Abstract
We investigated the effects of caloric restriction (CR) on growth of tumors and metastases in the 4T1 mammary tumor model and found that CR, compared with normal diet, reduced the growth of mammary tumors and metastases and the total number of metastases that originated both spontaneously from the primary tumor and also experimentally from i.v. injection of the tumor cells. CR also decreased proliferation and angiogenesis and increased apoptosis in tumors. CR reduced levels of insulin, leptin, insulin-like growth factor 1, insulin-like growth factor binding protein 3 and increased adiponectin in tumors. We also demonstrated that tumors from CR mice possessed lower levels of transforming growth factor-β, lower intratumor deposition of collagen IV and reduced invasiveness due to a decrease in tumor secretion of active matrix metalloproteinase 9. Our results suggest that CR-induced metabolic and signaling changes affect the stroma and the tumor cells resulting in a microenvironment that prevents proliferation of breast tumors and their metastases.
Introduction
Caloric restriction (CR) is the most widely studied model of extending lifespan (1,2,3). Since neoplasia is a major cause of premature mortality, it follows that CR would protect against neoplasia. Indeed, several studies have demonstrated that CR has beneficial effects on neoplasias in brain (4), liver (5), skin (6), colon (7), breast (8) and prostate (9). However, as noted in a recent review by Longo et al. (10), little is known about the extent to which CR reduces metastatic growth, although a recent report noted that lung metastases were reduced with CR in a model of breast cancer (11). The mechanisms mediating the beneficial effects are not completely elucidated, although it is known that CR reduces energy metabolism and decreases plasma concentrations of anabolic hormones and growth factors that are involved in tumorigenesis (12). It is also known that CR inhibits proliferation (13) and angiogenesis (9,14) and enhances apoptosis (15), mechanisms that reduce tumor growth. Less is known about how CR affects mechanisms mediating extracellular matrix (ECM) of tumors, which can also affect tumor growth (16,17).
Accordingly, the goal of this investigation was to study the effects of CR on experimental mammary tumor size and metastases to lung and to couple this with investigation into the mechanisms that affect tumor growth and metastases (i.e. proliferation, angiogenesis and apoptosis) and factors that affect the ECM of tumors [i.e. transforming growth factor-β (TGF-β), matrix metalloproteinases (MMP)-9 and (MMP)-2 and collagen IV]. We studied metastases that develop spontaneously from the primary tumor and also following i.v injection of tumor cells. The mammary tumor model we used, 4T1 cell line in BALB/c mice, resembles breast cancer in patients (18). CR is particularly relevant for the study of inhibition of breast cancer in view of the well-recognized relationship among breast cancer, hormones and obesity (12,19,20,21).
Materials and methods
Mice and diets
Female 8 week-old BALB/c mice were weighed and then randomly assigned to normal diet (ND) or CR for the duration of the experiment (AIN-93M Diets No. F05312 and F05314 40% dietary reduction, Bioserv). ND consisted of 90 kcal/week of a chemically defined control diet, which provides ∼10% fewer calories than are normally assumed to be required by a mouse. In all the experiments, during the first 2 weeks, for acclimation purposes, mice were fed with ND or CR (25% kcal/week reduction). After that, the CR diet was changed to 40% kcal/week reduction for the remainder of the experiments. The main composition for the ND and CR pellets is described in Table I. The ND is higher on the percentage of carbohydrates, whereas the CR is higher on percentage of protein, fat and fiber. The CR is not derived from the composition of the diet but rather the amount of diet administered to the ND and CR groups. CR mice received two pellets per day compared with ND mice that received three or four pellets on alternating days. In all experiments, mice were fed with ND or CR for 5 weeks prior to inoculation with 4T1 cells. At week 6, mice were injected with the mammary tumor cells and mice were closely monitored to evaluate tumor growth, spontaneous metastases, in vivo angiogenesis or experimental metastases. All mice were housed individually. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Table I.
Composition of diets
| Ingredients | ND (%) | CR (%) |
| Protein | 12.4 | 20.8 |
| Fat | 3.9 | 6.7 |
| Carbohydrates | 69.2 | 55.8 |
| Fiber | 3.8 | 5.5 |
| Micronutrients | ND (g/kg) | CR (g/kg) |
| Vitamins | 10.0 | 16.7 |
| Minerals | 35.0 | 58.3 |
Ingredients (top) are shown as a percentage of weight in a 1g pellet in the ND and CR diet. Micronutrients (bottom) are shown as g/kg added to the diets for ND and CR.
Body weight, food intake and blood measurements
Body weight was measured twice per week, whereas food intake was assessed daily. Mice were euthanized and blood was withdrawn 20 h after the last meal.
Cell culture
The 4T1 murine mammary tumor cell line, originally isolated from BALB/c mice, was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (American Type Culture Collection, Manassas, VA), 100 IU/ml penicillin and 100 μg/ml streptomycin (Invitrogen Corp., Carlsbad, CA). Cells were grown at 37°C in a humidified 5% CO2 incubator.
