Abstract
Cholangiocarcinoma (CCA) is a tumor with poor prognosis that is resistant to all currently available treatments. Whether curcumin, a nutraceutical derived from turmeric (Curcuma longa), has potential therapeutic activity against human CCA was investigated using three CCA cell lines (KKU100, KKU-M156 and KKU-M213). Examination of mitochondrial dehydrogenase activity, phosphatidylserine externalization, esterase staining, caspase activation and poly-adenosine diphosphate ribose polymerase cleavage demonstrated that curcumin inhibited proliferation of and induced apoptosis in these biliary cancer cells. Colony-formation assay confirmed the growth-inhibitory effect of curcumin on CCA cells. When examined for the mechanism, curcumin was found to activate multiple cell signaling pathways in these cells. First, all CCA cells exhibited constitutively active nuclear factor (NF)-κB, and treatment with curcumin abolished this activation as indicated by DNA binding, nuclear translocation and p65 phosphorylation. Second, curcumin suppressed activation of signal transducer and activator of transcription-3 as indicated by decreased phosphorylation at both tyrosine705 and serine727 and inhibition of janus kinase-1 phosphorylation. Third, curcumin induced expression of peroxisome proliferator-activated receptor gamma. Fourth, curcumin upregulated death receptors, DR4 and DR5. Fifth, curcumin suppressed the Akt activation pathway. Sixth, curcumin inhibited expression of cell survival proteins such as B-cell lymphoma-2, B-cell leukemia protein xL, X-linked inhibitor of apoptosis protein, c-FLIP, cellular inhibitor of apoptosis protein (cIAP)-1, cIAP-2 and survivin and proteins linked to cell proliferation, such as cyclin D1 and c-Myc. Seventh, the growth inhibitory effect of curcumin was enhanced in the IκB kinase-deficient cells, the enzyme required for nuclear factor-kappaB activation. Overall, our results indicate that curcumin mediates its antiproliferative and apoptotic effects through activation of multiple cell signaling pathways, and thus, its activity against CCA should be further investigated.
Introduction
Cholangiocarcinoma (CCA) is a highly malignant, generally fatal adenocarcinoma arising from the bile duct epithelial cells (cholangiocytes) of the intrahepatic or extrahepatic biliary system. It is one of the most highly metastatic cancers, characterized by poor prognosis and therapeutic inefficiency, and is an increasing health problem worldwide (1). The incidence of CCA varies across the world depending on prevalence of risk factors such as primary sclerosing cholangitis, Clonorchis sinensis and Opisthorchis viverrini infections, choledochal cysts and hepatolithiasis (2). The highest prevalence of O.viverrini infestations has been reported in northeastern Thailand, where CCA incidence is also highest (3). The therapeutic options for this hepatobiliary malignancy are limited because very few cases are diagnosed at an early stage. Currently, optimal treatment is surgical resection, but 90% of patients are not candidates because of widespread metastases and inadequate liver function (4). Moreover, recurrence after surgery is common and problematic. The available chemotherapeutic agents and radiation therapy are ineffective (1). Therefore, new therapeutic strategies directed against this malignancy are needed.
Chronic inflammation has been shown as a link between CCA and its risk factors (1). Therefore, targeting inflammatory pathways would offer a potential therapeutic strategy against this malignancy. Nuclear factor-kappaB (NF-κB) is a proinflammatory transcription factor that acts as the key mediator of carcinogenesis (5,6). The NF-κB signal transduction pathway is dysregulated and is constitutively active in a variety of human cancers (7–10). One of the key kinases involved in NF-κB activation pathway is IκB kinase (IKK). Activated NF-κB has been reported to protect cancer cells from apoptotic cell death (11) and is implicated in the expression of genes involved in inflammation (cyclooxygenase-2 and inducible nitric oxide synthase), proliferation (c-Myc and cyclin D1), metastasis (matrix metalloproteinase-9) and adhesion (intercellular adhesion molecule-1) of tumor cells (12).
Signal transducer and activator of transcription-3 (STAT-3) is another proinflammatory transcription factor that has been reported to regulate the expression of genes involved in survival (13), proliferation (14), invasion (15) and angiogenesis (16) of tumor cells. The activation of STAT-3 is regulated by phosphorylation at tyrosine and serine residues. Although phosphorylation at Tyr705 leads to STAT-3 dimerization, nuclear translocation, DNA binding and gene transcription, phosphorylation at Ser727 may regulate STAT-3 activity both negatively and positively. Peroxisome proliferator-activated receptor gamma (PPAR-γ) is a member of the nuclear receptor superfamily (17) and has been reported to play a role in lipid and glucose metabolism. Activation of PPAR-γ has been identified as an approach for inducing differentiation and inhibiting proliferation in a variety of cancers (18,19).
Natural products have played a significant role over the years in the development of anticancer drugs as>60% of the drugs are of natural origin (20,21). Natural products with the potential to inhibit NF-κB and STAT-3 activation and enhance PPAR-γ expression would have therapeutic potential against CCA. Curcumin (diferuloylmethane) is one such agent. Derived from the rhizomes of turmeric (Curcuma longa), curcumin has been shown to suppress activation of NF-κB and STAT-3 (22). It has also been shown to downregulate the expression of genes such as B-cell lymphoma (Bcl)-2, cyclooxygenase-2, matrix metalloproteinase-9, cyclin D1 and the adhesion molecules (23). Although numerous studies have demonstrated the chemopreventive potential of curcumin against a wide variety of tumors, studies concerning CCA are limited.
