Abstract
The High-Risk Plaque (HRP) Initiative is a research and development effort to advance the understanding, recognition, and management of asymptomatic individuals at risk for a near-term atherothrombotic event such as myocardial infarction or stroke. Clinical studies using the newest technologies have been initiated, including the BioImage Study in which novel approaches are tested in a typical health plan population. Asymptomatic at-risk individuals were enrolled, including a survey-only group (n = 865), a group undergoing traditional risk factor scoring (n = 718), and a group in which all were assessed for both risk factors and subclinical atherosclerosis (n = 6104). The latter two groups underwent baseline examination in a dedicated mobile facility equipped with advanced imaging tools suitable for noninvasive screening for subclinical atherosclerosis (coronary artery calcium by computed tomography [CT], carotid and aortic disease by ultrasound, and ankle-brachial index). Selected participants were offered advanced imaging (contrast-enhanced CT, magnetic resonance imaging, and positron emission tomography/CT). Plasma, PAXgene RNA, and DNA samples were obtained for biomarker discovery studies. All individuals will be followed until 600 major atherothrombotic events have occurred in those undergoing imaging.
Keywords: Primary prevention, Risk assessment, Cardiovascular disease, Atherosclerosis, High-risk plaque, Vulnerable plaque, Imaging, Biomarkers, BioImage study
Introduction
Heart attack and large artery stroke are usually caused by acute thrombosis precipitated by an underlying high-risk (vulnerable) atherosclerotic plaque [1–4]. The High-Risk Plaque (HRP) Initiative is a joint research and development effort to advance our understanding, recognition, and management of HRP, or rather individuals with HRP [5]. The overarching goal of the HRP Initiative is to find the asymptomatic adults who are at high risk of a near-term atherothrombotic event withhout knowing it and offer timely and adequate preventive care for the benefit of the individual and the society.
Atherothrombotic cardiovascular disease (CVD) is a leading cause of death and disability not only in affluent countries but globally and, as such, has a large economic and public health impact [6, 7]. The mortality of atherothrombotic CVD has fallen dramatically in the past decades, and the prolonged survival with chronic disease explains why the prevalence, burden, and costs of the disease remain high [8•, 9••]. The only effective approach to limit this undue loss of health and resources is to prevent the disease from developing in the first place (ie, primary prevention). Public health initiatives are important [10], but so is personalized prevention for those at highest risk.
Causal and modifiable risk factors for atherothrombotic CVD are known and constitute important therapeutic targets [11], but their ability to predict is limited [12]. Most heart attacks and strokes occur in individuals who would be classified as low or intermediate risk by the traditional risk factor–based approaches recommended in the United States (Framingham Risk Score [13, 14]) and Europe (SCORE [15]) [16, 17, 18•]. Conversely, many individuals with an apparently adverse risk factor profile remain asymptomatic. Thus, for contemporary medicine to improve, the challenge is to find and treat those unrecognized individuals at highest risk without harming those who do not need treatment (ie, limiting both undertreatment and overtreatment).
Novel circulating biomarkers may provide incremental prognostic information [19, 20], but their clinical utility remains to be established [21, 22••]. Despite great promise, genetic testing for “susceptibility” has not yet proven useful for risk stratification in clinical practice [22••, 23].
An alternative strategy of potentially greater impact would be assessment of subclinical (asymptomatic) atherosclerosis as proposed in recent guidelines [22••] and, in particular, detection of disease activity and plaques of the high-risk “vulnerable” type [24–27]. Such lesions are most often hidden in the arterial wall and not diagnosed until it is too late. In recent years, studies using advanced imaging technologies have provided insights into atherosclerotic plaque development and its progression to vulnerable plaque and rupture as a cause for acute atherothrombosis leading to myocardial infarction or stroke [28–31, 32••]. These insights are providing impetus for the discovery and development of novel screening and diagnostic tools and potential therapies.
Leading companies and recognized scientists in the field of cardiovascular disease, radiology, and other medical disciplines have come together to collectively design, fund, and execute a number of important studies to discover and validate methods and tests that will find individuals with HRP [5]. The goal of this effort, known as the HRP Initiative, is to identify and validate novel imaging and blood biomarkers of asymptomatic but high-risk atherosclerosis that could be used to spur the development of in vitro diagnostic tests, medical imaging devices, and therapeutic products for the more effective identification, assessment, and treatment of patients at high near-term risk of a major atherothrombotic event.
