Abstract
The Hedgehog (Hh) and Transforming Growth Factor-β (TGF-β) signaling pathways represent essential regulators of cell proliferation and differentiation during embryogenesis. Pathway deregulation is a characteristic of various cancers. Recently, evidence for a convergence of these pathways at the level of the GLI2 transcription factor in the context of tumor initiation and progression to metastasis has emerged. This short review summarizes recent knowledge about GLI2 function and mechanisms of action downstream of TGF-β in cancer.
Hedgehog signaling in cancer
Hedgehog (Hh) signaling components (Hh ligands (Sonic, Indian or Desert Hh) and cell surface receptors, Patched-1 (PTCH1) and Smoothened (SMO) play a major role during embryonic patterning and during tumor development. In the absence of Hh ligands, PTCH1 maintains SMO in an inactive state. Upon Hh binding to PTCH1, SMO repression by PTCH1 is alleviated, SMO translocates to the primary cilium and signaling is transduced, leading to activation and nuclear translocation of GLI transcription factors (see Figure 1 and (1)). The latter contribute to cancer progression via regulation of cell cycle progression and apoptosis.
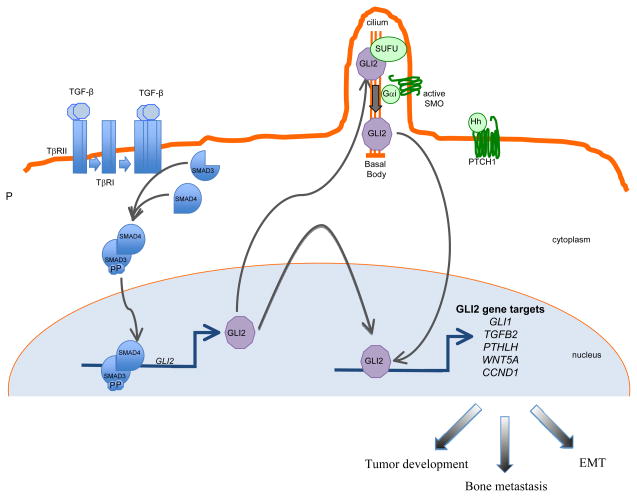
Figure 1. Schematic representation of TGF-β and Hedgehog (Hh) signaling in cancer.
TGF-β ligand activation of specific cell surface serine-threonine kinase receptors activates the SMAD cascade, resulting in transcriptional activation of the GLI2 gene. GLI2 protein may either regulate target gene expression and exert pro-oncogenic activities downstream of TGF-β signaling or may contribute to Hedgehog (Hh)-driven signaling which occurs via Smoothened (SMO) activation in the primary cilium following Hh binding to the pathway inhibitory receptor Patched-1 (PTCH1).
The direct implication of the Hh signaling pathway in tumorigenesis was initially established through the identification of loss-of-function mutations in the PTCH gene in patients with familial and sporadic basal cell carcinomas of the skin. Inappropriate Hh pathway activation, estimated as elevated GLI1 expression, has since been described in an ever-growing number of tumors, including esophageal squamous cell sarcomas, transitional cell carcinomas, small cell lung carcinomas, bladder, ovarian, gastro-intestinal and pancreatic carcinomas, as well as cutaneous melanoma (Reviewed in (2)). Hh signaling/GLI factors provide survival and advantage to tumor cells and have also been implicated in cancer stem cell renewal and survival.
Targeting the Hh pathway for cancer treatment by means of SMO antagonists has shown remarkable efficacy in the pre-clinical and clinical settings, against tumors (basal cell carcinoma (BCC) of the skin, pancreatic carcinoma) with identified mutations in the upstream components of the pathway. On the other hand, a number of tumors were found to be oblivious to Hh signaling inhibition despite exhibiting high expression of GLI1, suggesting the existence of alternate pathways leading to expression of downstream Hh mediators.
TGF-β signaling in cancer
TGF-β family members signal via membrane-bound heteromeric serine-threonine kinase receptor complexes whose activation by TGF-β ligands leads to phosphorylation of proteins of the SMAD family. The latter, in turn, accumulate in the nucleus and act as transcription factors to regulate target gene expression, acting either directly on SMAD-specific cis-elements on DNA, or via physical interaction with other transcription factors acting on their cognate DNA recognition sites (see Figure 1 and (3)). Negative control of the cell cycle drives the tumor suppressor functions of TGF-β in normal and pre-malignant tissues. On the other hand, TGF-β, which is secreted abundantly by tumors cells as well as by the local microenvironment, promotes invasion and metastases of various neoplasms through autocrine and paracrine mechanisms (4, 5). Notably, TGF-β induces epithelial to mesenchymal transition (EMT), whereby epithelial tumor cells acquire an invasive, mesenchymal-like, phenotype accompanied by changes in the expression of cell-cell adhesion molecules and secretion of metalloproteinases, leading to metastasis (6). TGF-β is a critical mediator of bone metastasis, whereby complex bidirectional interactions between tumor cells and the bone microenvironment increase bone destruction and establishment of metastases in the bone (7).