In the present study, we hypothesized that curcumin would suppress the growth of CCA through modulation of multiple cell signaling pathways. We present evidence that curcumin inhibits the growth of and induces apoptosis in CCA cell lines through (i) inhibition of the NF-κB and STAT-3 signaling pathway, (ii) upregulation of PPAR-γ expression, (iii) upregulation of death receptors and (iv) downregulation of cell survival proteins.
Materials and methods
Materials
Curcumin (Figure 1A, >98% pure) was supplied by Sabinsa (Piscataway, NJ). Penicillin, streptomycin, RPMI 1640 and fetal bovine serum were obtained from Invitrogen (Carlsbad, CA). Tris, glycine, NaCl, sodium dodecyl sulfate and bovine serum albumin were obtained from Sigma–Aldrich (St Louis, MO). Antibodies against p50, p65, cyclin D1, STAT-3, survivin, cellular inhibitor of apoptosis protein (cIAP)-1 and -2, c-Myc, Bcl-2, B-cell leukemia protein xL (Bcl-xL), phospho-specific STAT-3 (Tyr705 and Ser727), caspase-3, -8, and -9, poly-adenosine diphosphate ribose polymerase (PARP) and p-Akt were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The p-p65 (Ser536) antibody was purchased from Cell Signaling Technology (Beverly, MA).
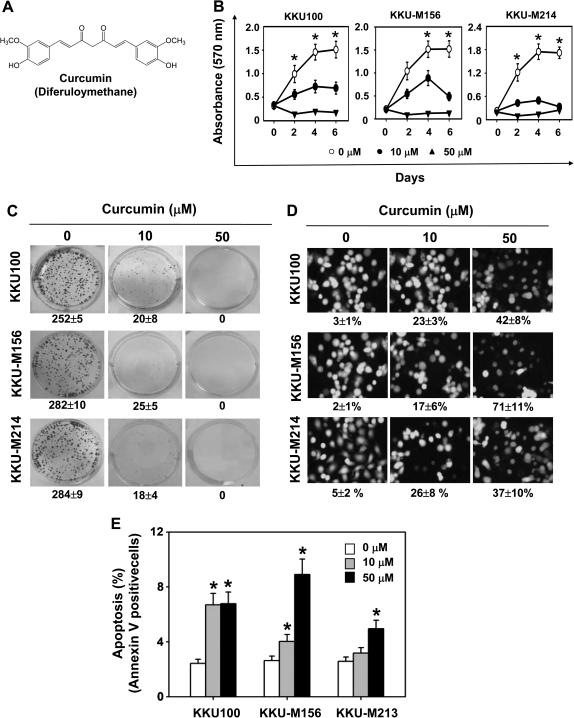
Fig. 1.
Curcumin induces apoptosis and inhibits proliferation of CCA cells. (A) The chemical structure of curcumin. (B) Cells were treated with indicated concentrations of curcumin, and cell viability was determined by measuring mitochondrial dehydrogenase activity on days 0, 2, 4 and 6. (C) Curcumin suppressed long-term colony formation in CCA cells. Cells treated with indicated concentrations of curcumin were allowed to form colonies for 6 days. The values given are the means ± standard errors of the mean of three replicates. One of three independent experiments is shown. (D) Curcumin enhanced apoptosis in CCA cells as determined by the Live/Dead assay reagent. Cells were treated with the indicated concentrations of curcumin for 24 h, stained with a Live/Dead assay reagent for 30 min and then analyzed under a fluorescence microscope. Values below each photomicrograph represent percentage of apoptotic cells. (E) Curcumin induced early apoptosis in CCA cells, as determined by the annexin V assay. Cells were treated with indicated concentrations of curcumin for 24 h. The cells were then incubated with a fluorescein isothiocyanate-conjugated annexin V antibody, and early apoptotic cells were analyzed by flow cytometry. Asterisk indicates significance of the difference compared with control; P < 0.05.
Cell lines
Three human CCA cell lines representing different stages of adenocarcinoma were used in this study: poorly differentiated (KKU100), moderately differentiated (KKU-M156) and well-differentiated (KKU-M214) adenocarcinoma. The cell lines were established and characterized from CCA patients hospitalized at the Faculty of Medicine, Khon Kaen University and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. IKK-α and IKK-β knockout cells and their wild-type parental cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin.
Measurement of cell proliferation by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide method
The effect of curcumin on the proliferation of CCA cells was determined by measuring mitochondrial dehydrogenase activity, using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) as the substrate (24). Briefly, 3000 cells were incubated with 0, 10 or 50 μM curcumin for 2, 4 or 6 days in 96-well plates. After termination of treatment, MTT solution was added to each well and the cells were incubated for 2 h at 37°C. An extraction buffer comprising 20% sodium dodecyl sulfate and 50% dimethyl formamide was added, and the cells were incubated overnight at 37°C to dissolve the formazan formed during the reaction. Absorbance of the colored product was then measured at 570 nm using a 96-well multiscanner (MRX Revelation; Dynex Technologies, Chantilly, VA).
Clonogenic assay
The clonogenic assay tests every cell in a given population for its ability to undergo ‘unlimited’ division and form colonies. The CCA cells were treated with 0, 10 or 50 μM curcumin. After 24 h, cells were transferred to the normal medium and allowed to form colonies. After 6 days, colonies were fixed in a solution of methanol and acetic acid (3:1), stained with 0.5% crystal violet and counted manually.
