Abstract
Nonsurgical management of patients with symptomatic mitral valve stenosis has been established as the therapeutic modality of choice for two decades. Catheter-based balloon dilation of the stenotic valvular area has been shown, at least, as effective as surgical interventions. Unfavorable results of catheter-based interventions are largely due to unfavorable morphology of the valve apparatus, particularly leaflets calcification and subvalvular apparatus involvement. A mitral valve score has been proposed in Boston, MA, about two decades ago, based on morphologic assessment of mitral valve apparatus by two-dimensional (2D) echocardiography to predict successful balloon dilation of the mitral valve. Several other scores have been developed in the following years in order to more successfully predict balloon dilatation outcome. However, all those scores were based on 2D echocardiography, which is limited by ability to distinguish calcification and subvalvular involvement. The introduction of new matrix-based ultrasound probe has allowed 3D echocardiography (3DE) to provide more detailed morphologic analysis of mitral valve apparatus including calcification and subvalvular involvement. Recently, a new 3DE scoring system has been proposed by our group, which represents an important leap into refinement of the use of echocardiography guiding mitral valve interventions.
Keywords: Echocardiography, Three-dimensional, Mitral valve, Stenosis, Score
Introduction
Although the incidence of rheumatic fever and its complications have declined in developed countries, the disease is still a major health problem in many developing countries. The mitral valve (MV) is the most commonly and severely affected (65%–70% of patients) by rheumatic process by stenosis and/or regurgitation [1]. Percutaneous balloon mitral valvuloplasty (BMV) was introduced in 1984 by Inoue et al. [2••] for the treatment of selected patients with mitral stenosis. BMV is a minimally invasive, nonsurgical procedure that has been established in several clinical studies to be a safe and effective therapeutic modality in selected patients with mitral stenosis (MS) [3–5] and is equivalent to or even better than surgical commissurotomy [6, 7]. With successful BMV, there is generally a twofold increase in the mitral valve area and an associated dramatic fall in transmitral valve gradient, left atrial pressure, and pulmonary artery pressure [3, 4, 8, 9]. These hemodynamic benefits are associated with postprocedural improvement in the patients’ symptoms and exercise tolerance [10]. The safety and success of BMV techniques is mostly dependent on the selection of patients. There are multiple predictors of the outcome, including age, functional class, previous commissurotomy, preprocedure MV area, valve anatomy, and balloon size used [11]. Morphology of the MV is considered as the main predictor of a successful BMV and hence MV scoring system using echocardiography is of crucial importance [11]. Many scores are derived targeting patient selection before BMV and correlated the severity with the immediate and long-term outcome. In this review article, we will summarize main characteristics of new MV scoring systems in a comparison with old scores.
MV Anatomy and Pathology
The MV is highly complex structure made up of several individual parts which need to function in harmony to maintain its competency. The parts are the annulus, the leaflets, the chordae tendinea cords, and the papillary muscles. The most important component of the valve is the leaflets. Anatomists usually described the two leaflets as being anterior and posterior. However, the appropriate orientation of the MV is seen to occupy a more oblique position within the cardiac short axis than these traditional names suggest. When the valve is closed, both leaflet edges form an arc-shaped closure line that is obliquely situated relative to the orthogonal planes of the body. Both ends of closure line are referred to as commissures (anterolateral and posteromedial). The anterolateral commissure is next to the left fibrous trigone and the left atrial appendage, while the posteromedial commissure is next to the right fibrous trigone and the interatrial septum. From the usual practice of the pathologist and anatomist, each leaflet is divided by indentations into three unequal segments, the so-called “scallops” [12–14].
Chordae tendineae are string-like structures that take origin from the papillary muscles, bifurcate several times, and attach to the ventricular aspects of both leaflets. The cords are thinnest at their sites of insertion on the leaflets. Primary cords normally insert in uniform fashion along the free margin of both leaflets. Secondary cords insert in layered fashion along the rough zone of the anterior leaflet. The coapting surfaces meet during systolic closure. As the papillary muscles contract and relax, the chordae tendineae transmit the resulting increase and decrease in tension to the MV causing open and closure [14, 15].
