50–53].
Overall the clinical update from the recent Vienna series on local control compares well with what has been reported so far from partly smaller series with partly more limited follow up [31–33,41–44] and indicates the large clinical benefit to be expected in regard to local control from this new image guided adaptive approach, in particular for advanced disease.
The improved local control in tumours >5 cm seems to be associated with some increase in cancer specific survival at 3 years from 57%/40% in 1993–97/98–2000 [13,45] to 70% in 2001–2008, respectively, whereas no change was detectable in tumours 2–5 cm. CSS (3y) is 77%/80% and 83% for stage IB, 78%/71% and 84% in stage IIB, 59%/28% and 52% in stage IIIB, in the historical and recent periods, respectively (Table 4, Fig. 1c). These findings may be interpreted as a trend to improved CSS, in particular for not very far advanced disease (stage IIB), whereas this is not seen in very advanced disease (stage IIIB). Such findings have clearly to be further clarified, also with longer follow up, as more events after the 3-year period will follow. At present the impact of the local image guided adaptive approach on cancer specific survival is not straightforward and demands clearly prospective clinical studies.
It is worthwhile to notice that morbidity was not increased in the protocol period. The tendency is rather a decrease (Tables 3 and 4, compared to [13,45]). Severe morbidity (G3/G4) was low despite a very high radiation dose in the HR CTV: for the bladder the actuarial rate was 2%/3%, for the rectum 4%/4%, for the bowel 0%/0%, for the vagina 1%/3% at 3/5 years, respectively. This is in accordance with the recent findings from the STIC trial [44].
There are limitations for these findings from the Vienna series: the current clinical results were achieved within a single institutional experience and are compared to a historical control of this institution with all the well known critical implications for historical comparisons [compare footnote 2]. The prognostic variables and the staging procedures were not comprehensively evaluated (e.g. lymph nodes, histology), the prospective evaluation focussed on tumour size, tumour volume and dose volume relations. The results may therefore be biased by such uncontrolled patient, tumour and staging parameters. The growing experience in CT assisted and then MRI guided adaptive brachytherapy of the Vienna investigators over more than 15 years is also a factor not well controlled. Such limitations will be partly overcome in the foreseeable future. A prospective collaborative International observational study on the parameters and the effects of MRI guided brachytherapy in locally advanced cervical cancer (EMBRACE) was initiated [54] and also a retrospective multi-institutional review following the same principles for reporting (RetroEMBRACE). These studies will altogether provide data on minimum 1200 patients treated with IGABT during the next years [55,56] in addition to the data soon becoming available from a large prospective French trial (STIC) [44].
Despite the growing importance of IGABT during the last years and multiple research activities in the field [32,33,39,40,52,53,57–68], there are still major issues to be clarified in order to bring IGABT to the next more mature levels. The most important issues are on DVH parameters and their correlation to outcome, both for CTV and OAR. This is of particular interest for limited and more advanced disease with good and poor response. The issue of dose volume constraints has to be clarified for the CTV, both for favourable and unfavourable advanced disease, with likely dose de-escalation and escalation [69]. Furthermore, there is the dissemination issue as IGABT is at present mainly linked to MRI with limited availability in the western world and even less all over the world. It has to be clarified, to what extent imaging technologies like CT and/or US will be efficient enough to become an alternative and/or a complementary tool within IGABT, next to MRI.
Despite the significantly improved local control, the assumed associated improvement in (cancer specific) survival needs to be clarified, also in the frame of chemoradiation [19]. Image guided adaptive brachytherapy will reduce local failure significantly, which will further make distant metastases the predominant pattern of failure (overall 52 with 34 distant compared to 18 loco-regional in this series, Table 1). This needs new strategies to overcome distant failure more efficiently which may be intensified simultaneous chemo-radiotherapy [21] and/or adjuvant chemotherapy [70] and/or any form of “targeted therapies”, in particular for patients identified – based on poor prognostic factors – to be at high risk of distant metastases. For future combined modality trials in cervix cancer (cytotoxic chemotherapy, targeted therapies), image guided adaptive brachytherapy should become a mandatory integral part in order to take advantage of the large clinical impact of this new treatment method in regard to local control and likely also survival.
