Abstract
Prostate cancer disparities have been reported in men of African descent who show the highest incidence, mortality, compared with other ethnic groups. Few studies have explored the genetic and environmental factors for prostate cancer in men of African ancestry. The glutathione-S-transferases family conjugates carcinogens before their excretion and is expressed in prostate tissue. This study addressed the role of GSTM1 and GSTT1 deletions on prostate cancer risk in populations of African descent. This multi-institutional case–control study gathered data from the Genetic Susceptibility to Environmental Carcinogens (GSEC) database, the African-Caribbean Cancer Consortium (AC3) and Men of African Descent and Carcinoma of the Prostate Consortium (MADCaP). The analysis included 10 studies (1715 cases and 2363 controls), five in African-Americans, three in African-Caribbean and two in African men. Both the GSTM1 and the GSTT1 deletions showed significant inverse associations with prostate cancer [odds ratio (OR): 0.90, 95% confidence interval (CI) 0.83–0.97 and OR 0.88, 95% CI: 0.82–0.96, respectively]. The association was restricted to Caribbean and African populations. A significant positive association was observed between GSTM1 deletion and prostate cancer in smokers in African-American studies (OR: 1.28, 95% CI: 1.01–1.56), whereas a reduced risk was observed in never-smokers (OR: 0.66, 95% CI: 0.46–0.95). The risk of prostate cancer increased across quartiles of pack-years among subjects carrying the deletion of GSTM1 but not among subjects carrying a functional GSTM1. Gene–environment interaction between smoking and GSTM1 may be involved in the etiology of prostate cancer in populations of African descent.
Introduction
Prostate cancer is a major global public health problem that disproportionately affects individuals of African ancestry, more than their white and Asian counterparts (1). An incidence rate of 156.7 per 100 000 and a mortality rate of 24.6 per 100 000 men was observed for whites in the USA between 2001 and 2007 (2). In the African-American population, the incidence rate was 248.5 per 100 000 and mortality rate of 59.4 per 100 000, i.e. almost 1.6-fold greater incidence and 2.4-fold greater mortality than that of the white population (2). Studies have shown that in other populations of African descent, the risk of developing prostate cancer is also relatively high when compared with other races (3,4). It is only recently that prevalence, incidence and mortality rate data on populations of African descent have emerged from outside the USA. Prostate cancer incidences are higher in black Caribbean, black African and other blacks compared with mixed white and African, mixed white and Caribbean, Pakistani and ‘all white’ men in the United Kingdom, respectively (5–7). These individuals of African descent live in diverse surroundings around the world, but still have in common an increased risk of developing prostate cancer, but less is known about risk of native Africans; this suggests that some genetic factors and/or lifestyle factors such as cigarette smoking and/or diet interacting with a specific genetic makeup may contribute to the elevated risk.
Polymorphisms in genes responsible for the metabolism of environmental and endogenous carcinogens could be associated with prostate cancer and thus, these polymorphisms could be used as biomarkers for identifying men at risk for prostate cancer (8). One of the most promising candidates is the glutathione S-transferases (GSTs) family, a group of phase II detoxifying enzymes that catalyze reactions taking place between the cytosolic glutathione and electrophilic compounds (9). GSTs are expressed in prostate tissue; thus, the lack of activity could lead to local accumulation of toxic compounds (10). For two of these genes, GSTM1 (GenBank: BC024005.2) and GSTT1 (GenBank: BC007065.1), a complete deletion of the gene eliminates the gene function, thus leading to the inability to eliminate electrophilic carcinogens as efficiently. Although the GST family has the general task of detoxification through conjugation, each GST is specialized in specific substrates; GSTM1 seems to be directly implicated in DNA adduct formation caused by benzo(a)pyrene, the main component of cigarette smoke (11). On the other hand, GSTT1 is involved in conjugating smaller molecules, such as epoxides (12), thus being involved in oxidative processes such as those caused by inflammation. Although the predominant role of GSTs is the conjugation of reactive metabolites, they may also be involved in producing reactive derivatives from the metabolism of certain chemicals, such as dichloromethane and other halogenated alkanes (13). Thus, the final direction of the effect on carcinogenesis, as well as the strength of the effect, if any, is difficult to predict.
The relationship between GST deletions and prostate cancer has been studied previously; however, most published results are limited to white populations. A previous meta-analysis (14) suggested an inverse association between GSTM1 and GSTT1 deletion and prostate cancer in men of African descent; however, only two studies were published at that time, making it impossible to draw firm conclusions.