The characteristic lesion of acute rheumatic fever is the Aschoff body, consisting of necrotic foci surrounded by activated histiocytes and lymphocytes and ultimately heals by fibrosis. These foci may be found in the pericardium, the myocardium, or in the valves. Chronic cardiac manifestations include fibrocalcific valvulitis [16]. Chronic rheumatic affection of the MV leaflets especially with recurrent episodes includes chronic scarring. Chronic scarring of the valve leaflets converts a translucent, pliable valve into a stiff, thickened structure and leads to one or all of the following deformities:
Fusion of the leaflet commissures.
Thickening, fibrosis, and calcification of the leaflet cusps.
These changes become progressive over time and result in funnel-shaped stenotic MV, which is often called “fish mouthed.” Rheumatic involvement of subvalvular apparatus includes fusion, thickening, retraction, shortening, and calcification. As a consequence, the free interchordal space diminishes and the opened “leaflet–chordae tendineae tunnel” available for diastolic flow is limited [16].
Percutaneous Balloon Mitral Valvuloplasty
Since the advent of the Inoue balloon in 1984 [2••], BMV became a feasible option for many patients with symptomatic MS. During BMV, the balloon is introduced across the mitral valve and its inflation fractures the rheumatic fusion of the valve leaflets at the commissures, dramatically improving leaflet excursion and orifice area [17]. Numerous large series have reported excellent short, medium, and long-term outcome with a low incidence of serious complications [18].
Randomized trials comparing BMV to closed commissurotomy show it to be superior to closed commissurotomy, providing an often larger valve area and exhibiting excellent long-term durability. In patients with MV score ≤8 (see later) and sinus rhythm, both BMV and open commissurotomy were comparable [19].
Metallic valvulotome achieved encouraging results with the reduction of the procedure cost [20, 21••]. BMV is now the treatment of choice for many patients with symptomatic mitral stenosis. Applications are expanding to include several categories of patients previously considered ineligible for the procedure [22].
MV Scoring Systems by Two-Dimensional Echocardiography
Transthoracic two-dimensional echocardiography (2DE) allows classification of patients into anatomic groups to predict immediate and long-term outcome of BMV. Several 2DE scoring systems have been suggested for evaluation of MV anatomy. Wilkins scoring system evaluates leaflet thickening, mobility, calcification, and subvalvular involvement on a scale of 0–4 (Table 1) [23••]. The MV morphology is considered favorable if the mitral echocardiographic score is ≤8.
Table 1.
Grading of mitral valve characteristics from the echocardiographic examination according to Wilkins (Boston) score
| Grade | Mobility | Thickening | Calcification | Subvalvar thickening |
|---|---|---|---|---|
| 1 | Highly mobile valve with only leaflet tips restricted | Leaflets near normal in thickness (4–5 mm) | A single area of increased echo brightness | A single area of increased echo brightness |
| 2 | Leaflet mid and base portions have normal mobility | Mid-leaflets normal, considerable thickening of margins (5–8 mm) | Scattered areas of brightness confined to leaflet margins | Scattered areas of brightness confined to leaflet margins |
| 3 | Valve continues to move forward in diastole, mainly from the base | Thickening extending through the entire leaflet (5–8 mm) | Brightness extending into the mid-portion of the leaflets | Thickening extending to the distal third of the chords |
| 4 | No or minimal forward movement of the leaflets in diastole | Considerable thickening of all leaflet tissue (>8–10 mm) | Extensive brightness throughout much of the leaflet tissue | Extensive thickening and shortening of all chordal structures extending down to the papillary muscles |
(Modified from Wilkins et al. [23••]; with permission.)
Another 2DE score by Chen et al. [24] is a modified Wilkins score parameter for subvalvular thickening according to the involved segment of chordal length: (1) if less than 1/3, (2) if more than 1/3, (3) if more than 2/3, and (4) if involved the whole chordal length with no separation.