Conflict of interest notification
The Department of Radiotherapy at the Medical University of Vienna receives/received financial and/or equipment support for research and educational purposes from Nucletron B.V., Varian Medical Systems, Inc., and Isodose Control B.V.
Acknowledgement
This study was partly supported by the FWF Grant No. L562.
Footnotes
[Both published Vienna series (1998–2000: MRI learning period [13]; 1993–1997: radiotherapy alone/CT assisted BT treatment planning [45] are taken for comparison, as both together are considered to represent best the full picture of the treatment in the preceding decade. The results from the period 1998–2000 were particularly poor].
[Actuarial pelvic control is given because the publication in 2000 only provides actuarial pelvic control data, with the majority of events being local, 22/26 (Table 1[45]). For the current series this is 7/8 pelvic failures being local, (Tables 1 and 2)].
References
- 1.Bentzen S.M. Radiation oncology health technology assessment: the best is the enemy of the good. Nat Clin Pract Oncol. 2008;5:563. doi: 10.1038/ncponc1203. [DOI] [PubMed] [Google Scholar]
- 2.Thwaites D.I., Verellen D. Vorsprung durch Technik: evolution, implementation, QA and safety of new technology in radiotherapy. Radiother Oncol. 2010;94:125–128. doi: 10.1016/j.radonc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Verellen D., De Ridder M., Linthout N., Tournel K., Soete G., Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 4.Bortfeld T., Schmidt-Ullrich R., DeNeve W., Wazer D. Springer; 2005. Image-Guided IMRT. [Google Scholar]
- 5.Webb S. Institute of Physics Publishing; Bristol: 2004. Contemporary IMRT: Developing Physics and Clinical Implementation. [Google Scholar]
- 6.Haie-Meder C., Pötter R., Van Limbergen E. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Potter R., Haie-Meder C., Van Limbergen E. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos J.C., Schard G., Berger D. Systematic evaluation of MRI findings in different stages of treatment of cervical cancer: potential of MRI on delineation of target, pathoanatomic structures, and organs at risk. Int J Radiat Oncol Biol Phys. 2006;64:1380–1388. doi: 10.1016/j.ijrobp.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan A.N., Dimopoulos J., Kirisits C., Berger D., Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Kirisits C., Pötter R., Lang S., Dimopoulos J., Wachter-Gerstner N., Georg D. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62:901–911. doi: 10.1016/j.ijrobp.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos J.C., Kirisits C., Petric P. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys. 2006;66:83–90. doi: 10.1016/j.ijrobp.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Jurgenliemk-Schulz I.M., Tersteeg R.J., Roesink J.M. MRI-guided treatment-planning optimisation in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol. 2009;93:322–330. doi: 10.1016/j.radonc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Pötter R., Dimopoulos J., Georg P. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Keys H.M., Bundy B.N., Stehman F.B. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 15.Morris M., Eifel P.J., Lu J. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 16.Peters W.A. 3rd, Liu, PY, Barrett, RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 17.Rose P.G., Bundy B.N., Watkins E.B. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 18.Whitney C.W., Sause W., Bundy B.N. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 19.Vale C., Thierny J.F., Stewart L.A. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michiels S., Le Maitre A., Buyse M. Surrogate endpoints for overall survival in locally advanced head and neck cancer: meta-analyses of individual patient data. Lancet Oncol. 2009;10:341–350. doi: 10.1016/S1470-2045(09)70023-3. [DOI] [PubMed] [Google Scholar]
- 21.Duenas-Gonzalez A., Zarba J.J., Patel F. Phase III, Open-Label, Randomized Study Comparing Concurrent Gemcitabine Plus Cisplatin and Radiation Followed by Adjuvant Gemcitabine and Cisplatin Versus Concurrent Cisplatin and Radiation in Patients With Stage IIB to IVA Carcinoma of the Cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 22.Quinn M.A., Benedet J.L., Odicino F. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S43–103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 23.Hunter, RD, Davidson, SE. Low dose-rate brachytherapy for treating cervix cancer: changing dose rate. In: Joslin CAF FA, Hall E, editor. Principles and practice of brachytherapy using afterloading systems, London: Arnold. 2001;343–353.