Tumor growth
In each experiment, mice were fed with ND or CR for 5 weeks previous to inoculation with 4T1 cells. At week 6, syngeneic mammary tumors were established orthotopically by implanting 105 4T1 cells suspended in 0.2 ml culture medium in the mammary gland of female BALB/c mice (n = 7 per group). Mice were then continued on the same diets until the end of the experiments. Tumor growth was assessed twice a week by caliper measurement of tumor diameter in the longest dimension (L) and at right angles to that axis (W). Tumor volumes were estimated using the formula, L × W × W × π/6. At the end of the experiment, half of each tumor was fixed by immersion in 10% phosphate-buffered formalin, dehydrated and embedded in paraffin. Major organs were subjected to gross pathology analysis to determine if metastases have developed. When metastases were found, the organ was removed and fixed for quantification and histopathology analysis. Then, representative slides from different areas of the tumors were stained with hematoxylin–eosin.
Spontaneous and experimental metastases
For spontaneous metastases, ND and CR mice were injected with 105 4T1 cells per 0.2 ml culture medium orthotopically in the mammary gland and continued on diets for the remainder of the time. Four weeks after injection, mice were euthanized and primary tumors and lungs were removed. Lungs were fixed in Bouin's-Picric acid solution (Sigma Aldrich Corp., St. Louis, MO) for quantification of the incidence of metastases. For experimental metastases, ND and CR mice were injected with 5 × 104 4T1 cells per 0.2 ml culture medium in the tail vein and 21 days later, lungs were harvested to quantify the number of lung nodules. In both experiments, lung metastases were quantified under a dissecting microscope using a millimeter paper, each nodule was measured and tallied into a graph by size: 0.5 to >1.5 mm.
Cell proliferation and microvessel density
Tumor cell proliferation and angiogenesis were evaluated by immunostaining for Ki-67 (Neo Markers, Fremont, CA) and CD31 (Santa Cruz Biotechnology, Santa Cruz, CA) proteins, respectively. Proliferation was assessed by counting the number of tumor cells with positive nuclei (for Ki-67) at ×400 magnification in representative regions of the tumor. Ten fields were counted per each sample and results were expressed as the proliferation index: proportion of positively staining cells over the total number of cells. Microvessel density was assessed by counting the number of microvessels positive for CD31 at x400 magnification in the fields that have the highest vascularization.
Tunel assay
The rate of tumor apoptosis was measured by using terminal deoxyribonucleotide transferase-mediated dUTP nick-end labeling (TUNEL) (Roche Diagnostics, Indianapolis, IN). First, slides were incubated with Proteinase K and then treated with 2 nmol/l biotin-conjugated deoxyuridine triphosphate and 0.1 U/μl terminal deoxyribonucleotide transferase for 1 h at 37°C followed by incubation with FITC-ExtrAvidin (1:200) (Sigma Aldrich Corp.). At the end of the procedure, slides were mounted in a Vector DAPI medium (Vector Labs, Burlingame, CA) for the fluorescent microscope. Ten fields were counted per each sample. Morphometric measurements were performed blinded. Results were expressed as the mean of apoptotic rates: proportion of positively staining cells over the total number of cells.
In vivo angiogenesis
Mice-fed ND or CR diets for 5 weeks were injected with 4T1 tumor cells to induce in vivo angiogenic response (22). The 4T1 (105) cells in medium with trypan blue were injected intradermally in the dorsal midscapular part of the mice (n = 5 per group). Mice were fed ND or CR for another 5 days. Mice were then euthanized, and the injection area was identified in the skin and the vascular area was photographed using a dissection microscope. A microscope micrometer was used to calibrate length in millimeters. Using the Imagine Pro Plus software, the total length of vessels was calculated based on that calibration. In vivo angiogenesis was represented by the total length of vessels around the tumors.
Metabolism and immunoassays
Protein levels of insulin were measured with the Ultra Sensitive Mouse Insulin ELISA Kit from Crystal Chem (Downers Grove, IL). Protein levels of vascular endothelial growth factor (VEGF), leptin, adiponectin, insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein 3 (IGFBP-3) and TGF-β were measured by Quantikine immunoassay Kits (R&D Systems, Minneapolis, MN). MMP-2 and MMP-9 activity levels were quantified by MMPs activity Biotrak assay Kits (GE Healthcare Life Sciences, Piscataway, NJ).
Zymography
In order to study the gelatinolytic MMP activity in tumor homogenates from ND and CR mice, sodium dodecyl sulfate–polyacrylamide gels were copolymerized with gelatin. Frozen tumor samples were homogenized at 4°C in an extraction buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2P2O7, 1 mM NaF, 1 mM benzamidine, 0.5% ethyl phenyl polyethylene glycol and 5 mM Na3VO4 in autoclaved water without protease inhibitors.