Reid score includes leaflet motion, leaflet thickness, subvalvular disease, and commissural calcium. Leaflet motion was expressed as a slope by dividing the height (H) by the length (L) of doming of anterior leaflet. Leaflet thickness was expressed as the ratio between the thickness of the tip of MV and thickness of posterior wall of aortic root. The score was assigned as 0 for mild affection, 1 for moderate, and 2 for severe affection [25•].
Nobuyoshi score included leaflet pliability, commissural disease, and subvalvular apparatus (Table 2) [26•].
Table 2.
Nobuyoshi score
| Component | Score | Definition |
|---|---|---|
| Leaflet mobility | 1 | Pliable leaflets with minimal restriction of leaflet tip mobility |
| 2 | Semi-pliable leaflets with restriction of leaflet body mobility | |
| 3 | Minimal forward movement of the leaflets | |
| Commissural disease | 1 | No commissural disease |
| 2 | One commissural disease | |
| 3 | Both commissural disease | |
| 4 | Diffuse commissural disease | |
| Subvalvular disease | 1 | Minimal thickening of chordae |
| 2 | Thickening and shortening of chordae | |
| 3 | Fused subvalvular apparatus |
Cormier score divided the patients into three groups depending on leaflets mobility and calcification and subvalvular affection: group 1, pliable noncalcified anterior mitral leaflet and mild subvalvular disease (i.e., thin chordae >10 mm long); group 2, pliable noncalcified anterior mitral leaflet and severe subvalvular disease (i.e., thickened chordae <10 mm long); and group 3, calcification of MV of any extent, as assessed by fluoroscopy, whatever the state of subvalvular apparatus [27].
However, none of the available 2DE scores has been shown to be superior to any of the others [28]. Most cardiologists use the Wilkins score. The available scoring systems, particularly Wilkins score, have many limitations [22, 29, 30], including:
Echocardiography limited in ability to differentiate nodular fibrosis from calcification.
Assessment of commissural involvement is not included or underestimated.
Doesn’t account for uneven distribution of pathologic abnormalities.
Doesn’t account for relative contribution of each variable (no weighting of variables).
Frequent underestimation of subvalvular disease.
Doesn’t use results from TEE or 3D echocardiography.
An ideal echo scoring system has to have many criteria:
Global and segmental evaluation (qualitative and quantitative) of each MV apparatus component separately to localize the deformity in a specific portion of MV apparatus.
Inclusion of all points that proved to predict and affect the PMV outcome via large study.
Validation in large studies that include patients with different age groups (not only young).
Easily applicable and interpretable by most cardiologists within a reasonable time.
High reproducibility and reliability.
Unified for both transthoracic and transesophageal approaches.
MV Assessment by Three-Dimensional Echocardiography
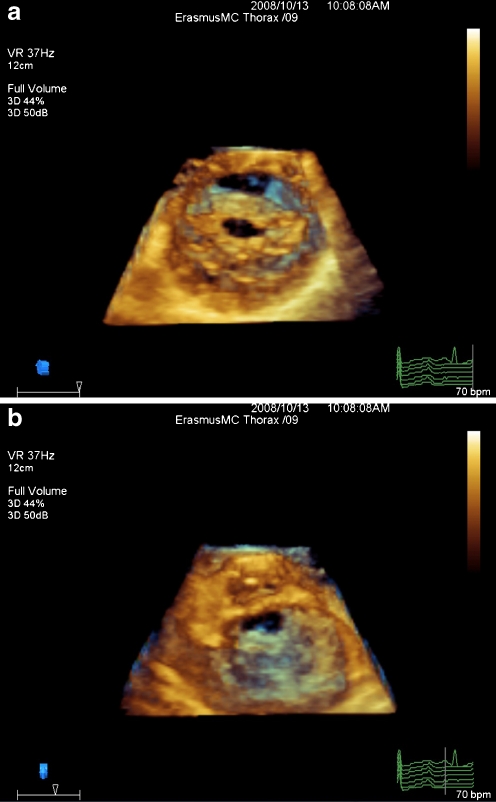
Three-dimensional echocardiography (3DE) has established a clear understanding of the functional anatomy of the MV. Real-time 3DE (RT3DE) provided detailed morphologic display and analysis of the mitral valve apparatus in 3D (Fig. 1). In addition, RT3DE provided rapid, accurate quantification of mitral valve structure, particularly for determining the valvular orifice area, as it facilitates the orientation of any slice plane to locate the minimal MV area. The improvement of RT3DE probe technology, especially the latest innovation in transesophageal (Fig. 2) 3DE probes, raised the need to construct a scoring system using RT3DE because it can be used independently before, during, and after BMV.