The HRP Initiative has initiated a number of important research studies that use the newest technologies, including the BioImage Study. In just 18 months, the BioImage Study completed enrollment of 7,687 participants in June 2009 using a novel and exciting approach for conducting critical medical research. The BioImage Study brought the study to the people, rather than using the traditional opposite approach.
BioImage Study
The BioImage Study (NCT00738725) is a prospective, observational study designed to evaluate associations among imaging and circulating biomarkers (cross-sectional) and their ability to predict atherothrombotic events (longitudinal) in asymptomatic at-risk subjects in the primary prevention of myocardial infarction and stroke [33]. In collaboration with Humana, a large US health benefits company, the BioImage Study used a unique and innovative approach to identify and recruit a typical at-risk population (Fig. 1). In contrast to other large-scale epidemiologic studies that used either a network of academic research centers or a well-defined population in a limited geographic area, the BioImage Study has recruited subjects from two geographic locations intended to represent the US population at large. In each location, we established a dedicated temporary research facility with mobile imaging equipment. The mobile equipment and dedicated staff moved from one location to the next, facilitating consistency of image acquisition.
Fig. 1.
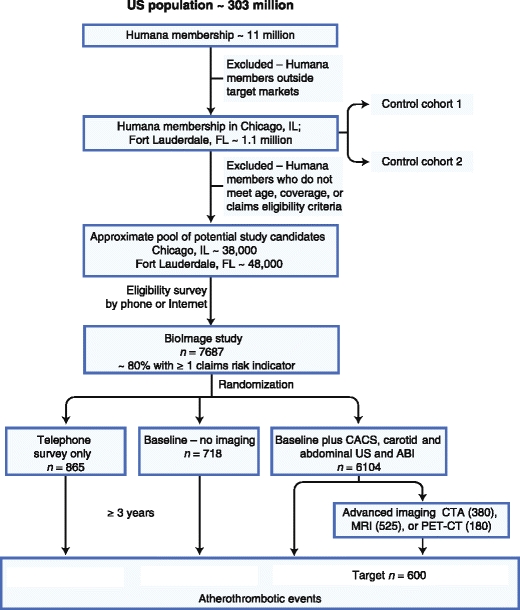
Flowchart for the BioImage Study. ABI ankle-brachial index; CACS coronary artery calcium score; CTA computed tomography angiography; MRI magnetic resonance imaging; PET-CT positron emission tomography–computed tomography; US ultrasound
As previously highlighted [34], age and gender are the strongest determinants of cardiovascular risk. Hence, in designing the BioImage Study, we considered all subjects over a certain gender-specific age without known cardiovascular disease as potential candidates. We included currently available noninvasive modalities for vascular imaging, such as ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). Certain modalities and examinations were limited to certain subsets, whereas others were used on the overall study population.
The BioImage Study and method of selection and recruitment were approved by the Western Institutional Review Board, Olympia, WA. All study participants provided written informed consent and Health Insurance Portability and Accountability Act authorization before enrollment.
Study Objectives
The primary objective of the BioImage Study is to identify imaging biomarkers that predict near-term (3-year) atherothrombotic events, with incremental improvement over traditional risk assessment (Framingham Risk Score). The secondary objectives of the study are to 1) correlate imaging data of subclinical atherosclerosis and blood-based markers, 2) provide standardized high-quality image sets for the development and refinement of quantitative digital image analysis, 3) provide standardized high-quality image sets for the optimization of image protocols and correction algorithms, 4) compare event rates in patients randomized to undergo imaging studies with those in patients who do not undergo such studies, 5) collect and store biological specimens for future evaluation, 6) postulate a novel screening and diagnostic pathway based on the discovered new risk markers, and 7) acquire pertinent data for the development of an economic and health impact model in the primary prevention of CVD. Another objective of the BioImage Study is to assess possible lifestyle changes induced by study procedures and knowledge of the presence of subclinical disease.