Measurement of apoptosis by Live/Dead assay
To measure apoptosis, we used the Live/Dead assay, which determines intracellular esterase activity and plasma membrane integrity. It is a two-color fluorescence assay that simultaneously determines numbers of live cells and dead cells. Intracellular esterases from live cells convert non-fluorescent cell-permeable calcein acetoxymethyl ester to the intensely fluorescent calcein, which is retained within cells. This assay also highlights dead cells, which have damaged membranes; the ethidium homodimer-1 enters damaged cells, is fluorescent when bound to nucleic acids, and produces a bright red fluorescence. The assay was performed as described elsewhere (25).
Measurement of apoptosis by phosphatidylserine externalization assay
The annexin V assay provides a simple and effective method for detecting apoptosis at a very early stage. This assay takes advantage of the facts that phosphatidylserine (PS) is translocated from the inner (cytoplasmic) leaflet of the plasma membrane to the outer surface soon after induction of apoptosis, and that the annexin V protein has a strong specific affinity for PS. We measured the loss of membrane asymmetry that occurs when PS moves to the extracellular surface of the membrane by using an annexin V staining kit (Santa Cruz Biotechnology). The assay was performed by following the manufacturer’s instructions.
Electrophoretic mobility shift assay
To determine NF-κB activation, nuclear extracts were prepared from control and treated cells and subjected to electrophoretic mobility shift assay (EMSA) as described previously (24). For supershift assays, nuclear extracts prepared from KKU-M156 cells were incubated with antibodies against p50 and p65 of NF-κB, either alone or in combination, for 30 min at 37°C. The complex was then analyzed by EMSA. Preimmune serum was included as the negative control. The specificity of binding was also examined by competition with the unlabeled oligonucleotide. The dried gels were visualized, and the radioactive bands were quantitated with Storm 820 and ImageQuant software (GE Healthcare, Piscataway, NJ).
Immunocytochemical analysis for NF-κB p65 localization
We performed an immunocytochemical analysis to determine the effect of curcumin on p65 nuclear translocation. Control and curcumin-treated KKU-M156 cells were plated on glass slides by centrifugation (Cytospin 4; Thermoshendon, Pittsburgh, PA), air dried for 1 h at room temperature and fixed with paraformaldehyde. After a brief washing with phosphate-buffered saline, cells were blocked with 5% normal goat serum for 1 h and then incubated with rabbit polyclonal p65 antibody. After overnight incubation, cells were washed and then incubated with goat anti-rabbit IgG–Alexa 594 for 1 h and counterstained for nuclei with Hoechst for 5 min. Stained cells were mounted with mounting medium (Sigma–Aldrich) and analyzed under a fluorescence microscope (Labophot 2; Nikon, Tokyo, Japan).
Western blot analysis
To determine the effect of curcumin on caspase activation, janus kinase-1 (JAK-1) activation, AKT8 in rodent T-cell lymphoma (Akt) activation, PARP cleavage, p65 phosphorylation, STAT-3 phosphorylation and expression of tumor necrosis factor receptor-activated factor (TRAF)-1 and -2, PPAR-γ, death receptors 4 and 5 (DR4, DR5), antiapoptotic proteins and proliferative proteins, whole cell extracts were prepared and western blot analysis was carried out as described previously (26). Briefly, cellular extracts containing equal amounts of proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were probed with relevant primary antibodies followed by horseradish peroxidase-conjugated secondary antibody, and signals were detected by enhanced chemiluminescence reagent (GE Healthcare).
Statistical analysis
Various parameters were monitored in normal and treated cells. Experiments were repeated a minimum of three times. Data are given as the mean ± standard error of the mean. The statistical analyses used the two-tailed unpaired Student’s t-test. The percentages of positively immunostained cells were compared by using the Mann–Whitney U-test. SPSS version 11.5 software was used for statistical analysis of data. A value of P < 0.05 was considered statistically significant.
Results
Our goal in this study was to determine whether curcumin modulates the growth of CCA cell lines and, if so, to delineate various mechanisms by which it may mediate its effects. We examined the effects of curcumin on NF-κB activation, STAT-3 activation, NF-κB- and STAT-3-regulated gene products and cell growth in CCA cells. Because moderately differentiated adenocarcinoma is the most common CCA, we selected KKU-M156 cells for most of the studies.
Curcumin suppresses proliferation of CCA cells
Whether curcumin has the potential to inhibit proliferation of CCA cells was investigated by measuring mitochondrial dehydrogenase activity. Curcumin inhibited proliferation of CCA cells in a dose-dependent manner. The suppression of cell proliferation was significant at a curcumin concentration of 50 μM (P < 0.05, Figure 1B). These results indicate that curcumin has potent antiproliferative effects in CCA cells.
Curcumin inhibits colony-forming ability of CCA cells
Since colony formation of tumor cells is closer to its physiology and growth in vivo, we investigated the effect of curcumin on suppression of long-term colony formation. Exposure of CCA cells to curcumin was associated with significant repression of colony-forming ability (Figure 1C). At 10 μM of curcumin, the number of colonies was significantly reduced (P < 0.05) from 252 to 20 in KKU100 cells, from 282 to 25 in KKU-M156 cells and from 284 to 18 in KKU-M214 cells. Colony formation was completely suppressed at 50 μM of curcumin.