Picric acid Sirius red staining
Samples were fixed in 10% formalin and cut into 7 μm thick sections. Tissue sections were stained with picric acid Sirius red (Sigma Aldrich Corp.) to identify collagen (23). The surface of tissue section covered by extracellular collagen was photographed on a Nikon E800 Eclipse microscope and analyzed by Imagine Pro Plus software. Results were reported as the percentage of inhibition in collagen volume fraction in the tissue sections.
Immunohistochemical staining
For the study of TGF-β, collagen I and collagen IV, serial tissue sections were cut and immunostained with the following primary antibodies: rabbit antibody against TGF-β (dilution 1:400; Santa Cruz Biotechnology), rabbit antibodies against collagen I and collagen IV (dilution 1:250; Abcam, Cambridge, MA). In each case, proper negative controls without the primary antibody were performed to show specificity of the antibody.
Western blotting
Cell lysates were prepared by treating cells with lysis buffer 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2P2O7, 1 mM NaF, 1 mM benzamidine, 0.5% ethyl phenyl polyethylene glycol, 5 mM Na ethyl phenyl polyethylene glycol3VO4, 10 μg/ml aprotinin, 10 μg/ml trypsin inhibitor and 10 μg/ml leupeptin. Lysates were sonicated for 20 s on ice and centrifuged at 10 000g for 10 min to sediment the particulate material. The protein concentration of the supernatant was measured and then samples were loaded and run on polyacrylamide gels under non-reducing or reducing conditions. The resolved proteins were transferred onto nitrocellulose sheets and the nitrocellulose membranes were then incubated with primary antisera according to the manufacturer’s instructions. Monoclonal antibody was used against β-actin (Sigma Aldrich Corp.). Anti-IgG secondary antibody conjugated to horseradish peroxidase was used for detection. Blots were developed with the enhanced chemiluminescence western blot detection system from NEN Life Sciences Products (Boston, MA).
Statistics
Results are expressed as the mean ± SE. All comparisons were made by the two-tailed Student’s t-test or Mann–Whitney rank sum test. Difference between groups of P < 0.05 was considered significant.
Results
Food intake and body weights
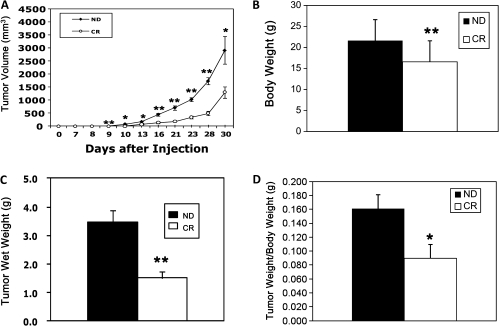
By experimental design, food intake in CR mice was lower than in ND mice. The total energy intake of ND mice was ∼12–14 kcal/day and in CR mice, it was ∼11 kcal/day for the first 2 weeks and then it decreased to 7 kcal/day. ND mice sometimes did not eat all of their food, whereas CR mice always consumed all the pellets given. At the end of the study, CR mice weighed significantly less (P < 0.01) and this regime produced a 23% reduction in total body weight compared with ND mice (ND: 21.6 ± 0.7 g and CR: 16.6 ± 0.3 g) (Figure 1B).
Fig. 1.
CR causes a significant inhibition of 4T1 murine mammary tumor formation in syngeneic mice. (A) Tumor volume was significantly increased over 30 days after inoculation with 4T1 cells and this growth was significantly reduced in CR mice. (B) CR mice weighed significantly less than ND mice. (C) The average of tumor wet weight at 30 days was significantly lower in CR mice. (D) Tumor wet weights normalized by body weight were also significantly different. *P < 0.05, **P < 0.01 versus ND; n = 7 per group.
Tumor growth
CR reduced 4T1 murine mammary tumor growth in syngeneic mice (P < 0.05 versus ND) (Figure 1A). The average wet tumor weight was significantly lower (P < 0.01) in CR mice (1.5 ± 0.3 g) versus ND mice (3.5 ± 0.4 g) (Figure 1C). These values were also different when normalized by body weight (P < 0.05) (Figure 1D).
Spontaneous lung metastases
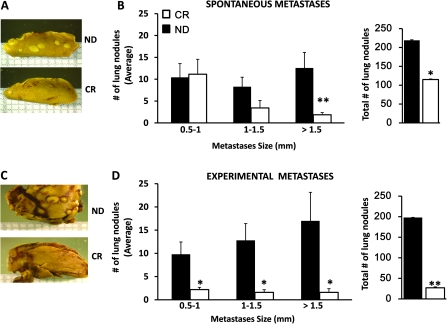
CR caused a significant lag in the development of spontaneous lung metastases, both in terms of numbers and size of metastases (Figure 2A and B). The differences were most prominent in the numbers of larger metastases >1.5 mm (CR: 1.9 ± 0.5 versus ND: 12.6 ± 3.6; P ≤ 0.01).