- 24.Meredith W.J. Livingstone; Edinburgh: 1967. Radium Dosage: The Manchester System. [Google Scholar]
- 25.Perez C.A., Grigsby P.W., Chao K.S., Mutch D.G., Lockett M.A. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998;41:307–317. doi: 10.1016/s0360-3016(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher G.H., Hamburger A.D. Female Pelvis. Squamos cell carcinoma of the Uterine Cervix. In: Fletcher G.H., editor. Textbook of Radiotherapy. Lea and Febiger; Philadelphia: 1980. pp. 720–789. third edition. [Google Scholar]
- 27.Perez C.A., Breaux S., Bedwinek J.M. Radiation therapy alone in the treatment of carcinoma of the uterine cervix. II. Analysis of complications. Cancer. 1984;54:235–246. doi: 10.1002/1097-0142(19840715)54:2<235::aid-cncr2820540210>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Eifel P.J., Moughan J., Owen J., Katz A., Mahon I., Hanks G.E. Patterns of radiotherapy practice for patients with squamous carcinoma of the uterine cervix: patterns of care study. Int J Radiat Oncol Biol Phys. 1999;43:351–358. doi: 10.1016/s0360-3016(98)00401-5. [DOI] [PubMed] [Google Scholar]
- 29.Gerbaulet A., Michel G., Haie-Meder C. The role of low dose rate brachytherapy in the treatment of cervix carcinoma. Experience of the Gustave-Roussy Institute on 1245 patients. Eur J Gynaecol Oncol. 1995;16:461–475. [PubMed] [Google Scholar]
- 30.Haie-Meder C., Chargari C., Rey A., Dumas I., Morice P., Magne N. DVH parameters and outcome for patients with early-stage cervical cancer treated with preoperative MRI-based low dose rate brachytherapy followed by surgery. Radiother Oncol. 2009;93:316–321. doi: 10.1016/j.radonc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Tan L.T., Coles C.E., Hart C., Tait E. Clinical impact of computed tomography-based image-guided brachytherapy for cervix cancer using the tandem-ring applicator - the Addenbrooke’s experience. Clin Oncol (R Coll Radiol) 2009;21:175–182. doi: 10.1016/j.clon.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Narayan K., van Dyk S., Bernshaw D., Rajasooriyar C., Kondalsamy-Chennakesavan S. Comparative study of LDR (Manchester system) and HDR image-guided conformal brachytherapy of cervical cancer: patterns of failure, late complications, and survival. Int J Radiat Oncol Biol Phys. 2009;74:1529–1535. doi: 10.1016/j.ijrobp.2008.10.085. [DOI] [PubMed] [Google Scholar]
- 33.Kang H.C., Shin K.H., Park S.Y., Kim J.Y. 3D CT-based high-dose-rate brachytherapy for cervical cancer: clinical impact on late rectal bleeding and local control. Radiother Oncol. 2010;97:507–513. doi: 10.1016/j.radonc.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Wittekind C., HMeyer H-J. Springer; Berlin Heidelberg New York: 2002. UICC: TNM Klassifikation maligner Tumoren. [Google Scholar]
- 35.Kirisits C., Lang S., Dimopoulos J., Berger D., Georg D., Pötter R. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65:624–630. doi: 10.1016/j.ijrobp.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 36.Dimopoulos J.C., Pötter R., Lang S. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311–315. doi: 10.1016/j.radonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Dimopoulos J.C., Lang S., Kirisits C. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:56–63. doi: 10.1016/j.ijrobp.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Lang S., Kirisits C., Dimopoulos J., Georg D., Pötter R. Treatment planning for MRI assisted brachytherapy of gynecologic malignancies based on total dose constraints. Int J Radiat Oncol Biol Phys. 2007;69:619–627. doi: 10.1016/j.ijrobp.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Georg P., Lang S., Dimopoulos J.C. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:356–362. doi: 10.1016/j.ijrobp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Georg, P, Potter, R, Georg, D, et al. Dose Effect Relationship for Late Side Effects of the Rectum and Urinary Bladder in Magnetic Resonance Image-Guided Adaptive Cervix Cancer Brachytherapy. Int J Radiat Oncol Biol Phys, in press, doi:10.1016/j.ijrobp.2010.12.029. [DOI] [PubMed]