TGF-β pathway targeting in melanoma and breast cancer cells
TGF-β signaling blockade has proven efficient in preventing the development of a variety of tumor types. For example, the small molecule TβRI kinase inhibitor SD-208 increased survival following orthotopic implantation of glioma cells (8). Most recently, we showed that systemic administration of SD-208 to mice bearing human melanoma bone metastases significantly reduced the development and progression of osteolytic lesion area. This was associated with decreased tumor burden as well as increased survival in a dose-dependent manner (9). SD-208 was similarly effective in preventing the development and progression of MDA-MB-231 breast cancer bone metastases. (10). Another TβRI kinase inhibitor, SM16, has showed remarkable efficacy to inhibit the growth of TGF-β-producing primary 4T1 murine mammary carcinoma, as well as metastasis to lung, via immune-mediated mechanism(s) (11).
TGF-β signaling blockade by other modalities, overexpression of either a dominant-negative form of TGF-β receptor type II (12) or SMAD7 (13) in breast cancer cells and in melanoma cells (14, 15), also was effective in reducing bone metastases. Noteworthy, a TGF-β/SMAD-dependent gene bone metastasis signature initially identified by Kang et al. in breast cancer cells (16) was found in highly metastatic melanoma cell lines (15) and was inhibited by both SMAD7 overexpression (15) and by the TβRI kinase inhibitor SD-208 (9). Thus, similar mechanisms are likely to be involved in TGF-β-driven bone metastases from melanoma and breast cancer cells.
Anti-TGF-β therapies with promising results in both the preclinical and clinical settings are diverse and include systemic administration of small-molecule TβRI/RII kinase inhibitors (see above examples), neutralizing antibodies and soluble receptors that act as ligand traps and inhibit the activity of all three TGF-β isoforms by preventing their binding cell surface TGF-β receptors, and tumor delivery of antisense oligonucleotides targeting TGF-β expression for the treatment of metastatic tumors overexpressing active TGF-β (Reviewed in (17)).
GLI2 is a target of multiple signaling pathways – Direct induction by the TGF-β/SMAD pathway
While GLI activation may result from Hh ligand- or Hh receptor-induced signaling, there is mounting evidence for non-canonical signaling events leading to the expression of Hh mediators of the GLI family. These include TGF-β, FGF, EGF and MAPK signaling (reviewed in (18, 19)). Evidence that GLI-dependent transcription may occur even in the absence of upstream Hh signals is suggested for example by the fact that Gli2 and Gli3 are widely expressed in the developing embryo, including in regions that are far from Shh production and may be expressed downstream of FGF signaling (20).
We have identified GLI2 as a direct TGF-β/SMAD target independent of Hedgehog signaling in a variety of cell types including primary and immortalized skin and lung fibroblasts, keratinocytes, MDA-MB-231 breast adenocarcinoma cells, as well as other human cancer cell lines derived from pancreatic carcinoma, glioblastoma and cutaneous melanoma (21). Using several experimental approaches including siRNA knockdown, we demonstrated that GLI2 induction by TGF-β is directly under the control of SMAD3. Subsequent GLI1 induction by TGF-β was shown to require GLI2 and to occur independently from SMO activity. Cloning of the 5′ regulatory region of the human GLI2 gene identified both SMAD and β-catenin recruitment to the TGF-β responsive region of the promoter (22), hinting that the WNT/β-catenin pathway may also be able to regulate GLI2 expression.
In a study comparing the pro-apoptotic and cytostatic effects of Hh pathway inhibition in a series of human pancreatic carcinoma cell lines, a subset of cancer cells expressing high GLI levels yet resistant to cyclopamine, a compound that interferes with SMO activity and prevents Hh-induced GLI1 expression, was identified (23). This suggested to us that high GLI expression was not due to constitutive Hh activation, and we hypothesized that constitutive TGF-β pathway activation may actually be responsible for GLI expression in these cyclopamine-resistant cell lines. We then demonstrated that pharmacologic inhibition of autocrine TGF-β signaling efficiently slowed their growth and reduced GLI2 expression (21), and that siRNA knockdown of GLI2 in these cyclopamine-resistant cell lines also caused growth inhibition. These experiments were the first to identify TGF-β signaling as a relevant target for therapeutic intervention in a cellular context characterized by high GLI expression and lack of responsiveness to Hh targeting (21).