Fig. 2.
CR causes a decrease in the number of spontaneous and experimental lung metastases. (A) Representative reduced spontaneous metastases in lung from a ND and a CR mouse. (B) The average ± SE of spontaneous lung metastases showed that the differences in reduction between CR with respect to ND mice were greater for larger metastases. In addition, the total number of lung nodules was significantly reduced in the CR mice. (C) Representative reduced experimental metastases in lung from a ND and a CR mouse. (D) The average ± SE of experimental lung metastases showed that the differences in reduction between CR with respect to ND mice were greater for larger metastases. In addition, the total number of lung nodules was significantly reduced in the CR mice. **P ≤ 0.01 versus ND; *P < 0.05 versus ND; n = 7 per group (spontaneous); n = 5 per group (experimental).
Experimental lung metastases
CR mice showed a delay in the development of experimental lung metastases but, most significantly, a decrease in the total number of tumor nodules (Figure 2C and D). The differences were more prominent in the numbers of larger metastases >1.5 mm (CR: 1.6 ± 0.8 versus ND: 17 ± 6.2; P < 0.05).
Proliferation and apoptosis
Tumors from CR mice demonstrated a 64% decrease in the cell proliferation index (CR: 7.9 ± 0.8 versus ND: 22.2 ± 0.9; P < 0.01). Apoptosis was also evaluated by the TUNEL assay in tumors from mice fed with ND or CR to determine the effect of CR on programmed cell death in this model. Tumors from CR mice displayed a significantly larger apoptotic rate (CR: 1.6 ± 0.1 versus ND: 0.9 ± 0.1, P < 0.05).
Microvessel density and in vivo angiogenesis
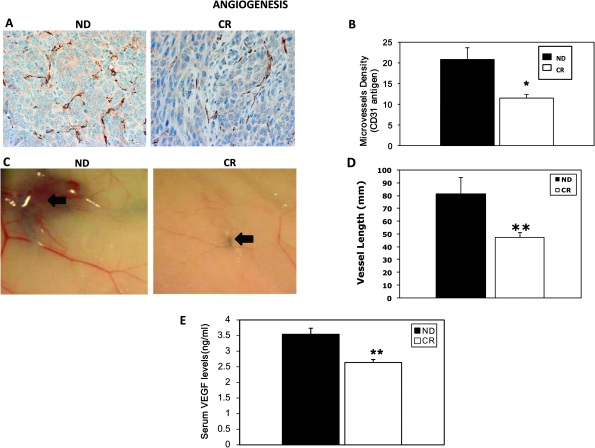
CR mice displayed significantly lower intratumor microvessel density than ND mice (CR: 11.5 ± 0.9 versus ND: 20.8 ± 3.0; P < 0.05) (Figure 3A and B). Because of the significant difference in microvessel density, we evaluated in vivo angiogenesis. Tumors from CR mice possessed significantly less angiogenesis than ND mice, as measured by total vessel length (CR: 47.4 ± 3.8 mm versus ND: 81.4 ± 12.9 mm, P < 0.01) (Figure 3C and D). Circulating levels of VEGF in serum were measured and results showed that CR mice have significantly lower levels than ND mice (CR: 2.6 ± 0.1 versus ND: 3.5 ± 0.2 ng/ml; P < 0.01) (Figure 3E), consistent with lower microvessel density and angiogenic response.
Fig. 3.
CR reduces intratumor microvessel density and in vivo angiogenesis induced by 4T1 tumors. (A) CD31 antigen staining to assess vascular density was reduced in CR mice. (B) Quantification of microvessel density shows a reduction in CR mice versus ND mice. (C) Representative photographs showing reduced vessels in an example of CR mouse. Black arrows indicate the vessels in the vicinity of tumor implant. (D) Reduced total length of vessels around tumor implants in CR mice. (E) Serum VEGF is also reduced in CR mice. *P < 0.05 versus ND; n = 4 per group (microvessel density); **P < 0.01 versus ND; n = 5 per group (in vivo angiogenesis); **P < 0.01 versus ND; n = 7 per group (VEGF).
Metabolic markers
CR mice showed a 36% reduction in insulin levels (P < 0.01) and a 38% reduction in leptin (P < 0.05) levels compared with ND mice (Table II). However, CR mice displayed a 17.5% increase in adiponectin (P < 0.05). In addition, CR mice displayed a 63% decrease in both circulating IGF-1 and IGFBP-3 protein (P < 0.01) (Table II).
Table II.