We present here data on GSTM1 and GSTT1 gene deletion and prostate cancer in individuals of African ancestry in the largest existing multi-institutional case–control study, in an attempt to shed light on susceptibility factors and gene–environment interaction in these men.
Materials and methods
Medline search of published studies
To make this study as inclusive as possible, a PubMed search was conducted to identify case–control studies of prostate cancer conducted on men of African ancestry and published before October 23 2010. The search strategy used was (prostate) AND (cancer OR neoplasms OR tumour* OR tumour* OR carcinoma* OR carcinogenesis) AND (‘glutathione transferase’ OR ‘glutathione s transferase’ OR ‘glutathione S-transferase’ OR GSTM1 OR GSTT1). This literature search led to the identification of 449 abstracts, which were systematically reviewed. Inclusion/exclusion of abstracts and their respective studies were made for the PubMed search based on pre-established selection criteria. These inclusion criteria were (i) association studies on GSTM1 and GSTT1 and prostate cancer; (ii) case–control studies and (iii) studies that included African, African-American or African-Caribbean individuals. The exclusion criteria were (i) studies including only cases or only controls and (ii) studies with patients that overlapped. In the latter case, i.e. studies that partially included the same population, only the most recent updated study was retained. Nineteen articles were identified for further consideration after title screening. The abstracts and full text of these articles were read to determine whether they met the inclusion/exclusion criteria. Two articles were excluded for not having individuals of African descent in the sample (15,16), six for using GST polymorphisms not under study (17–22) and three for not being case–control studies (23–25). Another was excluded because the study subjects included controls only (26). Studies with fewer than 10 cases or controls were excluded (27), but the authors were contacted to verify if more data were available, prior to exclusion. This left five studies identified in the PubMed search (28–32); of these, two papers had overlapping data thus constituting one data set (30,31), bringing the number of potential data sets that could be involved in the present effort to four, for a total of 402 cases and 888 controls.
Data collection
A multi-institutional case–control study of prostate cancer in individuals of African descent and GSTM1 and GSTT1 was conducted under the partnership of the Genetic Susceptibility to Environmental Carcinogens study (GSEC, www.gsec.net) and the African-Caribbean Cancer Consortium (AC3, www.ac-ca-consortium.org) (33), which constituted the GSEC-AC3 consortium. Studies identified through the Medline search were sought by sending a formal invitation to the senior authors of the papers asking to join the GSEC-AC3 consortium and share their data set according to the well-established rules and policies of the GSEC study (34). Members of the African-Caribbean Cancer Consortium with ongoing prostate cancer studies that include black men were invited to contribute their data to the GSEC-AC3 database; in addition, a list of prostate cancer researchers was obtained from the Men of African Descent and Carcinoma of the Prostate Consortium (MADCaP), consisting of 92 investigators who were also invited to participate in the study and to contribute data to the GSEC-AC3 database.
The final analysis thus included 10 data sets, 4 of which were previously published and identified through Medline (28,29,30–32), for a total of 1715 cases and 2363 controls.
Covariate assessment
Smokers were defined as those subjects who smoke at least 100 cigarettes in their lifetime. Smoking status was defined in the individual studies as never-, ex, current or as never- and ever smokers. In order to make the various data sets comparable, smoking status was reclassified as never- and ever smokers, with the latter category including ex and current smokers. Gleason score was available in 7 of the 10 data sets and was classified as a discrete number (categorized as ≤ 7 versus > 7). Self-reported height and weight were used to calculate body mass index as kilograms per square meter. Smoking dose is reported in pack-years and is categorized in quartiles of distribution among controls. Race was self-reported in all studies and was defined as African-American, African or African-Caribbean. Age was grouped into quartiles of the distribution in the studied population.
Laboratory methods
The GSTM1 and GSTT1 deletions were detected by polymerase chain reaction for the presence or absence of bands at 480 and 215 bp, respectively. The polymerase chain reaction products were analyzed by 2% agarose gel electrophoresis stained with ethidium bromide (10 mg/ml). An extra set of primer were included in the multiplex as an internal control leading to an amplification of a 268 bp band.
In the Lavender population (32), GSTM1 and GSTT1 deletions were identified using TaqMan® Copy Number assays (Applied Biosystems, Foster City, CA). These predesigned assays include primers and probes that are able detect the GST genes deletions as well as a reference sequence known to be present in two copies in a diploid genome. This method of relative quantitation is used to determine the relative copy number of the target of interest in a gDNA sample, normalized to the known copy number of the reference sequence.