Targeted at-Risk Population
We wanted to enrich the study population with individuals with a meaningful probability of developing events in the near term. To do so, we identified members in the Humana database who were 55–80 years of age (men > 55 years and women > 60 years), as this is the group where most cardiovascular events occur and where many were expected to have at least one additional risk factor [34]. Study participants were recruited among members of the Humana Health Plan (> 11 million members nationwide) with a male to female ratio of 1:1 and a racial/ethnic distribution corresponding to US Census data (approximately 69% white, 12% African American, 13% Hispanic, 4% Asian, and 2% other) [35]. To ensure a diverse study population, enrollment occurred in two cities: Chicago, IL and Fort Lauderdale, FL.
Recruitment Population Stratified by Claims
Preliminary observations indicate it is possible to risk-stratify Humana members using information on file, including medical and pharmacy health insurance claims [33]. Candidates had to be free of claims-based evidence of prior major cardiovascular disease, active cancer treatment, or certain other indicators of major intercurrent disease. We used medical and pharmacy claims to create risk indicators of established cardiovascular risk, such as medical claims or prescription medication use for hypertension, diabetes, or hyperlipidemia. One of the limitations of claims-based risk assessment is that certain risk factors, such as smoking and physical inactivity, cannot be determined or inferred from claims. The most common claims-based risk indicators were hypertension (65%) and hyperlipidemia (57%). The majority (57%) had two or more claims-based risk indicators.
We used medical claims for acute myocardial infarction and stroke to identify putative major cardiovascular events. The relationships between claims-based risk indicators and claims-based event rates are significant [33]. With the exception of hyperlipidemia, the presence or absence of specific claims-based risk indicators is also strongly related with claims-based event rates. Thus, despite the limitations of claims data, the concept of using claims-based risk indicators to estimate the average risk of a study population appears to be valid in individuals in the age range of 55–80 years without prior history of CVD.
Study Population
Potential study participants were contacted by phone and interviewed to evaluate eligibility, obtain verbal informed consent and authorization, and obtain selected personal and health information. Of those meeting the entrance criteria, approximately 750 were randomly selected to set aside as “survey-only group” (no risk assessment). All other individuals meeting the entrance criteria were invited to undergo a baseline examination in a dedicated mobile facility. Those who agreed to participate were randomized into a Framingham-only group (traditional risk factor scoring; target n = 750) and the full study group in which all underwent testing for subclinical atherosclorosis (risk factor scoring plus testing for subclinical atherosclerosis; target n = 6000). Among those in whom substantial arterial disease was detected, selected participants were offered advanced imaging for subclinical atherosclerosis (see Fig. 1).
As part of the informed consent procedure, participants granted authorization and consent to access their medical/pharmacy claims and related financial information. Pharmacy claims will be used to construct the medication history. In addition, the participants granted consent and authorization to verify outcomes on the basis of medical chart review at the facility where such care was provided. At completion of enrollment in June of 2009, a total of 24,149 Humana members had been surveyed by telephone or mail, of which 9866 met the eligibility criteria for the study and agreed to participate. Of this population, 7687 subjects completed enrollment, of which 865 were enrolled as “survey-only” participants, 718 were enrolled in the Framingham-only group, and 6104 were enrolled in the full study group (see Fig. 1).
Generalization of Study Results
As previously described in detail [33], preliminary data from the Chicago location indicate that the BioImage Study population is representative of the eligible Chicago population with respect to age, gender, business line (commercial vs Medicare), median household income, prevalence of risk factors, clinical conditions, comorbidities, and prescription drug use as determined based on medical or pharmacy claims.
Baseline Examination
The examination approach was also novel and proactive, bringing the necessary research facilities, experienced staff, and advanced imaging equipments to the study participants rather than the opposite. The mobile facility consisted of two dedicated trailers, one containing a 64-slice CT scanner and the other a 3.0 T MRI scanner. In addition, a fixed building was used for the other baseline study procedures, including blood sampling, US imaging, and ABI measurement.
All participants underwent measurement of customary physical parameters, including height, weight, waist to hip ratio, brachial blood pressure, ankle blood pressure, and a 12-lead electrocardiogram. A venous blood sample was processed for the collection of plasma, serum, RNA, and DNA (stored for later biomarker discovery studies) and routine chemistry tests (Table 1).