Induction of GLI2 by TGF-β has been confirmed by several independent groups. For example, in an experimental model of breast cancer, it was recently found that progression from ductal carcinoma in situ (DCIS) to invasive carcinoma implicates TGF-β signaling with increased GLI2 expression (24). Consistent with our initial observations (21), the authors found that TGF-β increases GLI2 expression and GLI-dependent transcription, influencing myoepithelial cell differentiation and progression to invasion. Recently, it was found that ovarian-specific Bmpr1a Bmpr1b double-mutant mice develop granulosa cell tumors that exhibit dysregulated TGF-β signaling associated with increased GLI2 (and GLI1) expression (25). The authors also showed that GLI2 is a TGF-β regulated gene in normal granulosa cells and suggested that GLI factors may contribute to cancer progression in the ovary.
There is ample evidence that GLI2 plays a direct functional role in the development of solid tumors. Overexpression of Gli2 in mouse skin by use of a Keratin 5 promoter is sufficient to produce BCCs (26). Inversely, GLI2 knockdown in prostate cancer cells reduces anchorage-independent colony formation, delays tumor xenograft growth in vivo and enhances paclitaxel chemosensitivity (27, 28). Likewise, in hepatocellular carcinoma cell lines, GLI2 knockdown inhibits cell proliferation through the regulation of genes implicated in cell cycle and apoptosis (29). GLI2 knockdown also reduces melanoma cell invasiveness through downregulation of E-cadherin and their capacity to form bone metastases (30). Noteworthy, pharmacologic interference with GLI function, or GLI1 knockdown, both lead to robust cytotoxicity in human colon carcinoma cells, whereas a SMO inhibitor had little effect (31).
GLI2 in melanoma progression
We recently established that GLI2 modulates critical events associated with melanoma progression (30). We showed that basal GLI2 expression in melanoma cells largely depends upon autocrine TGF-β signaling and that high GLI2 expression was associated with a “mesenchymal transition” and loss of E-cadherin expression. In epithelial cancers, loss of E-cadherin is a hallmark of epithelial to mesenchymal transition, EMT, a complex phenotypic conversion that involves changes in morphology, differentiation and cell-cell adhesion, and acquisition of a motile behavior, functionally associated with poor prognosis in various cancers (32). Likewise, a mesenchymal transition is characteristic of melanoma switch from an early radial growth phase to vertical growth phase of primary melanomas, a critical event leading to metastatic spreading (33).
Interestingly, GLI1, a direct GLI2 target, was shown previously to induce an EMT in rat kidney epithelial cells, via induction of the E-cadherin repressor SNAIL (34). In melanoma, we found that high GLI2 expression was associated with increased cell invasiveness in vitro and capacity to form bone metastases in mice (30). Within human melanoma primary tumors, GLI2 expression was heterogeneous: tumor areas expressing high levels of GLI2 exhibited little E-cadherin expression and were often at the invasive tumor front, while regions with low GLI2 expression showed strong pericellular E-cadherin staining. We also found that GLI2 expression increased with disease progression. A direct link between GLI2 expression and aggressiveness of melanoma cells was established, as GLI2 knockdown in highly invasive melanoma cells, i.e. strongly expressing GLI2, dramatically reduced their capacity to invade Matrigel and to form bone metastases in nude mice, thus providing direct evidence for the relevance of GLI2 targeting to treat melanoma skeletal metastases. Retrospectively, we found that both SMAD7 overexpression and pharmacologic inhibition of Tβ RI activity, which are both efficient in reducing melanoma bone metastases (see below and (9, 15)), reduced both basal and TGF-β-induced GLI2 expression in melanoma cells, an event that likely contributes to its anti-metastatic activity.
TGF-β and bone metastasis
The link between TGF-β signaling and the osteoclastogenic factor PTHrP has long been established and implicates both SMAD- and non-SMAD-dependent mechanisms (reviewed in (7)). Targeted inhibition of the TGF-β pathway is effective to inhibit breast cancer bone metastasis in nude mice, partly due to reduced PTHrP expression (12). Recently, Johnson et al. confirmed our initial identification of GLI2 as a TGF-β-regulated gene in the MDA-MB-231 breast carcinoma cell line (21), and determined that stable overexpression of a repressor form of GLI2 in these cells inhibited formation of osteolytic bone metastases in mice, as well as PTHrP expression (35). Additional TGF-β-dependent events are likely critical for MDA-MB-231 metastasis to bone as, in a thorough analysis of genes overexpressed in osteolytic bone metastases generated by MDA-MB-231 cells in nude mice, IL11 and CTGF were identified as critical TGF-β-regulated genes that cooperate with OPN and CXCR4 to act as metastasis-enhancing genes (16). In that setting, PTHrP itself was not expressed in the highly metastatic cell populations. In our own studies of melanoma metastasis to bone, we identified PTHrP, IL-11, CXCR4 and OPN as genes regulated by TGF-β whose expression was greatly reduced by both SMAD7 overexpression (15) and by systemic administration of the TβRI inhibitor SD-208 (9), two approaches that severely reduced melanoma bone metastasis formation and/or diminished established metastases. These reports highlight a remarkable similarity between the mechanisms involved in TGF-β-driven bone metastases from melanoma and breast cancer.