Metabolic markers
| ng/ml | ND | CR |
| Insulin | 0.014 ± 0.001 | 0.009 ± 0.001** |
| Leptin | 0.8 ± 0.1 | 0.5 ± 0.01* |
| Adiponectin | 2640 ± 84 | 3102 ± 149* |
| IGF-1 | 243 ± 8 | 89 ± 29** |
| IGFBP-3 | 202 ± 35 | 75 ± 13** |
Effects of CR on serum levels of insulin, leptin, adiponectin, IGF-1 and IGFBP-3. Circulating serum levels of insulin, leptin and adiponectin were measured by enzyme-linked immunosorbent assay immunoassays. Serum from tumor-bearing ND or CR mice were used to evaluate the levels of these metabolic markers. Values shown are mean ± SE; *P < 0.05 and **P < 0.01 versus ND; n = 7 per group. Circulating levels of IGF-1 and IGFBP-3 were also measured in serum from ND and CR mice.
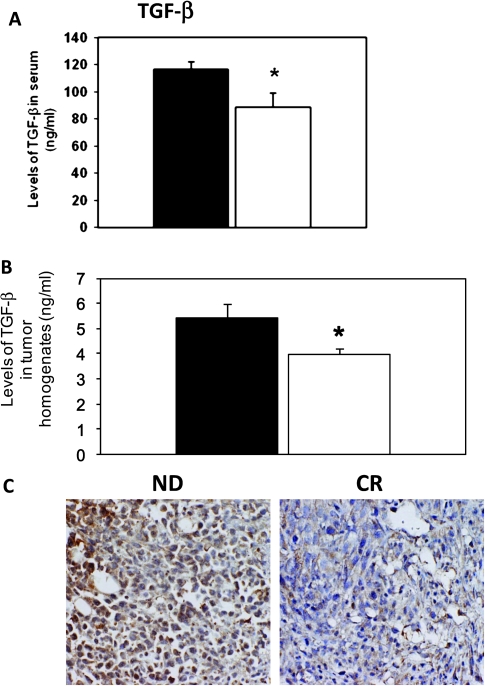
Levels of TGF-β
CR mice showed a 24% lower level of serum TGF-β compared with ND mice (ND: 116.4 ± 5.5 and CR: 88.3 ± 10.6 ng/ml; P < 0.05) (Figure 4A). The levels of TGF-β in tumor homogenates and intratumor levels of TGF-β were also significantly lower in the CR mice (ND: 5.4 ± 0.6 and CR: 3.9 ± 0.2 ng/ml; P < 0.05) (Figure 4B). In addition, immunohistochemistry analysis also confirmed that TGF-β expression was lower in tumors from CR mice (Figure 4C).
Fig. 4.
Levels of TGF-β are reduced in CR mice. (A) Reduced serum TGF-β in CR (white bars) with respect to the ND (black bars) mice. (B) Intratumor levels of TGF-β are also reduced in CR mice. (C) TGF-β staining is reduced in tumors from CR mice. *P < 0.05 and versus ND; n = 7 per group.
MMP activities
CR mice demonstrated a lower tumor-associated MMP-9 activity by zymography (ND: 500 ± 54 and CR: 280 ± 18 A.U; P < 0.01) and also by Biotrak assay Kit (ND: 1.1 ± 0.1 and CR: 0.7 ± 0.1 ng/μg protein; P < 0.05). In contrast, MMP-2 levels were not changed among tumors from ND and CR mice (data not shown). However, reductions in serum MMP activities were observed for both MMP-2 (ND: 3.2 ± 0.2 and CR: 0.5 ± 0.4 ng/ml; P < 0.01) and MMP-9 (ND: 0.9 ± 0.1 and CR: 0.4 ± 0.1 ng/ml; P < 0.05).
Intratumor collagen deposition
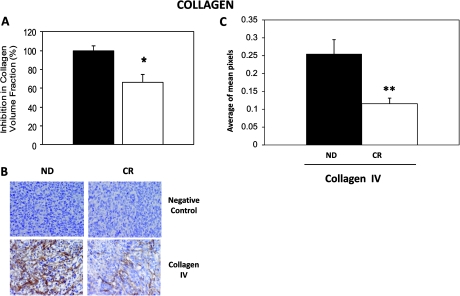
CR mice showed a 34% lower total collagen intratumor volume fraction compared with ND mice (ND: 42.6 ± 2.4 and CR: 28.3 ± 3.6; P < 0.05) (Figure 5A). Collagen IV expression was significantly reduced in tumors from CR mice (Figure 5B). The average mean of pixels for collagen IV in CR mice was 0.12 ± 0.02 compared with 0.25 ± 0.04 in ND (P < 0.01) (Figure 5C). Western blot analysis confirmed the reduced expression of collagen IV in tumors from CR mice (data not shown). However, collagen I did not change (data not shown).
Fig. 5.