Curcumin induces apoptosis in CCA cells
We investigated by several assays whether curcumin could induce apoptosis in CCA cells. Measurement of intracellular esterase activity showed that curcumin-induced apoptosis in CCA cells in a dose-dependent manner. Exposure of CCA cells to 10 and 50 μM curcumin significantly increased the number of apoptotic cells from 23 to 42% in KKU100 cells, from 17 to 71% in KKU-M156 cells and from 26 to 37% in KKU-M214 cells (P < 0.05, Figure 1D).
We also investigated early apoptosis by PS externalization assay. As shown in Figure 1E, significant increases in annexin V-positive cells were observed in cells treated with curcumin as compared with controls (P < 0.05). Comparison of cells treated with curcumin and controls indicated that annexin V-positive cells increased from 2 to 7% in KKU100 cells, from 2 to 10% in KKU-M156 cells and from 2 to 5% in KKU-M213 cells at 50 μM of curcumin (Figure 1E).
Overall, these results indicate that curcumin induces apoptosis in CCA cells and that KKU-M156 cells are more sensitive to curcumin-induced apoptosis than the other CCA cells tested. Therefore, we selected KKU-M156 cell for further studies.
Curcumin induces caspase activation and PARP cleavage in CCA cells
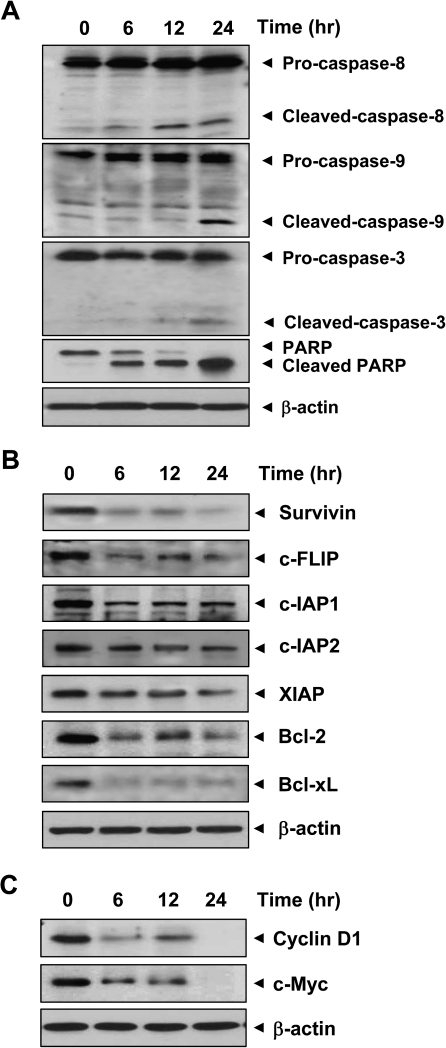
Activation of caspases is a hallmark of apoptosis. While caspase-8 activation constitutes the extrinsic pathway, the intrinsic pathway requires caspase-9 activation. Activated caspase-8 and -9 activate caspase-3, which in turn induces PARP cleavage. As shown in Figure 2A, curcumin induced activation of caspase-3, -8 and -9 and PARP cleavage in CCA cells. These results indicate that curcumin induces apoptosis in CCA cells by both the extrinsic and intrinsic pathways.
Fig. 2.
Curcumin induces caspase activation and PARP cleavage and downregulates expression of antiapoptotic and proliferative gene products in KKU-M156 cells. Cells were treated with 50 μM curcumin for the indicated times, and whole cell extracts were prepared and analyzed using indicated antibodies. β-Actin was used as an internal control.
Curcumin downregulates expression of cell survival proteins
One of the key mechanisms through which tumor cells show resistance to apoptosis is overexpression of cell survival proteins. Therefore, we investigated the effect of curcumin on expression of cell survival proteins such as survivin, cellular FLICE-like inhibitory protein (c-FLIP), cIAP-1, cIAP-2, X-linked inhibitor of apoptosis protein, Bcl-2 and Bcl-xL. Results indicate that curcumin inhibits expression of these proteins in CCA cells in a time-dependent manner (Figure 2B).
Curcumin suppresses expression of proteins associated with proliferation in CCA cells
We examined whether suppression of proliferation of CCA cells by curcumin is due to downregulation of proteins involved in cell proliferation, such as cyclin D1 and c-Myc. We found that curcumin inhibited expression of cyclin D1 and c-Myc in CCA cells in a time-dependent manner (Figure 2C).
Curcumin inhibits constitutive NF-κB activation in CCA cells
Since NF-κB is involved in regulation of proteins involved in cell survival and proliferation, we investigated whether CCA cells express constitutive NF-κB activation. As shown in Figure 3A (left), all three CCA cell lines exhibited significant NF-κB activation. Curcumin inhibited constitutive NF-κB in KKU-M156 cells in a dose-dependent manner (Figure 3A, middle).
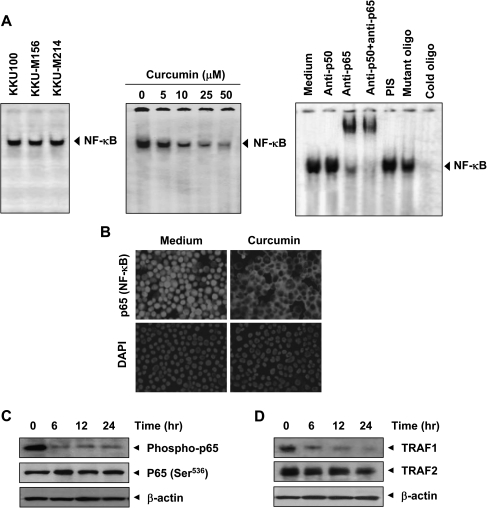
Fig. 3.