Statistical analysis
All statistical analyses were carried out using STATA SE (version 8.0 and version 10.0) software (StataCorp LP, College Station, TX). For each gene, crude odds ratios (ORs) for the association with prostate cancer were calculated. Summary ORs were adjusted for study, age and smoking status using multivariable logistic regression models. The crude and adjusted ORs were also calculated for each gene polymorphism stratified by smoking status and race. In addition, the association between prostate cancer and smoking was assessed according to genotype status.
Interactions between GST genotypes and ethnicity across levels of smoking status were assessed and tested with multiparameter Wald tests.
The association between prostate cancer and the combined GSTM1–GSTT1 genotype was also performed; the reference group was constituted of subjects carrying both functional genes, then categories were created of subjects with one GST functional and the other deleted, finally a category with subjects carrying both genes deletion.
Data on benign prostate hyperplasia in controls were received from the investigators but excluded from the present analysis. Statistical heterogeneity between and within groups was tested using the Q statistic (35), to determine whether to use the fixed- or random-effects model for calculating the summary ORs. Fixed-effects methods were used if the result of the Q-test was not significant. Otherwise, we calculated pooled estimates and confidence intervals (CIs) assuming a random-effects model with inverse-variance weighting, using the DerSimonian and Laird method (35). The proportion of total variability attributed to between-study heterogeneity, the I2 statistic, and its corresponding 95% CI (uncertainty) were also calculated (36,37). This statistic is useful when deciding whether there is too much heterogeneity to combine the studies and derive a pooled estimate. Although publication bias was not expected, we assessed this possibility using Begg funnel plots and Egger’s bias test (38).
Results
Individual data from 1715 prostate cancer cases and 2363 controls of African descent were available, 467 cases from the USA, 1168 from the Caribbean Islands and 80 from West Africa (Table I). The source of DNA was peripheral blood in 9 of 10 distinct studies; one used DNA from prostate cancer tissue blocks.
Table I.
Description of populations included in the analysis
| Control source | Study site | Cases | Controls |
GSTM1 |
GSTT1 |
|||
| % Null in controls | OR (95% CI) | % Null in controls | OR (95% CI) | |||||
| African-American | ||||||||
| Rebbeck (30,31) | Clinic | PA | 41 | 123 | 28.5 | 0.73 (0.46–1.15) | 40.5 | 0.74 (0.49–1.11) |
| Lavender (32)a | Clinic | DC | 188 | 637 | 30.3 | 1.02 (0.81–1.28) | 22.0 | 1.21 (0.93–1.57) |
| Parka | Clinic | FL | 61 | 61 | 13.3 | 1.52 (0.95–2.43) | –– | –– |
| Taioli | Healthy volunteers | NY | 30 | 32 | 21.9 | 0.95 (0.51–1.74) | –– | –– |
| Agalliu (29)a | Healthy | WA | 147 | 84 | 31.7 | 0.84 (0.47–1.50) | 27.4 | 0.74 (0.40–1.37) |
| OR (95 % CI)b | 467 | 937 | 1.0 (0.85 –1.19)c | 1.01 (0.78–1.24)d | ||||
| Caribbean | ||||||||
| Bunkera | Healthy | Tobago | 277 | 449 | 25.6 | 0.91 (0.76–1.08) | 30.3 | 0.97 (0.82–1.14) |
| Jacksona | Clinic | Jamaica | 128 | 145 | 26.2 | 1.07 (0.82–1.40) | 35.2 | 0.95 (0.74–1.22) |
| Mallick (28)a | Clinic | Guadeloupe | 134 | 134 | 26.9 | 0.65 (0.37–1.16) | 36.6 | 0.50 (0.29–0.86) |
| Romanaa | Population | Guadeloupe | 629 | 622 | 31.7 | 0.85 (0.76–0.95) | 30.9 | 0.82 (0.73–0.92) |
| OR (95 % CI)b | 1168 | 1350 | 0.88 (0.81–0.96)e | 0.86 (0.79–0.94)f | ||||
| Africa | ||||||||
| Lavender (32)a | Clinic | USA | 14 | 21 | 25.0 | 0.74 (0.30–1.83) | 25.0 | 0.55 (0.17–1.72) |
| Ukoli | Healthy | Nigeria | 66 | 55 | 23.6 | 0.71 (0.45–1.14) | 40.0 | 0.93 (0.64–1.34) |
| OR (95 % CI)b | 80 | 76 | 0.72 (0.47–1.08) | 0.89 (0.62–1.26) | ||||
| Totalb | 1715 | 2363 | 0.90 (0.83–0.97)g | 0.88 (0.82–0.96)h | ||||
DNA extracted from peripheral blood.