Table 1.
Baseline characteristics of participants in selected population studies
| Characteristic | BioImage (n = 7687) | MESA (n = 6814) | ARIC (n = 15792) | CHS (n = 5201) | Framingham Heart Study | ||
|---|---|---|---|---|---|---|---|
| Original (n = 5209) | Offspring (n = 5124) | 3rd Generation (n = 4095) | |||||
| Representative cohort | Imaging subset (n = 6101) | Subset (n = 6526) | Subset (n = 13145) | Subset (n = 4536) | Total (n = 5209) | Total (n = 5124) | Total (n = 4094) |
| Age, y | 68.8 (6.0) | 62 (10) | 54 (5.8) | 72.8 | 44 (9) | 36.5 (10.5) | 40 (9) |
| Sex, women,% | 56.4 | 53 | 56.8 | 56.7 | 55.2 | 51.5 | 53.3 |
| Race,% | |||||||
| White | 74 | 39 | 74.8 | 100 | |||
| Asian | 2 | 12 | |||||
| Black | 15.3 | 27 | 25.2 | ||||
| Hispanic | 6.1 | 22 | |||||
| Other | 2.6 | ||||||
| BMI, kg/m 2 | 29.1 (5.6) | 28.3 (5) | 27.36 (5.1) | 26.4 (4.5) | 25.6 (4.2) | 25.2 (4.2) | 26.9 (5.4) |
| Systolic blood pressure, mm Hg | 139.5 (18.5) | 127 (22) | 120.72 (18.6) | 135 (21) | 135.4 (21.8) | 121.9 (16) | 116.7 (13.5) |
| Diastolic blood pressure, mm Hg | 78.2 (9.1) | 72 (10) | 73.46 (11.2) | 70 (11) | 84.9 (12.6) | 78.9 (10.5) | 75.3 (9) |
| Diabetes mellitus,% | 15.6 | 14 | 10.1 | 14.5 | |||
| Current smoker,% | 8.5 | 13 | 26.1 | 10.9 | 57.6 | 44.5 | 17.4 |
| Total cholesterol, mg/dL | 202.5 (38.7) | 194.1 (35.3) | 214 (41.4) | 212 (40) | 221 (44.7) | 196.4 (39.5) | 188.7 (35.4) |
| LDL, mg/dL | 114.1 (33.3) | 117 (32) | 136.8 (39.0) | 130 (36) | |||
| HDL, mg/dL | 55.6 (15.3) | 51 (15) | 52.6 (17.1) | 54 (16) | 50.2 (13.5) | 54.5 (14.1) | |
| Study period | 2008–2009 | 2000–2002 | 1987–1989 | 1989–1990 | 1948–1953 | 1971–1975 | 2002–2005 |
| References | [39, 40] | [41] | [42] | [43] | [43] | [43] | |
Data shown as mean (standard deviation) or prevalence %.
ARIC Atherosclerosis Risk In Communities; BMI body mass index; CHS Cardiovascular Health Study; HDL high-density lipoprotein; LDL low-density lipoprotein; MESA Multi-Ethnic Study of Atherosclerosis
The full study (imaging) group was also screened for subclinical atherosclerosis, including assessment of coronary artery calcification score (CACS) by CT, measurement of carotid intima-media thickness (IMT), presence of carotid atherosclerotic plaques and abdominal aortic aneurysm (AAA) by US, and ankle brachial index (ABI) (see Fig. 1). Subjects who meet one or more predefined criteria for CACS, carotid IMT, presence of carotid plaque and AAA, or ABI were offered advanced imaging for subclinical atherosclerosis [33]. The choice of advanced imaging method was based on logistical considerations, patient preference, and presence/absence of certain clinical criteria that would render a particular method unsuitable or contraindicated. The advanced imaging methods included contrast-enhanced or noncontrast MRI for carotid and aortic plaques, contrast-enhanced coronary CT angiography for noncalcified plaques and stenoses, and 18 F-fluorodeoxyglucose PET/CT for carotid and aortic inflammation.