Curcumin inhibits constitutive NF-κB activation in CCA cells. (A, left) Nuclear extracts prepared from KKU100, KKU-M156 and KKU-M214 cells were analyzed for NF-κB activation by EMSA. (A, middle) Effect of curcumin on constitutive NF-κB activation. KKU-M156 cells were incubated with curcumin at the indicated concentrations for 4 h. Nuclear extracts were prepared and analyzed for NF-κB activation by EMSA. (A, right) The binding of NF-κB to the DNA is specific and consists of p50 and p65 subunits. Nuclear extracts were prepared from KKU-M156 cells, incubated for 30 min with indicated antibodies, preimmune serum and mutant or unlabeled NF-κB oligonucleotide probe and then assayed for NF-κB by EMSA. (B) Curcumin induced redistribution of p65. KKU-M156 cells were incubated without or with curcumin (50 μM) for 4 h and then analyzed for the distribution of p65 by immunocytochemistry. Red stain indicates p65, and blue stain indicates nucleus. (C, D) Curcumin inhibited phosphorylation of p65 and expression of TRAF1 and TRAF2. KKU-M156 cells were treated with 50 μM curcumin for the indicated times, and whole cell extracts were prepared and analyzed by western blotting using antibodies against phospho-p65 (Ser536), TRAF1 and TRAF2. β-Actin was used as an internal control. Figures are representative of three independent experiments.
To examine whether the NF-κB visualized by EMSA in KKU-M156 cells was indeed NF-κB, we incubated the nuclear extracts from these cells with antibodies against p50 or p65 subunits and performed the EMSA. Antibody against the p65 subunit of NF-κB shifted the band to one of higher molecular weights. Preimmune serum had no effect on DNA binding, and addition of excess unlabeled NF-κB (cold oligonucleotide; 100-fold excess) completely abolished the intensity of the band, whereas addition of a mutated oligonucleotide had no effect on DNA binding (Figure 3A, right). These results indicate that the band visualized by EMSA was indeed NF-κB.
Curcumin inhibits nuclear retention and phosphorylation of p65
When NF-κB is activated, the p65 subunit is phosphorylated and translocated to the nucleus. Immunocytochemical analysis indicated a constitutive presence of p65 in the nucleus of KKU-M156 cells (Figure 3B). When these cells were treated with curcumin, p65 was redistributed from nucleus to cytoplasm. Curcumin treatment also inhibited p65 phosphorylation at Ser536 in KKU-M156 cells (Figure 3C).
Curcumin inhibits TRAF1 and TRAF2 expression in KKU-M156 cells
Activation of NF-κB requires sequential recruitment of adapter proteins including TRAF1 and TRAF2. As shown in Figure 3D, exposure of KKU-M156 cells to curcumin was associated with a time-dependent decrease in TRAF1 expression. The effect of curcumin on TRAF2 was less prominent than that on TRAF1.
Curcumin inhibits constitutive STAT-3 activation in CCA cells
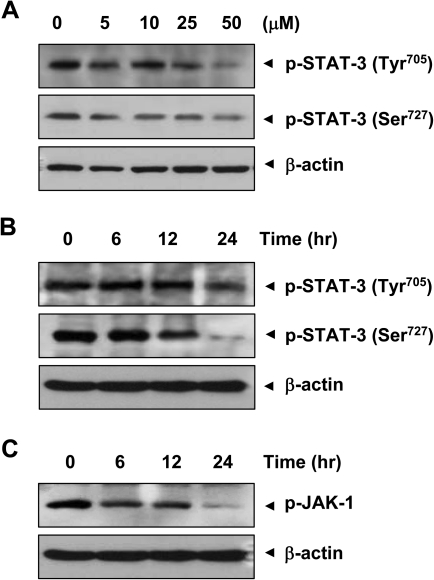
To investigate whether curcumin can modulate constitutive STAT-3 phosphorylation in CCA cells, KKU-M156 cells were incubated with different concentrations of curcumin for 24 h. Whole cell extracts were prepared and examined for STAT-3 phosphorylation by western blotting using an antibody that recognizes phosphorylation at Tyr705 and Ser727. As shown in Figure 4A, curcumin inhibited the constitutive phosphorylation of STAT-3, with maximum inhibition occurring at 50 μM. We also determined optimum time required for curcumin to suppress STAT-3 activation in KKU-M156 cells. The inhibition was time dependent, and maximum inhibition occurred at 24 h (Figure 4B).
Fig. 4.
Curcumin inhibits STAT-3 and JAK-1 phosphorylation in KKU-M156 cells. (A) Cells (1 × 106) were treated with indicated concentrations of curcumin for 6 h, whole cell extracts were prepared and analyzed for STAT-3 phosphorylation using p-STAT-3 (Tyr705, Ser 727) antibody. (B and C) KKU-M156 cells were treated with 50 μM curcumin for the indicated times, and whole cell extracts were prepared and analyzed for STAT-3 phosphorylation (at Tyr705 and Ser727) and JAK-1 phosphorylation. Data shown are representative of three independent experiments.