Adjusted for study, age (quartiles) and smoking(ever/never); meta ORs from fixed models are reported.
Q-test P-value: 0.261; I2 (%): 24% (0.0–69.0); Eggers-Test P-value: 0.828
Q-test P-value: 0.081; I2 (%): 60% (0.0–89.0); Eggers-Test P-value: 0.330.
Q-test P-value: 0.303; I2 (%): 18% (0.0–87.0); Eggers-Test P-value: 0.828.
Q-test P-value: 0.068; I2 (%): 58% (0.0–86.0); Eggers-Test P-value: 0.683.
Q-test P-value: 0.294; I2 (%): 16% (0.0–56.0); Eggers-Test P-value: 0.883.
Q-test P-value: 0.066; I2 (%): 45% (0.0–75.0); Eggers-Test P-value: 0.593.
The frequency of the GSTM1 homozygous deletion in the controls varied from 13 to 31.7%; the homozygous deletion of GSTT1 ranged from 22 to 40% (Table I). Mean age of the cases was 65.2 ± 8.7 years, of the controls 57.8 ± 10.1 years (P < 0.0001). For studies that provided prostate specific antigen levels (717 cases, 1343 controls), the mean value among cases was 118.2 ng/μl ± 548.6 SD (median: 9 ng/μl); for controls, it was 1.7 ng/μl ± 5.1 SD (median: 1.0 ng/μl; P < 0.0001; data not shown).
GSTM1 deletion and prostate cancer
Data on GSTM1 were available for 1308 cases and 1852 controls; there was an inverse association between GSTM1 deletion and prostate cancer in men of African ancestry overall (OR: 0.90; 95% CI: 0.83–0.97); such a reduced risk was observed in Caribbean and in African men but not in African-Americans (Table I). No significant heterogeneity across studies was observed in the analyses.
The analysis according to smoking status shows a significant inverse association between GSTM1 deletion and prostate cancer among never-smokers, with no statistical interaction between the genotype and ethnicity (OR: 0.87; 95% CI: 0.77–0.97); this result was even more evident among African-American men (adjusted OR: 0.66; 95% CI: 0.46–0.95), where a statistically significant positive association between GSTM1 deletion and prostate cancer among ever smokers was also observed (adjusted OR: 1.28; 95% CI: 1.01–1.56). There was statistical interaction between genotype and ethnicity among ever smokers (P = 0.05). These associations were not present in Caribbean or African subjects, where smoking seems not to influence the association between GSTM1 deletion and prostate cancer.
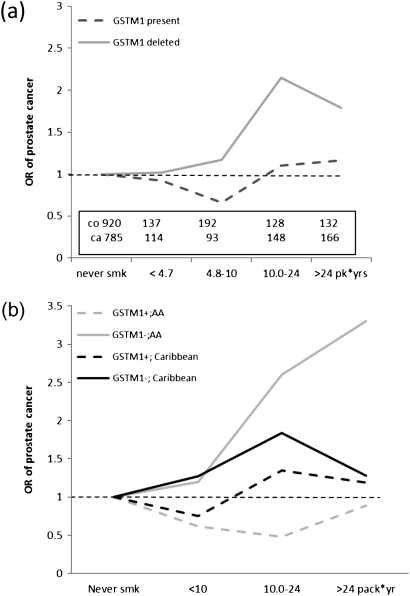
The analysis according to smoking dose (Table II) suggests that the association between GSTM1 deletion and prostate cancer does not significantly change across levels of pack-years of cigarette consumption. When the association between prostate cancer and pack-years of cigarettes smoked was stratified according to GSTM1 deletion (Figure 1a), the risk of prostate cancer increased across quartiles of pack-years among subjects with the deletion of GSTM1; however, this result was not observed among subjects carrying a functional GSTM1. The result was stronger in African-American men: compared with never-smokers, the risk of prostate cancer was 1.21 among men with GSTM1 deletion who smoked up to 10 pack-years, was 2.63 for smokers of 10–24 pack-years and 3.33 in men smoking over 25 pack-years. These results were not observed in Caribbean men (Figure 1b).
Table II.