- 41.Chargari C., Magne N., Dumas I. Physics Contributions and Clinical Outcome with 3D-MRI-Based Pulsed-Dose-Rate Intracavitary Brachytherapy in Cervical Cancer Patients. Int J Radiat Oncol Biol Phys. 2009;74:185–193. doi: 10.1016/j.ijrobp.2008.06.1912. [DOI] [PubMed] [Google Scholar]
- 42.Mahantshetty U., Banerjee S., Chopra S., Engineer R., Shrivastava S.K. Clincal outcome of patients treated with template based high dose rate (HDR) interstitial brachytherapy boost in gynecological malignancies. Radiother Oncol. 2011;99:S117. [Google Scholar]
- 43.Beriwal S., Bhatnagar A., Heron D.E. High-dose-rate interstitial brachytherapy for gynecologic malignancies. Brachytherapy. 2006;5:218–222. doi: 10.1016/j.brachy.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Charra-Brunaud C., Levitchi M., Delannes M. Dosimetric, clinical results of a French prospective study of 3D brachytherapy for cervix carcinoma. Radiother Oncol. 2011;99:S57. [Google Scholar]
- 45.Pötter R., Knocke T.H., Fellner C., Baldass M., Reinthaller A., Kucera H. Definitive radiotherapy based on HDR brachytherapy with iridium 192 in uterine cervix carcinoma: report on the Vienna University Hospital findings (1993–1997) compared to the preceding period in the context of ICRU 38 recommendations. Cancer Radiother. 2000;4:159–172. doi: 10.1016/S1278-3218(00)88900-3. [DOI] [PubMed] [Google Scholar]
- 46.Pötter R., Dimopoulos J., Bachtiary B. 3D conformal HDR-brachy- and external beam therapy plus simultaneous cisplatin for high-risk cervical cancer: clinical experience with 3 year follow-up. Radiother Oncol. 2006;79:80–86. doi: 10.1016/j.radonc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Lee C.B., Stinchcombe T.E., Rosenman J.G., Socinski M.A. Therapeutic advances in local-regional therapy for stage III non-small-cell lung cancer: evolving role of dose-escalated conformal (3-dimensional) radiation therapy. Clin Lung Cancer. 2006;8:195–202. doi: 10.3816/CLC.2006.n.047. [DOI] [PubMed] [Google Scholar]
- 48.Duprez F., Bonte K., De Neve W., Boterberg T., De Gersem W., Madani I. Regional relapse after intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;79:450–458. doi: 10.1016/j.ijrobp.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Deutsch I., Zelefsky M.J., Zhang Z. Comparison of PSA relapse-free survival in patients treated with ultra-high-dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy. 2010;9:313–318. doi: 10.1016/j.brachy.2010.02.196. [DOI] [PubMed] [Google Scholar]
- 50.Lang S., Nulens A., Briot E. Intercomparison of treatment concepts for MR image assisted brachytherapy of cervical carcinoma based on GYN GEC-ESTRO recommendations. Radiother Oncol. 2006;78:185–193. doi: 10.1016/j.radonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Jurgenliemk-Schulz I.M., Lang S., Tanderup K. Variation of treatment planning parameters (D90 HR-CTV, D 2cc for OAR) for cervical cancer tandem ring brachytherapy in a multicentre setting: comparison of standard planning and 3D image guided optimisation based on a joint protocol for dose-volume constraints. Radiother Oncol. 2009;94:339–345. doi: 10.1016/j.radonc.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 52.De Brabandere M., Mousa A.G., Nulens A., Swinnen A., Van Limbergen E. Potential of dose optimisation in MRI-based PDR brachytherapy of cervix carcinoma. Radiother Oncol. 2008;88:217–226. doi: 10.1016/j.radonc.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Lindegaard J.C., Tanderup K., Nielsen S.K., Haack S., Gelineck J. MRI-guided 3D optimization significantly improves DVH parameters of pulsed-dose-rate brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:756–764. doi: 10.1016/j.ijrobp.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 54.Pötter R., Kirisits C., Fidarova E.F. Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma. Acta Oncol. 2008;47:1325–1336. doi: 10.1080/02841860802282794. [DOI] [PubMed] [Google Scholar]
- 55.Tanderup K., Lindegaard J.C., Kirisits C. EMBRACE. Radiother Oncol. 2011;99:S22. [Google Scholar]