Fig. 1.
An example of rheumatic mitral valve stenosis as seen from left atrial side (a) and left ventricular side (b)
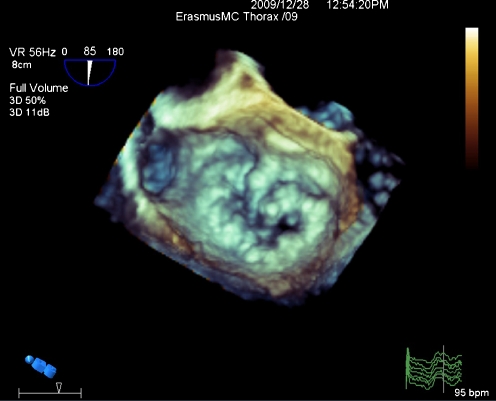
Fig. 2.
An example of mitral valve stenosis seen from left ventricular side on transesophageal RT-3DE
MV Score by 3DE
Recently, Anwar et al. [31••] introduced the first scoring system using real-time transthoracic 3DE (RT3D-TTE) in patients with MS candidate for BMV. The authors compared the RT3D-TTE score with Wilkins score regarding the selection of patients through the PMV outcome [31••].
Description
The new RT3DE morphology score was derived to include both MV leaflets and subvalvular apparatus (Table 3).
Table 3.
Mitral valve score based on real-time three-dimensional echocardiography
| Leaflets | ||||||
| Anterior leaflet | Posterior leaflet | |||||
| A1 | A2 | A3 | P1 | P2 | P3 | |
| aThickness (0–6) | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 |
| aMobility (0–6) | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 |
| bCalcification (0–10) (0 = no, 1–2 = calcified) | 0–2 | 0–1 | 0–2 | 0–2 | 0–1 | 0–2 |
| bSubvalvular apparatus | ||||||
| Proximal third | Middle third | Distal third | ||||
| Thickness (0–3) (0 = normal, 1 = thickened) | 0–1 | 0–1 | 0–1 | |||
| Separation (0–6) (0 = normal, 1 = partial, 2 = no) | 0–2 | 0–2 | 0–2 | |||
aNormal = 0, mild = 1–2, moderate = 3–4, severe >5
bNormal = 0, mild = 1–2, moderate = 3–5, severe >6
(Modified from Anwar et al. [31••]; with permission.)
Scoring of Leaflets
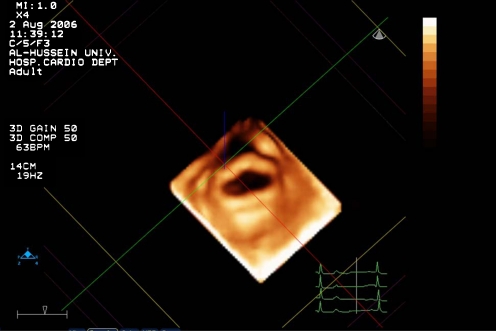
Each leaflet was divided into three scallops (anterolateral A1–P1, middle A2–P2, and posteromedial A3–P3) and scored separately for thickness, mobility, and calcification. Thickness, mobility, and calcification for each scallop were scored as follows: normal thickness and mobility was scored as 0, and abnormal thickness or restricted mobility was scored as 1. Absence of calcification was scored as 0, calcification in middle scallop (A2 or P2) was scored as 1, and calcification of commissural scallops of both leaflets (A1, A3–P1, P3) was scored as 2. Figure 3 displays an example of leaflets thickening on RT-TT3DE in a patient with rheumatic heart disease.