Association of GSTM1, GSTT1 deletion and prostate cancer in populations of African descent according to smoking habits
| Controls (N) | Cases (N) | Adjusted ORa (95% CI) | |
| GSTM1 (null versus present) | |||
| Never-smokersb | 920 | 785 | 0.87 (0.77–0.97) |
| Ever smokersc | 817 | 769 | 1.03 (0.91–1.15) |
| Pack × years | |||
| ≤4.7 | 137 | 114 | 0.86 (0.63–1.18) |
| 4.8–10 | 142 | 93 | 1.19 (0.87–1.87) |
| 10–24 | 128 | 148 | 1.19 (0.89–1.58) |
| >24 | 132 | 166 | 1.02 (0.78–1.34) |
| African-American | |||
| Never-smokers | 95 | 90 | 0.66 (0.46–0.95) |
| Ever smokers | 223 | 243 | 1.28 (1.01–1.56) |
| Caribbean | |||
| Never-smokers | 786 | 668 | 0.89 (0.79–1.01) |
| Ever smokers | 960 | 498 | 0.94 (0.81–1.09) |
| African | |||
| Never-smokers | 33 | 21 | 0.56 (0.24–1.31) |
| Ever smokers | 27 | 23 | 0.50 (0.14–1.86) |
| GSTT1 (null versus present) | |||
| Never- Smokersd | 887 | 744 | 0.84 (0.75–0.94) |
| Ever smokerse | 810 | 764 | 0.91 (0.80–1.02) |
| Pack × years | |||
| ≤4.7 | 130 | 113 | 0.99 (0.74–1.33) |
| 4.8–10 | 132 | 87 | 0.89 (0.64–1.24) |
| 10–24 | 109 | 137 | 0.77 (0.56–1.05) |
| >24 | 104 | 135 | 1.30 (0.94–1.79) |
| African-American | |||
| Never-smokers | 68 | 56 | 0.99 (0.61–1.60) |
| Ever smokers | 159 | 181 | 0.83 (0.65–1.05) |
| Caribbean | |||
| Never-smokers | 786 | 668 | 0.84 (0.77–0.95) |
| Ever smokers | 556 | 498 | 0.93 (0.81–1.07) |
| African | |||
| Never-smokers | 33 | 19 | 0.58 (0.26–1.33) |
| Ever smokers | 27 | 23 | 0.77 (0.36–1.65) |
Adjusted for study and age (quartiles).
Interaction GSTM1 × ethnicity: P = 0.38.
Interaction GSTM1 × ethnicity: P = 0.05.
Interaction GSTT1 × ethnicity: P = 0.79.
Interaction GSTT1 × ethnicity: P = 0.38.
Fig. 1.
Association prostate cancer and smoking dose according to GSTM1 status, overall (a) and according to place of origin (b).
When the data were stratified by grade, there was no substantial difference in the association between prostate cancer and GSTM1 deletion between cases with a Gleason score <7 and cases with a score ≥7 (OR: 0.96, 95% CI: 0.86–1.09 and OR: 0.96, 95% CI: 0.82–1.12, respectively).
GSTT1 deletion and prostate cancer
GSTT1 was tested in 1609 cases and 1761 controls; GSTT1 deletion was significantly inversely associated with prostate cancer in men of African ancestry, overall and among Caribbean and African men (Table I) but not in African-American men. Statistical heterogeneity among studies was observed overall and in the stratified analyses according to ethnicity. The effect of GSTT1 deletion was equally observed in both smokers and non-smokers overall. No statistical interaction between genotype and ethnicity was observed. Among smokers, no dose–response was observed across pack-years of cigarette smoked (Table II). When the data were stratified by both ethnicity and smoking status, the significant reduction in risk associated with GSTT1 deletion and prostate cancer was restricted to never-smoker Caribbean men, although the CIs were very similar to those observed in smokers.
When the data were stratified by grade, the inverse association between prostate cancer and GSTT1 deletion was restricted to cases with a Gleason score <7 (OR: 0.87, 95% CI: 0.77–0.98) and not observed among cases with a score ≥7 (OR: 0.98, 95% CI: 0.83–1.14). The estimates however are very similar and the CIs partially overlapping.
The analysis of the combined GSTM1 and GSTT1 deletion suggests that GSTM1 deletion is the main genotype responsible for the observed reduction in prostate cancer risk (data not shown).