Since STAT-3 phosphorylation requires JAK-1 activation, we investigated the effect of curcumin on expression of p-JAK-1 in KKU-M156 cells. As is evident from Figure 4C, curcumin inhibited JAK-1 activation at 50 μM in a time-dependent manner.
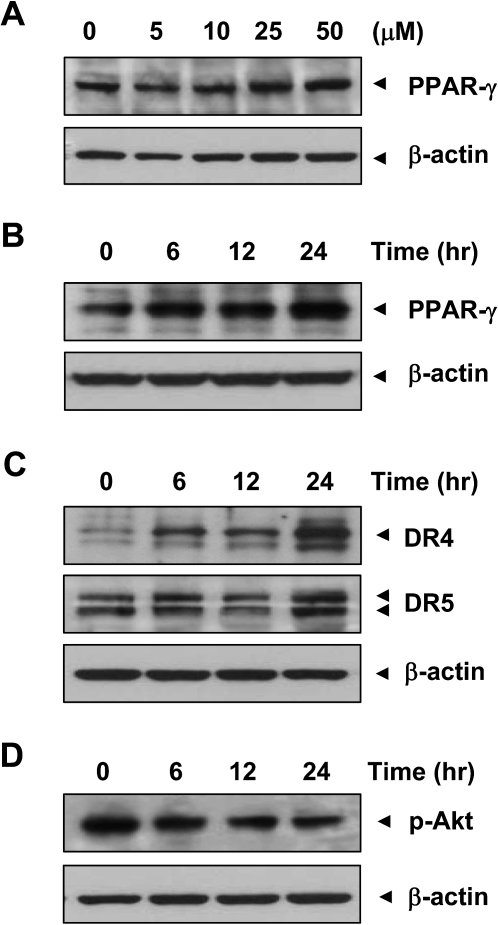
Curcumin enhances PPAR-γ expression in KKU-M156 cells
Since PPAR-γ agonists are known to exhibit growth-inhibitory effects in tumor cells, including CCA cells (18,19), we investigated the effect of curcumin on PPAR-γ expression in CCA cells. Curcumin enhanced PPAR-γ expression in KKU-M156 cells in a dose- and time-dependent manner (Figure 5A and B).
Fig. 5.
Curcumin upregulates PPAR-γ and death receptors and downregulates p-Akt in KKU-M156 cells. (A) Cells were treated with indicated concentrations of curcumin for 6 h or (B–D) with 50 μM curcumin for indicated times. Whole cell extracts were prepared and analyzed using indicated antibodies. Data shown are representative of two independent experiments.
Curcumin induces expression of death receptors in KKU-M156 cells
The extrinsic pathway of apoptosis requires induction of death receptors. Therefore, we investigated the effect of curcumin on expression of death receptors, DR4 and DR5. Curcumin enhanced DR4 and DR5 expression in KKU-M156 cells in a time-dependent manner (Figure 5C).
Curcumin inhibits Akt activation in KKU-M156 cells
Since activation of NF-κB requires kinases including Akt, we investigated the effect of curcumin on Akt activation. Administration of curcumin was associated with an inhibition of Akt activation in KKU-M156 cells in a time-dependent manner (Figure 5D).
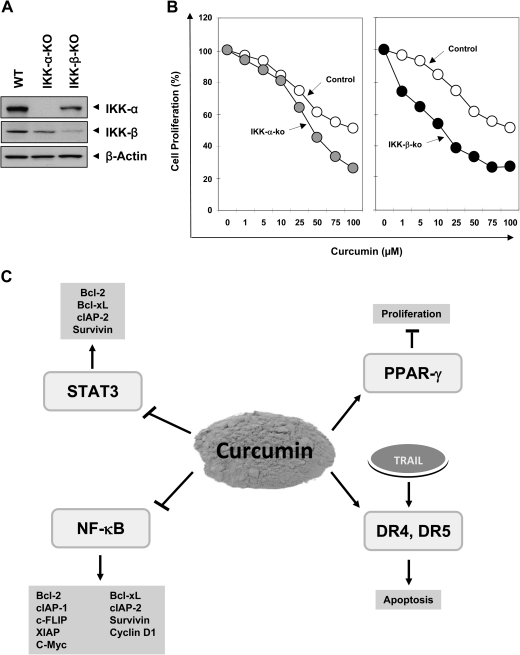
Growth inhibitory effect of curcumin is enhanced in IKK-α- and IKK-β-deficient cell lines
Because IKK is required for NF-κB activation, we examined whether ablation of IKK-α and IKK-β, two subunits of IKK will increase the sensitivity of cells to curcumin. The wild-type and deficient cells were treated with different concentrations of curcumin for 3 days and cell growth was examined by measuring mitochondrial dehydrogenase activity. We confirmed the absence of IKK-α and IKK-β in deficient cells by western blot analysis (Figure 6A). As shown in Figure 6B, curcumin inhibited growth of IKK-deficient cells to a greater extent as compared with wild-type cells. However, IKK-β-deficient cells were more sensitive as compared with IKK-α-deficient cells. Overall, these results indicated that NF-κB activation is required for tumor cell proliferation.
Fig. 6.
IKK-deficient cells are more sensitive to growth inhibition by curcumin: (A) The whole cell extract prepared from wild-type and knockout cells were analyzed by western blotting using indicated antibodies. β-Actin was used as an internal control. (B) Wild-type and deficient cells were treated with indicated concentrations of curcumin for 3 days. Cell growth was examined by measuring mitochondrial dehydrogenase activity as described in Material and Methods. (C) A schematic diagram showing curcumin’s mechanism of action in CCA cells.