Notification of Baseline Findings
Study participants were informed that these study-procedures were conducted for research purposes only and that only those obvious findings requiring immediate medical attention would be reported back to the participant and their physicians. At the conclusion of the baseline visit, participants were provided with a summary of selected findings, including blood pressure, ABI, presence of carotid artery stenosis, and if the CACS exceeded the 75% percentile. A letter was mailed to the participant and their primary care physician with the routine blood chemistry results. No specific interventions were offered or recommended.
Surveillance and Event Detection
The study population will be followed until 600 major atherothrombotic events have occurred in the imaging group. These primary endpoints for the study include fatal and nonfatal myocardial infarction, coronary death, hospitalization for unstable angina (angina at rest with documented electrocardiographic changes), ischemic stroke (fatal and nonfatal), and arterial revascularization procedures (either percutaneous or surgical). Procedure-related complications are excluded. Potential cardiovascular events will be identified by querying the Humana claims and member databases for predefined event triggers at regular intervals throughout the follow-up period. The claims monitoring for event triggers will be conducted using Diagnosis-Related Groups (DRG), International Classification of Diseases (ICD)-9, and Current Procedure Terminology (CPT) codes for myocardial infarction, cerebrovascular events, unstable angina, peripheral vascular disease, revascularization procedures, and all deaths. Use of claims information for these conditions has previously been validated in numerous peer-reviewed studies comparing administrative claims information with clinical information obtained through chart reviews [36, 37]. Any study participants leaving the Humana health plan before the end of the follow-up period will be followed by telephone contact only. The study participants randomized to the “survey-only” group and the Humana members included in the two control cohorts will also be followed through the evaluation of claims and member data, as described above.
Discussion
More than 200 million people in the United States belong to some form of health insurance plan. The individual’s personal information and most health care and pharmacy transactions are known to the plan to adjudicate and pay the claims. Although the potential for doing studies within the health care system has long been recognized, few examples exist where the health care and research chasms have been successfully bridged, allowing health plans to play a critical role in addressing an important medical research question.
Study Advantages
The BioImage Study recruited volunteer participants from Humana’s health plan members, linking their research data with their broader health care data and then prospectively using this information to monitor for putative events. This represents a unique and novel approach that affords many advantages.
Scale and Pace
The study’s unique mobility combined with capabilities to quickly identify and recruit large numbers of eligible people made it possible for the BioImage Study to recruit and enroll faster than conventional studies. Dedicated, temporary, and mobile research facilities enabled the use of multiple advanced imaging modalities within a single convenient visit for the participants, and also allowed for the use of a few highly trained and dedicated technicians, avoiding operator or equipment variability.
Generalization
Geocoding the data using socioeconomic and ethnic characteristics from the US Census allowed us to enroll people representative of the US population. The BioImage Study involves a single baseline set of imaging, clinical, and biological measurements in a group of US participants with mostly moderate or high risk. Therefore, conclusions reached in this study will apply to relatively healthy elderly individuals, generally with above-average cardiovascular risk.
Minimizing Recruitment Bias
Possessing data on the entire recruitment population allows us to identify any selection bias in the enrolled population. Control cohorts unrelated to the BioImage Study can help evaluate any bias related to selection criteria, claims-based risk stratification, and recruitment process or event rate markers.
Understanding Economic Impact
We have the data needed to analyze the impact of primary outcomes as well as any influence of the study itself on utilization patterns of participants.
Other Benefits
One of the benefits of this approach is the ability to judge how the participants compare with nonparticipants, including those who were not invited or those who could not be reached or declined to participate. The BioImage Study will be able to answer questions about selection bias as well as whether the study induced health care changes. In particular, the risks of selection bias or study-induced provider or patient behavioral changes are important factors to consider. As an example, the preliminary results from the Chicago location regarding risk factor distribution demonstrate a significant difference in the proportions of individuals with zero risk factors between study participants and eligible subjects. This may indicate some selection bias, presumably due to those volunteering being more concerned about their health status than other similar individuals from the general population.