Discussion
This study reports for the first time a significant inverse association between GSTM1 and GSTT1 homozygous deletions and prostate cancer in men of African descent. The literature on genetic susceptibility to prostate cancer in men of African descent is limited. Two meta-analyses including white and Asian men (39,40) suggest that GSTM1 deletion is associated with increased risk of prostate cancer; however, the limited number of individual studies conducted in men of African ancestry seem to indicate an association in the opposite direction (28) or no association (29,32) in this ethnic group. This large multi-institutional study confirms the inverse association for GSTM1 deletion and in addition indicates a reduced risk of prostate cancer associated with GSTT1 deletion. This result points to a role of functional GSTM1 and GSTT1 in activating compounds that could act as prostate carcinogens, thus suggests a paradoxical inverse effect of the loss of GST function on prostate cancer risk. Such compounds could derive from exposures to endogenous hormones or exogenous toxins (for example alogenate compounds or diet) that are specific to certain geographic areas.
Another unique result of this analysis is the finding of an association between GSTM1 deletion and prostate cancer in African-American men who smoke but not in Caribbean or African smokers. Prostate cancer risk increases with increasing pack-years of smoke in a dose-dependent fashion in African-American men homozygous for the GSTM1 deletion in comparison with men with a functional GSTM1, although some of the CIs were very wide probably because of the small number of subjects in some of the categories. This effect however was not observed in groups from the Caribbean and Africa. A possible gene–environment interaction between the GSTs and smoking habits has been suggested by studies conducted in White prostate cancer cases (21,29) but has not been explored in men of African ancestry. Cigarette smoking contains many substrates for GSTM1; therefore, it is expected that a loss of GSTM1 function will affect the accumulation of genotoxic compounds from tobacco smoke. This assumption is further supported by the observation that GSTM1 deletion is associated with an increase in benzo(a)-pyrene DNA adducts from tobacco smoke (11).
The observed difference in the association with ethnicity may point to differences in lifetime exposure to other carcinogens that saturate the GST system, which therefore becomes less available for tobacco metabolism. Other possible explanations are the lower smoking dose to which Caribbean and African men are exposed in comparison with African-American men. In fact, in our study, the average pack-years of smoking in the latter group was 27.2 in comparison with 17.4 in Caribbean men. Such differences make some of the categories of the subgroup analysis very small, thus with limited statistical power. In addition, the composition of cigarettes is probably to vary across geographic areas. Furthermore, it is possible that other risk factors, such as chronic prostate viral infection (41), may be implicated in prostate cancer risk in the Caribbean men, whereas such factors may be less relevant in African-American men, where smoking may have a more predominant role. It is also possible that some of the differences observed across ethnicities reflect differences in clinical characteristics of the cases, which may constitute a mix of different disease aggressiveness features; although our data do not seem to suggest a difference in the association between GSTs and prostate cancer according to the Gleason score, the possibility of case mixing cannot be completely ruled out since information on tumor stage is lacking for most of the patients. In addition, several of the countries where the data were collected do not offer formal prostate cancer screenings; therefore, the chances of later diagnoses is very high in comparison with the USA. Prostate specific antigen values are highly correlated with the Gleason score in our data, underlying the fact that prostate specific antigen is used as confirmatory clinical test rather than as a periodic screening test in most of the cases.
Despite the large sample size, the study had several limitations: the number of African-American and African cases is still too limited for more detailed analyses of gene–environment interaction across different doses of tobacco smoke; information on other exposures that could be relevant for prostate cancer in addition to tobacco smoke are not available in this population; the lack of ability to rule out confounding for the smoking analysis, or to adjust for other risk factors due to the limited number of standardized, comparable variables available across the various data sets is a limitation that could not be overcome and that could have biased the results in an unpredictable direction. Information on genetic admixture within the more general category of African ancestry origin is also not available. The large variability in the frequency of both GSTM1 and GSTT1 deletion suggests a high degree of admixture of the population under study. How genetic admixture could have influenced the results is hard to predict from the information available in the present study. It can be hypothesized, for example, that the higher degree of genetic admixture present in African-American in comparison with Caribbean men could have introduced some genetic predisposition to prostate cancer that could be transmitted, by chance, along with the GSTM1 deletion, thus confounding the association with prostate cancer. The African population is pivotal for elucidating this possibility but unfortunately is not represented in a sufficiently large number in the present study.
The polymerase chain reaction method used by the studies included here cannot distinguish between subjects with two GSTM1 functional alleles versus those who carry only one functional allele. Although only the complete deletion of the gene causes loss of function, it has been suggested that the presence of one functional allele may induce modest but relevant changes of function that should be considered in association studies (42).