CR mice possess reduced intratumor collagen IV expression. (A) Quantification of the percentage of total collagen volume fraction shows a significant reduction in CR (white bar) versus ND (black bar) mice. (B) Tumors from CR mice express lower levels of collagen IV. (C) Quantification of the mean pixels shows that collagen IV levels were significantly lower in tumors from CR mice. *P < 0.05 and **P < 0.01 versus ND; n = 4 per group.
Discussion
We found that CR reduces growth of mammary tumors and metastases, involving several different mechanisms, confirming and extending prior work on this topic. It is well recognized that CR extends longevity, in part, by protecting against the major diseases that reduce lifespan, e.g. cancer. One century ago it was reported that growth of transplantable sarcomas was inhibited significantly in underfed mice (24). Now the literature abounds with studies showing that CR leads to a reduction in spontaneous and chemically induced tumors in rodent (25,26,27,28,29) models. However, all studies have not shown a favorable effect of CR on tumor growth. For example, a recent paper by Kalaany et al. (30) found that CR reduced tumor growth in some human cancer cell lines in nude mice, particularly ones including mammary tumors (MDA-MB-231 and MDA-MB-435) but not in others, suggesting that signaling pathways are important. Although several studies have shown that CR leads to decreased growth of several tumors (4,5,6,7,8,9), including mammary (8,9,10,11), the effect of CR on metastases has been studied less, as noted in a recent review (10). Recently Phoenix et al. (11), primarily examined the effects of metformin, a CR mimetic, but also included some data on CR and reported a reduction in the average number of metastatic nodules per lung. That study employed the model of the triple-negative 66c14 tumor cell line derived from a spontaneously arising mammary tumor from a Balb/c mouse. One major finding of our investigation was that CR not only reduced the primary tumor size but also the number and size of metastases. This suggests that the mechanisms responsible for the growth and the invasion of spontaneous micrometastases into adjacent lung tissue may be inhibited by CR. CR appears to affect the growth of metastases more than the frequency of micrometastases, particularly for spontaneous metastases, which suggests that nutrition is important for metastatic spread as well as for the growth of the primary tumor. Importantly, several mechanisms initiated by CR-mediating metastatic spread and growth are involved, e.g. the reduced angiogenesis observed in CR mice could be affecting the angiogenic switch (31) needed for lung colonization. Having noted all of the above, determining whether CR affects total number of micrometastasis is particularly difficult because of the inability to determine the number of micrometastasis that do not survive.
We examined several mechanisms by which CR induced its favorable effects on reducing tumor growth and metastases. For example, tumor growth depends on inhibition of proliferation (13), angiogenesis (9,14) and increase of apoptosis (15). It has been shown that CR reduces cell proliferation in several tumors (32,33), including rat mammary tumors (34). In this study, we evaluated the effect of CR on 4T1 tumor growth in the BALB/c syngeneic model. We observed that CR caused a significant reduction in 4T1 tumor growth, showing that the CR diet initiated before tumor cell inoculation reduces subsequent mammary tumor growth with a concomitant reduction in the tumor cell proliferation index.
Apoptosis can inhibit tumor growth and it has been shown that CR enhances rates of apoptosis concomitant with decreases in DNA synthesis, markedly reducing the number and volume of preneoplastic lesions (13). In general, several studies have shown that circulating factors, such as IGF-I, are the keys mediators of the antiproliferative and proapoptotic effects of CR (8,35). In our model of mammary tumors, CR increased apoptosis and decreased the levels of IGF-I.
Inhibiting angiogenesis is a major mechanism utilized to restrict tumor growth, as any growing tissue is dependent on a rapidly increasing blood supply through angiogenesis. Mukherjee et al. (13,15) demonstrated that CR reduced angiogenesis in brain and prostate (9) syngeneic tumor models. CR also reduced vascular density in mammary carcinogenesis (14). In the present study, we found that CR reduces microvessel density and angiogenesis in this mouse model of mammary tumor. VEGF triggers the onset of blood vessels and capillaries around the tumor that facilitates the dissemination of tumor cells and the growth of metastases in target organs. Levels of VEGF are reduced in serum of CR mice. We also demonstrated that CR reduces lung metastases and this reduction could be due to the reduction in VEGF levels and the significant reduction in angiogenesis.
Growth factors, like IGF-1 and TGF-β, have been also implicated in tumor growth and metastases (35,36). Increased IGF-1 serum levels are associated with an increased risk of breast cancer in menopausal women (37). We studied the effects of CR on insulin, IGF-1 and IGFBP-3, which play a key role in mammary tumorigenesis, proliferation, growth and apoptosis (35,38,39). CR decreases by ∼40%, the levels of serum IGF-1 concentration in rodents and this plays a role in protecting against cancer and aging (40). We show that CR diminishes serum levels of leptin and insulin and increases adiponectin in mammary tumor-bearing mice. Interestingly, these hormones are related to obesity and cancer (19), which is particularly relevant to the model we studied, as breast cancer and CR are related to obesity and hormones (12,19,20,21). In particular, the lower levels of leptin could be associated with an accelerated metabolism and high-energy requirements or with an adipose atrophy in the tumor-bearing mice (41,42). It has been shown that TGF-β regulates ECM and modulates cell invasion and primary tumor microenvironment (43). However, the role of TGF-β in tumor growth and metastasis has been controversial (36,44). More recently, several papers have shown that increased levels of TGF-β increase tumor progression and metastasis (43,44) and inhibition of TGF-β signaling may interfere with the capacity of tumor cells to metastasize (45,46). In our study, we found that CR mice have lower levels of TGF-β in tumors and serum along with protection against tumor growth and metastasis, supporting the concept that inhibition of TGF-β therapy could be beneficial.