Discussion
Although the worldwide incidence of and mortality due to CCA are increasing, available treatments have not substantially prolonged patient survival. Therefore, there is a need to develop novel therapeutic agents that can increase the susceptibility of CCA cells. Here, we provide evidence that curcumin may have chemopreventive potential against cholangiocarcinogenesis by affecting multiple cell signaling molecules. We found that this nutraceutical suppressed activation of proinflammatory transcription factors such as NF-κB and STAT-3 and the gene products involved in tumor cell survival and proliferation. Curcumin also inhibited proliferation of CCA cells and upregulated expression of PPAR-γ and death receptors (Figure 6).
We found that CCA cell lines tested exhibited constitutively active NF-κB, and curcumin suppressed this activation. Although curcumin has been shown to inhibit inducible and constitutive NF-κB activation in cell lines of various origins (27–29), this is the first report showing inhibition of constitutive NF-κB in CCA cells. Because activation of NF-κB leads to nuclear translocation of the p65 subunit, we confirmed the presence of nuclear p65 in KKU-M156 cells by immunocytochemistry. How curcumin inhibits constitutive NF-κB is not clear from these findings. Curcumin has been shown to inhibit constitutive IKK, the enzyme required for constitutive NF-κB activation in multiple myeloma cells (27). Therefore, one possibility, that the inhibitory effect of curcumin on constitutive NF-κB is mediated through inhibition of IKK activation, cannot be ruled out by the present study. IKK has been reported to phosphorylate the p65 subunit of NF-κB at Ser536. Inhibition of p65 phosphorylation by curcumin further suggests that its inhibitory effect on NF-κB activation is mediated through IKK.
STAT-3 was also constitutively activated in KKU-M156, and curcumin inhibited this activation. This agrees with previous reports showing STAT-3 phosphorylation in CCA cell lines (30,31). Constitutive activation of STAT-3 has been reported in a large variety of other tumors, moreover, including breast cancer, prostate cancer, head and neck squamous cell carcinoma, lymphomas and leukemia, brain tumors, colon cancer, gastric cancer, esophageal cancer, ovarian cancer, nasopharyngeal cancer and pancreatic cancer (32). The implication of these reports is that targeting the STAT-3 pathway could be a potent therapeutic strategy against CCA. This is supported by our recent report showing the ability of curcumin to inhibit STAT-3 activation in a hamster model of cholangiocarcinogenesis (33).
Our data also showed that curcumin suppressed antiapoptotic gene products that are regulated by NF-κB and STAT-3. STAT-3 activation has been reported to suppress apoptosis. These effects are mediated through expression of cell survival proteins, including Bcl-xL (13), Bcl-2 (34), survivin (35) and cIAP-2 (36). Most tumor cells that exhibit constitutive activation of STAT-3 also express these cell survival gene products (37). NF-κB is known for its potential to regulate antiapoptotic gene products and is associated with resistance to apoptosis in tumor cells, including CCA cells (38–40). NF-κB has been reported to regulate expression of cell survival proteins such as c-FLIP, Bcl-xL (41), Bcl-2 (42), X-linked inhibitor of apoptosis protein (43), cIAP-1 (44), cIAP-2 (44) and survivin (45). Bcl-xL can block cell death induced by a variety of chemotherapeutic agents (46), and its overexpression has been reported in CCA cells (47). That curcumin treatment was associated with downregulation of expression of cell survival proteins could be due to the observed inhibition of activation of NF-κB and STAT-3. Curcumin’s downregulation of c-FLIP, a caspase-8 inhibitor, agrees with a recent report showing induction of apoptosis in CCA cells through downregulation of c-FLIP by tamoxifen (48). Activation of caspase-3, -8 and -9 suggests that curcumin induced apoptosis through both the extrinsic and intrinsic pathways. The extrinsic pathway of apoptosis involves induction of death receptors such as DR5 and DR4. That curcumin has the potential to induce apoptosis through the extrinsic pathway was further confirmed by its ability to induce DR5 and DR4 in CCA cells.
We also found that CCA cells overexpressed gene products involved in cell proliferation (cyclin D1 and c-Myc). The inhibition of cyclin D1 and c-Myc expression by curcumin could contribute to the suppression of CCA cell proliferation. Since these gene products are regulated by NF-κB (49) and STAT-3 (14), one possible cause of the suppression of CCA cells proliferation could be due to inhibition of these transcription factors. Finally, the prominent upregulation of PPAR-γ by curcumin in the CCA cells studied here may explain the antiproliferative effect of curcumin, since PPAR-γ has been shown to have a growth-inhibitory effect in tumor cells, including CCA cells (18,19).
In conclusion, our findings demonstrate that curcumin mediates its antiproliferative and apoptotic effects through (i) inhibition of NF-κB and STAT-3 signaling pathways, (ii) downregulation of antiapoptotic proteins, (iii) upregulation of PPAR-γ expression and (iv) upregulation of DR4 and DR5 expression (Figure 6C). That the IKK-α- and IKK-β-knockout cells were more sensitive to growth inhibition as compared with wild-type cells further signifies the role of NF-κB activation in tumor cell proliferation. Taken together, our results indicate that curcumin could be an effective molecule and NF-κB and STAT-3 as novel target against CCA. Further experiments in animal models are needed, however, to fully realize the potential of curcumin in this disease.