In conclusion, this large multi-institutional effort reports an inverse association for both GSTM1 and GSTT1 deletions in relation to prostate cancer risk in men of African descent and suggests that smoking may have a role in prostate cancer risk in African-American men who carry certain gene susceptibility variants involved in tobacco metabolism; Caribbean and African men are exposed to lower doses of tobacco smoke, where the activity of GSTs may be less relevant; they may also be exposed to other exogenous and/or endogenous carcinogens that activate different genetic metabolic pathways. Information on such exposures was not collected and analyzed in the present study but deserves attention in future research efforts. Chronic inflammation, hormone levels, bacterial and viral infections and environmental chemicals (43) are some areas to concentrate future studies of gene–environment interaction in the etiology of prostate cancer in populations of African ancestry.
Funding
National Institutes of Health (R01-CA085074 and P50-CA105641 to T.R.R., R01-CA056678 and R01-CA092579 to J.L.S.); Department of Defense (PC094423 to C.R.R. and E.T.).
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AC3
African-Caribbean Cancer Consortium
- CI
confidence interval
- GSEC
Genetic Susceptibility to Environmental Carcinogens study
- GST
glutathione-S-transferase
- OR
odds ratio
References
- 1.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, et al. Bethesda, MD: National Cancer Institute; 2010. SEER Cancer Statistics Review, (1975-2007) [Google Scholar]
- 3.Bunker CH, et al. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol. Biomarkers Prev. 2002;11:726–729. [PubMed] [Google Scholar]
- 4.Chokunonga E, et al. Cancer incidence in the African population of Harare, Zimbabwe: second results from the cancer registry 1993-1995. Int. J. Cancer. 2000;85:54–59. doi: 10.1002/(sici)1097-0215(20000101)85:1<54::aid-ijc10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Ravery V, et al. Prostate cancer characteristics in a multiracial community. Eur. Urol. 2008;53:533–538. doi: 10.1016/j.eururo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shlomo Y, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur. Urol. 2008;53:99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Mallick S, et al. Prostate cancer incidence in Guadeloupe, a French Caribbean archipelago. Eur. Urol. 2005;47:769–772. doi: 10.1016/j.eururo.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Detchokul S, et al. Recent developments in prostate cancer biomarker research: therapeutic implications. Br. J. Clin. Pharmacol. 2011;71:157–174. doi: 10.1111/j.1365-2125.2010.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft D, et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39:D691–D697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin SS, et al. A review of genetic polymorphisms and prostate cancer risk. Ann. Epidemiol. 2002;12:182–196. doi: 10.1016/s1047-2797(01)00310-6. [DOI] [PubMed] [Google Scholar]
- 11.Sundberg K, et al. Glutathione conjugation and DNA adduct formation of dibenzo[a, l]pyrene and benzo[a]pyrene diol epoxides in V79 cells stably expressing different human glutathione transferases. Chem. Res. Toxicol. 2002;15:170–179. doi: 10.1021/tx015546t. [DOI] [PubMed] [Google Scholar]
- 12.Hayes JD, et al. Glutathione transferases. Ann. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson JT, et al. Detoxication enzymes and chemoprevention. Proc. Soc. Exp. Biol. Med. 1997;216:192–200. doi: 10.3181/00379727-216-44169. [DOI] [PubMed] [Google Scholar]
- 14.Ragin CC, et al. Review of studies on metabolic genes and cancer in populations of African descent. Genet. Med. 2010;12:12–18. doi: 10.1097/GIM.0b013e3181c8e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd LC, et al. Polymorphisms in glutathione-S-transferase genes (GST-M1, GST-T1 and GST-P1) and susceptibility to prostate cancer among male smokers of the ATBC cancer prevention study. Eur. J. Cancer Prev. 2003;12:317–320. doi: 10.1097/00008469-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Quinones LA, et al. Joint effect among p.53, CYP1A1, GSTM1 polymorphism combinations and smoking on prostate cancer risk: an exploratory genotype-environment interaction study. Asian J. Androl. 2006;8:349–355. doi: 10.1111/j.1745-7262.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 17.Mao GE, et al. Glutathione S-transferase P1 Ile105Val polymorphism, cigarette smoking and prostate cancer. Cancer Detect. Prev. 2004;28:368–374. doi: 10.1016/j.cdp.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Ning B, et al. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. Pharmacogenetics. 2004;14:35–44. doi: 10.1097/00008571-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Beer TM, et al. Polymorphisms of GSTP1 and related genes and prostate cancer risk. Prostate Cancer Prostatic Dis. 2002;5:22–27. doi: 10.1038/sj.pcan.4500549. [DOI] [PubMed] [Google Scholar]