A Hh/TGF-β vicious cycle?
As shown above, TGF-β is a potent transcriptional regulator of GLI2, which results in GLI1 activation independently from the Hh signaling cascade (21). In addition to facilitating direct GLI2-dependent oncogenic events, it is also plausible that TGF-β may prime or potentiate Hh responsiveness by elevating the available pool of GLI2, a critical substrate necessary for Hh response (Figure 1).
Functional crosstalks between the TGF-β and Hh pathways have been identified, whereby Hh signaling leads to the expression of TGF-β family members that may be necessary for SMO-dependent tumorigenesis. For example, it has been shown that Shh promotes motility and invasiveness of gastric cancer cells through TGF-β-mediated activation of the ALK5–Smad3 pathway (36). Similarly, in a mouse model of SMO-mediated BCC development, activation of the TGF-β signaling pathway was observed and appeared to be critical for SMO-mediated cancer development possibly via immunosuppressive mechanisms (37). Inversely, transcriptional upregulation of Shh was recently shown to contribute to TGF-β-induced EMT in non-small cell lung cancer cells (38).
Taken together, these reports indicate that TGF-β and Hh signaling may form a vicious cycle promoting and amplifying the metastatic process whereby GLI2, and its downstream target GLI1, play a major role in allowing promoting tumor cell invasion and resistance to apoptosis.
While a number of reports indicates the potential therapeutic benefit of targeting either the TGF-β/SMAD or Shh/GLI signaling pathways to counter the neoplastic process, consistent with their respective pro-oncogenic capacities, major drawbacks include widespread toxicity due to blockade of numerous vital functions associated with growth factor signaling together with possible off-target effects. Suppression of GLI2 expression or suppression of GLI2 function independently from its upstream activators may therefore represent a valuable therapeutic option for the treatment of several cancers (39), as it also circumvents the difficult identification of the relevant upstream signals leading to its expression.
In conclusion, the critical role played by GLI2 downstream of TGF-β signaling in driving cancer progression towards metastasis in a Hedgehog-independent manner has been documented in distinct cancer types. GLI2 promotes a mesenchymal transition of tumor cells characterized by loss of E-cadherin expression, as well as secretion of soluble factors such as matrix metalloproteinases and osteoclastic molecules including PTHrP, all these events contributing to the acquisition of a more aggressive phenotype and metastasis.
Acknowledgments
Supported by the Donation Henriette et Emile Goutière, Institut National du Cancer (INCa, PLBIO-2008), INSERM, CNRS, Ligue Nationale Contre le Cancer (Equipe Labellisée LIGUE), Université Paris XI (to A.M.), Indiana Life Sciences Grant (to T.A.G.), Jerry and Peggy Throgmartin Endowment of Indiana University Simon Cancer Center (to T.A.G.) and by funds from NIH RO1 CA101891 (to K.L.), NIH R01 CA69158 and Susan Komen Foundation (to T.A.G.). V.I.A. was the recipient of a Cancéropôle Ile-de-France/Région Ile-de-France post-doctoral fellowship. Due to limitation of the number of references to be incorporated in the manuscript, it was impossible to provide complete coverage of the relevant literature. We apologize to those whose pertinent contributions have not been cited.