- 56.Sturdza A., Lindegaard J.C., Fokdal L.U. Retro-EMBRACE: Preliminary results of image guided BT for cervical cancer in the last years of the 20st century. Radiother Oncol. 2011;99:S22. [Google Scholar]
- 57.Dimopoulos J.C., De Vos V., Berger D. Inter-observer comparison of target delineation for MRI-assisted cervical cancer brachytherapy: application of the GYN GEC-ESTRO recommendations. Radiother Oncol. 2009;91:166–172. doi: 10.1016/j.radonc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Dimopoulos J.C., Schirl G., Baldinger A., Helbich T.H., Pötter R. MRI assessment of cervical cancer for adaptive radiotherapy. Strahlenther Onkol. 2009;185:282–287. doi: 10.1007/s00066-009-1918-7. [DOI] [PubMed] [Google Scholar]
- 59.Hellebust T.P., Kirisits C., Berger D. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol. 2010;96:153–160. doi: 10.1016/j.radonc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Trnkova P., Potter R., Baltas D. New inverse planning technology for image-guided cervical cancer brachytherapy: description and evaluation within a clinical frame. Radiother Oncol. 2009;93:331–340. doi: 10.1016/j.radonc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Jurgenliemk-Schulz I.M., Lang S., Tanderup K. Variation of treatment planning parameters (D90 HR-CTV, D 2cc for OAR) for cervical cancer tandem ring brachytherapy in a multicentre setting: comparison of standard planning and 3D image guided optimisation based on a joint protocol for dose-volume constraints. Radiother Oncol. 2010;94:339–345. doi: 10.1016/j.radonc.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Tanderup K., Potter R., Lindegaard J.C., Berger D., Wambersie A., Kirisits C. PTV margins should not be used to compensate for uncertainties in 3D image guided intracavitary brachytherapy. Radiother Oncol. 2010;97:495–500. doi: 10.1016/j.radonc.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Viswanathan A.N. The Frank Ellis memorial lecture: the use of three-dimensional imaging in gynaecological radiation therapy. Clin Oncol (R Coll Radiol) 2008;20:1–5. doi: 10.1016/j.clon.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Lee L.J., Sadow C.A., Russell A., Viswanathan A.N. Correlation of point B and lymph node dose in 3D-planned high-dose-rate cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:803–809. doi: 10.1016/j.ijrobp.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanderup K., Nielsen S., Nyvang G. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing organs at risk in brachytherapy of cervical cancer. Radiother Oncol. 2010;94:173–180. doi: 10.1016/j.radonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Koom W.S., Sohn D.K., Kim J.Y. Computed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: preliminary demonstration of correlation between dose-volume parameters and rectal mucosal changes observed by flexible sigmoidoscopy. Int J Radiat Oncol Biol Phys. 2007;68:1446–1454. doi: 10.1016/j.ijrobp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Fidarova E.F., Berger D., Schussler S. Dose volume parameter D2cc does not correlate with vaginal side effects in individual patients with cervical cancer treated within a defined treatment protocol with very high brachytherapy doses. Radiother Oncol. 2010;97:76–79. doi: 10.1016/j.radonc.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Georg P., Kirisits C., Goldner G. Correlation of dose-volume parameters, endoscopic and clinical rectal side effects in cervix cancer patients treated with definitive radiotherapy including MRI-based brachytherapy. Radiother Oncol. 2009;91:173–180. doi: 10.1016/j.radonc.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Van de Kamer, JB, De Leeuw, AA, Moerland, MA, Jurgenliemk-Schulz, IM. Determining DVH parameters for combined external beam and brachytherapy treatment: 3D biological dose adding for patients with cervical cancer. Radiother Oncol 2010;94:248–253. [DOI] [PubMed]
- 70.Lorvidhaya V., Chitapanarux I., Sangruchi S. Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: a randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1226–1232. doi: 10.1016/s0360-3016(02)04405-x. [DOI] [PubMed] [Google Scholar]