- 20.Debes JD, et al. Gluthatione-S-transferase P1 polymorphism I105V in familial and sporadic prostate cancer. Cancer Genet. Cytogenet. 2004;155:82–86. doi: 10.1016/j.cancergencyto.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Nock NL, et al. Associations between smoking, polymorphisms in polycyclic aromatic hydrocarbon (PAH) metabolism and conjugation genes and PAH-DNA adducts in prostate tumors differ by race. Cancer Epidemiol. Biomarkers Prev. 2007;16:1236–1245. doi: 10.1158/1055-9965.EPI-06-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybicki BA, et al. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect. Prev. 2006;30:412–422. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agalliu I, et al. Polymorphisms in the glutathione S-transferase M1, T1, and P1 genes and prostate cancer prognosis. Prostate. 2006;66:1535–1541. doi: 10.1002/pros.20491. [DOI] [PubMed] [Google Scholar]
- 24.Woodson K, et al. A survey of gene-specific methylation in human prostate cancer among black and white men. Cancer Lett. 2004;205:181–188. doi: 10.1016/j.canlet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Woodson K, et al. Hypermethylation of GSTP1, CD44, and E-cadherin genes in prostate cancer among US Blacks and Whites. Prostate. 2003;55:199–205. doi: 10.1002/pros.10236. [DOI] [PubMed] [Google Scholar]
- 26.Garte S. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol. Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- 27.Cunningham JM, et al. Evaluation of genetic variations in the androgen and estrogen metabolic pathways as risk factors for sporadic and familial prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:969–978. doi: 10.1158/1055-9965.EPI-06-0767. [DOI] [PubMed] [Google Scholar]
- 28.Mallick S, et al. GSTM1 and GSTT1 polymorphisms and the risk of prostate cancer in a Caribbean population of African descent. Urology. 2007;69:1165–1169. doi: 10.1016/j.urology.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Agalliu I, et al. Glutathione S-transferase M1, T1, and P1 polymorphisms and prostate cancer risk in middle-aged men. Prostate. 2006;66:146–156. doi: 10.1002/pros.20305. [DOI] [PubMed] [Google Scholar]
- 30.Kelada SN, et al. The glutathione S-transferase-mu and -theta genotypes in the etiology of prostate cancer: genotype-environment interactions with smoking. Cancer Epidemiol. Biomarkers Prev. 2000;9:1329–1334. [PubMed] [Google Scholar]
- 31.Rebbeck TR, et al. Glutathione S-transferase-mu (GSTM1) and -theta (GSTT1) genotypes in the etiology of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 1999;8:283–287. [PubMed] [Google Scholar]
- 32.Lavender NA, et al. Examination of polymorphic glutathione S-transferase (GST) genes, tobacco smoking and prostate cancer risk among men of African descent: a case-control study. BMC Cancer. 2009;9:397. doi: 10.1186/1471-2407-9-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragin CC, et al. African-Caribbean cancer consortium for the study of viral, genetic and environmental cancer risk factors. Infect. Agent Cancer. 2007;2:17. doi: 10.1186/1750-9378-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taioli E. International collaborative study on genetic susceptibility to environmental carcinogens. Cancer Epidemiol. Biomarkers Prev. 1999;8:727–728. [PubMed] [Google Scholar]
- 35.Sutton AJ, et al. Chichester, UK: John Wiley and Sons Ltd; 2004. Methods for Meta-Analysis in Medical Research. [Google Scholar]
- 36.Higgins JP, et al. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Ioannidis JP, et al. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ntais C, et al. Association of GSTM1, GSTT1, and GSTP1 gene polymorphisms with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2005;14:176–181. [PubMed] [Google Scholar]
- 40.Mo Z, et al. An updating meta-analysis of the GSTM1, GSTT1, and GSTP1 polymorphisms and prostate cancer: a HuGE review. Prostate. 2009;69:662–688. doi: 10.1002/pros.20907. [DOI] [PubMed] [Google Scholar]
- 41.Taylor ML, et al. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam. Med. 2005;37:506–512. [PubMed] [Google Scholar]
- 42.Norskov MS, et al. Copy number variation in glutathione-S-transferase T1 and M1 predicts incidence and 5-year survival from prostate and bladder cancer, and incidence of corpus uteri cancer in the general population. Pharmacogenomics J. 2010 doi: 10.1038/tpj.2010.38. Jun 1. PMID: 2051407. [DOI] [PubMed] [Google Scholar]
- 43.Multigner L, et al. Chlordecone exposure and risk of prostate cancer. J. Clin. Oncol. 2010;28:3457–3462. doi: 10.1200/JCO.2009.27.2153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.