Tumor invasiveness depends on the balance between the deposition and the proteolytic degradation of the ECM and basement membrane. Alterations in the ECM, e.g. collagens IV and I, have been associated with the processes of angiogenesis, invasion and metastases (47,48). The ratio between MMPs and their endogenous inhibitors has been shown to play an important role in ECM remodeling, tumor microenvironment and progression (49). In particular, MMP-2 and MMP-9 degrade gelatin and type IV collagen, main components of basement membrane, which is the main barrier separating in situ and invasive carcinoma. Collagen, as one of the key molecules in ECM, has been involved in tumor microenvironment and progression (17,47,48). The effects of CR on TGF-β and collagen deposition in tumors have not been shown, and the link among CR, ECM and tumorigenesis has not been demonstrated. We found that tumors from CR mice exhibit lower levels of TGF-β, less intratumor collagen IV and less active MMP-9. Also, circulating levels of MMP-9 and MMP-2 are lower in CR mice serum. One of the targets of MMP-9 is the proangiogenic factor VEGF, which is activated by MMP-9 cleavage. Interestingly, CR mice have lower levels of VEGF in serum and their tumors show a marked decrease in vascular networks. Thus, lower levels of MMPs could result in a less invasive and less angiogenic phenotype; combined together, this could reduce the number of lung metastases in CR mice.
In summary, CR mice displayed multiple mechanisms that may contribute to decreased growth of mammary tumors and metastases, as well as numbers of metastases. These range from cellular alterations such as decreased proliferation and increased apoptosis to effects on critical mechanisms for invasion and metastasis, such as decreased angiogenesis and altered ECM regulation. The fact that CR of the animals prior to introduction of the tumor cells resulted in these strong effects on tumor growth and metastasis underscore the need for additional studies on the potential beneficial effects of CR for cancer prevention, not only on initial steps in tumorigenesis but also on critical stages in tumor progression and metastasis.
Funding
National Institutes of Health (AG027211 to S.F.V) and its Diversity Supplement Program (AG027211-02S1 to M.S.D.L.); Seed Grant (PC6-10 to M.S.D.L.) from the Foundation of UMDNJ and Pilot Research Grant from The Josiah Macy, Jr. Foundation (Award # 5-46787-B to M.S.D.L].
Acknowledgments
We would like to thank Tara McNulty for her contribution.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CR
caloric restriction
- ECM
extracellular matrix
- IGF-1
insulin-like growth factor 1
- IGFBP-3
insulin-like growth factor binding protein 3
- MMP
matrix metalloproteinases
- ND
normal diet
- TGF-β
transforming growth factor-β
- TUNEL
terminal deoxyribonucleotide transferase-mediated dUTP nick-end labeling
- VEGF
vascular endothelial growth factor
References
- 1.Bordone L, et al. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 2.Spindler SR. Caloric restriction: from soup to nuts. Ageing Res. Rev. 2010;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Fontana L, et al. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulrooney TJ, et al. Influence of caloric restriction on constitutive expression of NF-κB in experimental mouse astrocytoma. PLoS One. 2011;6:e18085. doi: 10.1371/journal.pone.0018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu PP, et al. Caloric restriction profoundly inhibits liver tumor formation after initiation by 6-nitrochrysene in male mice. Carcinogenesis. 1994;15:159–161. doi: 10.1093/carcin/15.2.159. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JW, et al. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005;26:1077–1084. doi: 10.1093/carcin/bgi051. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley KE, et al. Low-carbohydrate diet versus caloric restriction: effects on weight loss, hormones, and colon tumor growth in obese mice. Nutr. Cancer. 2008;60:61–68. doi: 10.1080/01635580701510150. [DOI] [PubMed] [Google Scholar]
- 8.Cleary MP, et al. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp. Biol. Med. (Maywood) 2007;232:70–80. [PubMed] [Google Scholar]
- 9.Mukherjee P, et al. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J. Natl Cancer Inst. 1999;91:512–523. doi: 10.1093/jnci/91.6.512. [DOI] [PubMed] [Google Scholar]