It is difficult to overstate the importance of finding asymptomatic individuals at near-term risk for atherothrombosis. It is the challenging purpose of the HRP Initiative. A simple and effective screening test for subclinical atherothrombosis could enable effective primary prevention in the months or years before the first event and has a potential impact on life expectancy that seems unattainable for any other major medical condition. It is estimated that the impact on life expectancy of such a test to enable preventive treatment would be comparable to the eradication of all forms of cancer [38]. Only recognition and treatment of at-risk atherosclerosis, not only risk factor assessment, offer the potential to reduce cardiovascular morbidity and mortality from its current level.
Limitations
One limitation of the BioImage Study is that only individuals with health benefit coverage with Humana were eligible for enrollment and, consequently, no uninsured individuals were enrolled. When we compared the household incomes of the insured participants from the Chicago area, it mirrored those for the state as a whole (insured and noninsured individuals). However, the uninsured individuals, independent of socioeconomic characteristics, have multiple disadvantages that in themselves are associated with poor health; hence, the findings of this study may not be applicable to uninsured individuals. Another limitation is the absence of traditional physician follow-up and reliance on administrative data to identify primary outcomes. However, and as mentioned above, claims-based outcomes have been extensively validated. Finally, we could not randomize people for the advanced imaging studies; and personal preference (eg, dislike for tight spaces, concerns about administration of contrast agents) or personal health concerns (eg, desire to know as much as possible) could have biased participation.
Conclusions
The BioImage Study represents a new model that brings important medical research to a health plan population and leverages the health plan data and systems to the benefit of research. This research model is broadly applicable to important medical questions that require study of a large population representing the population-at-large. The BioImage Study has the potential to materially contribute to our understanding of who is at risk for near-term atherothrombosis and enable a new paradigm of early disease recognition and treatment to augment what is potentially achievable through risk factor reduction.
Acknowledgment
We wish to thank Sanaz Rasouli for help with Table 1.
Disclosures
Valentin Fuster (chair), Erling Falk (co-chair), and Henrik Sillesen are members of the Scientific Program Board of the BioImage Study. Pieter Muntendam is an employee of BG Medicine and owns stock options and shares in BG Medicine.
Funding sources
The HRP Initiative is a precompetitive industry collaboration funded by BG Medicine, Abbott, AstraZeneca, Merck, Philips, and Takeda.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Erling Falk, Email: erling.falk@ki.au.dk.
Henrik Sillesen, Email: sillesen@mac.com.
Pieter Muntendam, Email: PMuntendam@bg-medicine.com.
Valentin Fuster, Email: valentin.fuster@mssm.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Fuster V, Lois F, Franco M. Early identification of atherosclerotic disease by noninvasive imaging. Nat Rev Cardiol. 2010;7:327–333. doi: 10.1038/nrcardio.2010.54. [DOI] [PubMed] [Google Scholar]
- 2.Falk E, Fuster V. Atherothrombosis - Disease burden, activity, and vulnerability. In: Fuster V, Walsh RA, Harrington RA, editors. Chapter 52 in Hurst's the Heart. 13. New York: McGraw-Hill Medical; 2011. pp. 1215–1223. [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.Schaar JA, Muller JE, Falk E, et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. 2004;25:1077–1082. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.The High-Risk Plaque Initiative. Available at www.hrpinitiative.com. Accessed April 2011.
- 6.Roger VL, Go AS, Lloyd-Jones DM, et al. American heart association statistics committee and stroke statistics subcommittee: heart disease and stroke statistics - 2011 update: a report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A race against time: the challenge of cardiovascular disease in developing economies. Columbia University, New York, 2004. Available at www.earth.columbia.edu/news/2004/images/raceagainsttime_FINAL_051104.pdf. Accessed April 2011.