Funding
This work was supported by a grant from the Clayton Foundation for Research to B.B.A.; the MD Anderson Cancer Center Core Support Grant from the National Institutes of Health (CA016672); a program project grant from the National Institutes of Health (CA-124787-01A2); a grant from the Center for Targeted Therapy of MD Anderson Cancer Center.
Acknowledgments
The authors are thankful to Kathryn Hale from the Department of Scientific Publications of The University of Texas MD Anderson Cancer Center (Houston, TX) for carefully proofreading the manuscript. We thank the Office of the Higher Education Commission, the Higher Education Research Promotion and National Research University Project of Thailand, and Thailand Research Fund for financial support. Dr Aggarwal is the Ransom Horne, Jr, Professor of Cancer Research.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- Akt
AKT8 in rodent T-cell lymphoma
- Bcl
B-cell lymphoma
- Bcl-xL
B-cell leukemia protein xL
- CCA
cholangiocarcinoma
- c-FLIP
cellular FLICE-like inhibitory protein
- cIAP
cellular inhibitor of apoptosis protein
- DR
death receptor
- EMSA
electrophoretic mobility shift assay
- IKK
IκB kinase
- JAK-1
janus kinase-1
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- NF-κB
nuclear factor-kappaB
- PARP
poly-adenosine diphosphate ribose polymerase
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- PS
phosphatidylserine
- STAT-3
signal transducer and activator of transcription-3
- TRAF
tumor necrosis factor receptor-activated factor
References
- 1.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis KN, et al. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Vatanasapt V, et al. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- 4.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 5.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 6.Orlowski RZ, et al. NF-kappaB as a therapeutic target in cancer. Trends Mol. Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 7.Fabre C, et al. A novel effect of DNA methyltransferase and histone deacetylase inhibitors: NFkappaB inhibition in malignant myeloblasts. Cell Cycle. 2008;7:2139–2145. doi: 10.4161/cc.7.14.6268. [DOI] [PubMed] [Google Scholar]
- 8.Lu T, et al. Cytokine overexpression and constitutive NFkappaB in cancer. Cell Cycle. 2004;3:1114–1117. [PubMed] [Google Scholar]
- 9.Weisz L, et al. Mutant .p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007;67:2396–2401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, et al. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr. Cancer Drug Targets. 2007;7:305–316. doi: 10.2174/156800907780809741. [DOI] [PubMed] [Google Scholar]
- 11.Beg AA, et al. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Catlett-Falcone R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 14.Masuda M, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 15.Xie TX, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 16.Niu G, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 17.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobuke T, et al. A Ligand for peroxisome proliferator-activated receptor gamma inhibits human cholangiocarcinoma cell growth: potential molecular targeting strategy for cholangioma. Dig. Dis. Sci. 2006;51:1650–1657. doi: 10.1007/s10620-005-9064-2. [DOI] [PubMed] [Google Scholar]
- 19.Sarraf P, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat. Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SC, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman DJ, et al. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, et al. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, et al. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 24.Bharti AC, et al. Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB-independent and TRAF6-dependent mechanism. J. Biol. Chem. 2004;279:6065–6076. doi: 10.1074/jbc.M308062200. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SC, et al. Modification of cysteine-179 of IkappaBalpha kinase by Nimbolide leads to downregulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010;285:35406–35417. doi: 10.1074/jbc.M110.161984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi G, et al. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 27.Bharti AC, et al. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 28.Plummer SM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 29.Sandur SK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isomoto H, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–396. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniai M, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann. N. Y. Acad. Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 33.Prakobwong S, et al. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int. J. Cancer. 2010 doi: 10.1002/ijc.25656. in press, doi:20824699. [DOI] [PubMed] [Google Scholar]
- 34.Zushi S, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int. J. Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Mahboubi K, et al. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab. Invest. 2001;81:327–334. doi: 10.1038/labinvest.3780241. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharya S, et al. Regulation of Stat3 nuclear export. J. Clin. Invest. 2003;111:553–559. doi: 10.1172/JCI15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoki Y, et al. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal BB, et al. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 39.Pinlaor S, et al. iNOS-dependent DNA damage via NF-kappaB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. Int. J. Cancer. 2006;119:1067–1072. doi: 10.1002/ijc.21893. [DOI] [PubMed] [Google Scholar]
- 40.Seubwai W, et al. Cepharanthine exerts antitumor activity on cholangiocarcinoma by inhibiting NF-kappaB. Cancer Sci. 2010;101:1590–1595. doi: 10.1111/j.1349-7006.2010.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zong WX, et al. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamatani M, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J. Biol. Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 43.Stehlik C, et al. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CY, et al. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 45.Zhu L, et al. Anti-apoptotic protein survivin plays a significant role in tubular morphogenesis of human coronary arteriolar endothelial cells by hypoxic preconditioning. FEBS Lett. 2001;508:369–374. doi: 10.1016/s0014-5793(01)03084-8. [DOI] [PubMed] [Google Scholar]
- 46.Simonian PL, et al. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–1216. [PubMed] [Google Scholar]
- 47.Onori P, et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int. J. Cancer. 2009;125:565–576. doi: 10.1002/ijc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawar P, et al. Molecular mechanisms of tamoxifen therapy for cholangiocarcinoma: role of calmodulin. Clin. Cancer Res. 2009;15:1288–1296. doi: 10.1158/1078-0432.CCR-08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhopadhyay A, et al. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]