- 10.Longo VD, et al. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phoenix KN, et al. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res. Treat. 2010;123:333–344. doi: 10.1007/s10549-009-0647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, et al. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee P, et al. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br. J. Cancer. 2002;86:1615–1621. doi: 10.1038/sj.bjc.6600298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson HJ, et al. Effect of dietary energy restriction on vascular density during mammary carcinogenesis. Cancer Res. 2004;64:5643–5650. doi: 10.1158/0008-5472.CAN-04-0787. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee P, et al. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin. Cancer Res. 2004;10:5622–5629. doi: 10.1158/1078-0432.CCR-04-0308. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, et al. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erler JT, et al. Three-dimensional context regulation of metastasis. Clin. Exp. Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslakson CJ, et al. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 19.Grossmann ME, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 20.Harvie M, et al. Energy balance adiposity and breast cancer—energy restriction strategies for breast cancer prevention. Obes. Rev. 2006;7:33–47. doi: 10.1111/j.1467-789X.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 21.Lorincz AM, et al. Molecular links between obesity and breast cancer. Endocr. Relat. Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 22.De Lorenzo MS, et al. Altered tumor angiogenesis and metastasis of B16 melanoma in transgenic mice overexpressing tissue inhibitor of metalloproteinases-1. In Vivo. 2003;17:45–50. [PubMed] [Google Scholar]
- 23.Junqueira LC, et al. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 24.Moreschi C. Beziehungen zwischen ernährung und tumorwachstum. Zeitschrift f Immunitätsforschung Originale. 1909;Bd.II:651–675. [Google Scholar]
- 25.Kritchevsky D. Caloric restriction and experimental mammary carcinogenesis. Breast Cancer Res. Treat. 1997;46:161–167. doi: 10.1023/a:1005960410225. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, et al. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan KP, et al. Diet, caloric restriction, and the rodent bioassay. Toxicol. Sci. 1999;52(2 suppl.):24–34. doi: 10.1093/toxsci/52.2.24. [DOI] [PubMed] [Google Scholar]
- 28.Kari FW, et al. Roles for insulin-like growth factor-1 in mediating the anti-carcinogenic effects of caloric restriction. J. Nutr. Health Aging. 1999;3:92–101. [PubMed] [Google Scholar]
- 29.Hursting SD, et al. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- 30.Kalaany NY, et al. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naumov GN, et al. Tumor-vascular interactions and tumor dormancy. APMIS. 2008;116:569–585. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 32.Lok E, et al. Dietary restriction, cell proliferation and carcinogenesis: a preliminary study. Cancer Lett. 1988;38:249–255. doi: 10.1016/0304-3835(88)90016-x. [DOI] [PubMed] [Google Scholar]
- 33.Lok E, et al. Caloric restriction and cellular proliferation in various tissues of the female Swiss Webster mouse. Cancer Lett. 1990;51:67–73. doi: 10.1016/0304-3835(90)90232-m. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Z, et al. Effect of energy restriction on tissue size regulation during chemically induced mammary carcinogenesis. Carcinogenesis. 1999;20:1721–1726. doi: 10.1093/carcin/20.9.1721. [DOI] [PubMed] [Google Scholar]
- 35.Rogozina OP, et al. Serum insulin-like growth factor-I and mammary tumor development in ad libitum-fed, chronic calorie-restricted and intermittent calorie-restricted MMTV-TGF-alpha mice. Cancer Prev. Res. (Phila Pa) 2009;2:712–719. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- 36.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugumar A, et al. Insulin-like growth factor (IGF)-I and IGF-binding protein 3 and the risk of premenopausal breast cancer: a meta-analysis of literature. Int. J. Cancer. 2004;11:293–297. doi: 10.1002/ijc.20253. [DOI] [PubMed] [Google Scholar]
- 38.Pollak MN, et al. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 39.Jogie-Brahim S, et al. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr. Rev. 2009;30:417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn SE, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 41.López-Soriano J, et al. Leptin and tumor growth in rats. Int. J. Cancer. 1999;81:726–729. doi: 10.1002/(sici)1097-0215(19990531)81:5<726::aid-ijc10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 42.Bing C, et al. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br. J. Cancer. 2006;95:1028–1037. doi: 10.1038/sj.bjc.6603360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheen-Chen SM, et al. Serum levels of transforming growth factor β1 in patients with breast cancer. Arch. Surg. 2001;136:937–940. doi: 10.1001/archsurg.136.8.937. [DOI] [PubMed] [Google Scholar]
- 45.Moore LD, et al. Silencing of transforming growth factor-β1 in situ by RNA interference for breast cancer: implications for proliferation and migration in vitro and metastasis in Vivo. Clin. Cancer Res. 2008;14:4961–4970. doi: 10.1158/1078-0432.CCR-07-4604. [DOI] [PubMed] [Google Scholar]
- 46.Padua D, et al. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 47.Mundel TM, et al. Type IV collagen-derived angiogenesis inhibitors. Microvasc. Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]