References
- 1.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–81. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 3.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–50. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 4.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javelaud D, Alexaki VI, Mauviel A. Transforming growth factor-beta in cutaneous melanoma. Pigment Cell Melanoma Res. 2008;21:123–32. doi: 10.1111/j.1755-148X.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Meulmeester E, Ten Dijke P. The dynamic roles of TGF-beta in cancer. J Pathol. 2011;223:205–18. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 7.Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2011;37 (Suppl 2):S2–14. doi: 10.1053/j.seminoncol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–61. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 9.Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, et al. TGF-{beta}-RI Kinase Inhibitor SD-208 Reduces the Development and Progression of Melanoma Bone Metastases. Cancer Res. 2011;71:175–84. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rausch MP, Hahn T, Ramanathapuram L, Bradley-Dunlop D, Mahadevan D, Mercado-Pimentel ME, et al. An orally active small molecule TGF-beta receptor I antagonist inhibits the growth of metastatic murine breast cancer. Anticancer Res. 2009;29:2099–109. [PMC free article] [PubMed] [Google Scholar]
- 12.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azuma H, Ehata S, Miyazaki H, Watabe T, Maruyama O, Imamura T, et al. Effect of Smad7 expression on metastasis of mouse mammary carcinoma JygMC(A) cells. J Natl Cancer Inst. 2005;97:1734–46. doi: 10.1093/jnci/dji399. [DOI] [PubMed] [Google Scholar]
- 14.Javelaud D, Delmas V, Moller M, Sextius P, Andre J, Menashi S, et al. Stable overexpression of Smad7 in human melanoma cells inhibits their tumorigenicity in vitro and in vivo. Oncogene. 2005;24:7624–9. doi: 10.1038/sj.onc.1208900. [DOI] [PubMed] [Google Scholar]
- 15.Javelaud D, Mohammad KS, McKenna CR, Fournier P, Luciani F, Niewolna M, et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res. 2007;67:2317–24. doi: 10.1158/0008-5472.CAN-06-3950. [DOI] [PubMed] [Google Scholar]
- 16.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 17.Korpal M, Kang Y. Targeting the transforming growth factor-beta signalling pathway in metastatic cancer. Eur J Cancer. 2010;46:1232–40. doi: 10.1016/j.ejca.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Lauth M, Toftgard R. Non-Canonical Activation of GLI Transcription Factors: Implications for Targeted Anti-Cancer Therapy. Cell Cycle. 2007:6. doi: 10.4161/cc.6.20.4808. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–34. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Brewster R, Mullor JL, Ruiz i Altaba A. Gli2 functions in FGF signaling during antero-posterior patterning. Development. 2000;127:4395–405. doi: 10.1242/dev.127.20.4395. [DOI] [PubMed] [Google Scholar]
- 21.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 22.Dennler S, Andre J, Verrecchia F, Mauviel A. Cloning of the human GLI2 Promoter: transcriptional activation by transforming growth factor-beta via SMAD3/beta-catenin cooperation. J Biol Chem. 2009;284:31523–31. doi: 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, et al. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol. 2010;24:1251–66. doi: 10.1210/me.2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–7. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 27.Thiyagarajan S, Bhatia N, Reagan-Shaw S, Cozma D, Thomas-Tikhonenko A, Ahmad N, et al. Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res. 2007;67:10642–6. doi: 10.1158/0008-5472.CAN-07-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narita S, So A, Ettinger S, Hayashi N, Muramaki M, Fazli L, et al. GLI2 knockdown using an antisense oligonucleotide induces apoptosis and chemosensitizes cells to paclitaxel in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:5769–77. doi: 10.1158/1078-0432.CCR-07-4282. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, Marcusson EG. Selective down-regulation of glioma-associated oncogene 2 inhibits the proliferation of hepatocellular carcinoma cells. Cancer Res. 2007;67:3583–93. doi: 10.1158/0008-5472.CAN-06-3040. [DOI] [PubMed] [Google Scholar]
- 30.Alexaki VI, Javelaud D, Van Kempen LC, Mohammad KS, Dennler S, Luciani F, et al. GLI2-Mediated Melanoma Invasion and Metastasis. J Natl Cancer Inst. 2010;102:1148–59. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazumdar T, DeVecchio J, Shi T, Jones J, Agyeman A, Houghton JA. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res. 2011;71:1092–102. doi: 10.1158/0008-5472.CAN-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Gruss C, Herlyn M. Role of cadherins and matrixins in melanoma. Curr Opin Oncol. 2001;13:117–23. doi: 10.1097/00001622-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, et al. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene. 2006;25:609–21. doi: 10.1038/sj.onc.1209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, Oyajobi BO, et al. TGF-{beta} promotion of Gli2 induced PTHrP expression is independent of canonical Hedgehog signaling. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–90. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 37.Fan Q, He M, Sheng T, Zhang X, Sinha M, Luxon B, et al. Requirement of TGFbeta signaling for SMO-mediated carcinogenesis. J Biol Chem. 2010;285:36570–6. doi: 10.1074/jbc.C110.164442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maitah MY, Ali S, Ahmad A, Gadgeel S, Sarkar FH. Up-regulation of sonic hedgehog contributes to TGF-beta1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One. 2011;6:e16068. doi: 10.1371/journal.pone.0016068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]