- 8.Fuster V, Mearns BM. The CVD paradox - mortality vs prevalence. Nat Rev Cardiol. 2009;6:669. doi: 10.1038/nrcardio.2009.187. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement From the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 10.Capewell S, O'Flaherty M: Rapid mortality falls after risk-factor changes in populations. Lancet. 2011 Mar 15. [Epub ahead of print]. [DOI] [PubMed]
- 11.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 12.Ware JH. The limitations of risk factors as prognostic tools. N Engl J Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 13.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation 2002;106:3143-421. Available at: www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf Accessed April 2011. [PubMed]
- 14.Grundy SM, Cleeman JI, Merz CN, et al.: Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227-39. Available at: www.nhlbi.nih.gov/guidelines/cholesterol/atp3upd04.pdf Accessed April 2011. [DOI] [PubMed]
- 15.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1–113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 16.Wald NJ, Morris JK, Rish S. The efficacy of combining several risk factors as a screening test. J Med Screen. 2005;12:197–201. doi: 10.1258/096914105775220642. [DOI] [PubMed] [Google Scholar]
- 17.Lauer MS. Primary prevention of atherosclerotic cardiovascular disease: the high public burden of low individual risk. JAMA. 2007;297:1376–1378. doi: 10.1001/jama.297.12.1376. [DOI] [PubMed] [Google Scholar]
- 18.• Murphy TP, Dhangana R, Pencina MJ et al.: Performance of Current Guidelines for Coronary Heart Disease Prevention: Optimal Use of the Framingham-based Risk Assessment. Atherosclerosis, online Feb 2011. This analysis of risk assessment in primary prevention of CVD provides a strong argument for lowering the treatment threshold and treating many more individuals with intensive risk factor modification. [DOI] [PubMed]
- 19.Nordestgaard BG, Adourian AS, Freiberg JJ, Guo Y, Muntendam P, Falk E. Risk factors for near-term myocardial infarction in apparently healthy men and women. Clin Chem. 2010;56:559–567. doi: 10.1373/clinchem.2009.139964. [DOI] [PubMed] [Google Scholar]
- 20.Blankenberg S, Zeller T, Saarela O, et al. MORGAM Project: Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 21.Wald NJ, Morris JK. Assessing risk factors as potential screening tests: a simple assessment tool. Arch Intern Med. 2011;171:286–291. doi: 10.1001/archinternmed.2010.378. [DOI] [PubMed] [Google Scholar]
- 22.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 24.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 25.Fuster V, Moreno PR, Fayad ZA, et al. Atherothrombosis and high-risk plaque –part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 26.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 27.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 28.Fuster V, Fayad ZA, Moreno PR, et al. Atherothrombosis and highrisk plaque: part II: approaches by noninvasive computed tomographic/magnetic resonance imaging. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 29.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 30.Saam T, Hatsukami TS, Takaya N, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 31.Rudd JH, Narula J, Strauss HW, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55:2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 32.Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators: A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 33.Muntendam P, McCall C, Sanz J, Falk E. Fuster V; High-Risk Plaque Initiative: The BioImage Study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease–study design and objectives. Am Heart J. 2010;160:49–57. doi: 10.1016/j.ahj.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419–1424. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Census Bureau: 2000 Census of population and housing, demographic profile. Profiles of general demographic characteristics.
- 36.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 37.Varas-Lorenzo C, Castellsague J, Stang MR, et al. Positive predictive value of ICD-9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf. 2008;17:842–852. doi: 10.1002/pds.1619. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RN: U.S. decennial life tables for 1989–91, vol 1 no 4, United States life tables eliminating certain causes of death. National Center for Health Statistics. Hyattsville, Maryland. 1999. Available at: http://www.cdc.gov/nchs/data/lifetables/life89_1_4.pdf Accessed April 2011.
- 39.Blaha MJ, Budoff MJ, Rivera JJ, et al. Relationship of carotid distensibility and thoracic aorta calcification: multi-ethnic study of atherosclerosis. Hypertension. 2009;54:1408–1415. doi: 10.1161/HYPERTENSIONAHA.109.138396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lloyd-Jones DM, Walsh JA, Prineas RJ, et al. Association of electrocardiographic abnormalities with coronary artery calcium and carotid artery intima-media thickness in individuals without clinical coronary heart disease (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;104:1086–1091. doi: 10.1016/j.amjcard.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiner AP, Carlson CS, Jenny NS, et al. USF1 gene variants, cardiovascular risk, and mortality in European Americans: analysis of two US cohort studies. Arterioscler Thromb Vasc Biol. 2007;27:2736–2742. doi: 10.1161/ATVBAHA.107.154559. [DOI] [PubMed] [Google Scholar]
- 43.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]