Fig. 3.
Displays an example of leaflets thickening on RT-TT3DE in a patient with rheumatic heart disease
Scoring of Subvalvular Apparatus
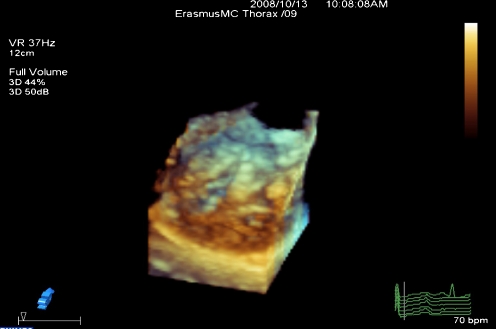
The anterior and posterior chordae were scored at three levels: proximal (valve level), middle, and distal (papillary muscle level). At each level, both anterior and posterior chordae were scored separately for thickness and separation in between as follows: normal thickness was scored as 0 and abnormal thickness was scored as 1, normal chordal separation (distance in between >5 mm) was scored as 0, partial separation (distance in between <5 mm) as 1, and absence of separation as 2. Figure 4 displays an example of chordal thickening on RT-TT3DE in a patient with rheumatic heart disease.
Fig. 4.
Displays an example of chordal thickening on RT-TT3DE in a patient with rheumatic heart disease
Total Score Grading
The individual RT3DE score points of leaflets and subvalvular apparatus RT3DE score were summed to calculate the total RT3DE score, ranging from 0 to 31 points. Total score of mild MV involvement was defined as <8 points, moderate MV involvement 8–13, and severe MV involvement >14.
Potential Advantages
The 3D score has many potential benefits that help for a detailed assessment of the MV.
Visualization of leaflets. By RT3DE, visualization and assessment of the whole length of both leaflets is possible through single image plane, especially in sinus rhythm. Leaflet mobility could be well assessed. RT3DE could detect the thickness of each leaflet scallop. The whole leaflet length could not be evaluated by a single 2DE image especially for the posterior leaflet, which is short and naturally less mobile than the anterior one. As shown in Table 4, many views from different cut section planes are needed to assess all scallops.
Leaflet calcification. Scoring of leaflet calcification using Wilkins score depends on the bright areas and the extension of calcification along the leaflet length [32, 33]. Multiple cut planes are needed for detecting calcification in all scallops of both MV leaflets. RT3DE could predict the extent and distribution of calcification in each scallop from a single short axis cut plain. The new RT3DE score described calcification at the commissural parts of leaflet by a higher score than the middle leaflets calcification because it was proved that calcification of commissures is one of the strong predictors of outcome after PMV because it affects the degree of commissural splitting (Fig. 5) [34, 35].
Subvalvular apparatus. RT3DE score included the chordal thickness and separation, which is a good independent predictor for BMV outcome. Both chordal thickness and separation are scored at three levels by dividing their length into three parts (proximal, middle, and distal). This detailed information, especially for chordal separation, was not obtained by most 2D scoring systems, including Wilkins score [36].
Score applicability. Compared to Wilkins score, the RT3DE score is simple and more helpful, particularly for less experienced operators as it provides a simple number for each leaflet scallop and subvalvular apparatus segment separately. This was evident by good interobserver and intraobserver agreements for most of the score components.
Score approach. The score can be applied using both transthoracic and transesophageal approaches because the image orientation and interpretation are not different.
Table 4.
Observed scallop in each two-dimensional echocardiographic view
| View | A1 | A2 | A3 | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Parasternal long-axis | − | + | − | − | + | − |
| Apical 4-chamber | − | + | + | + | − | − |
| Apical 2-chamber | + | + | − | − | + | − |
| Apical long-axis | − | + | − | − | + | − |
| Parasternal short-axis | ± | + | ± | + | + | + |
+, visualized; −, not visualized; ±, may be visualized
Fig. 5.
Displays an example of chordal splitting after balloon mitral valvuloplasty on RT-TT3DE in a patient with rheumatic heart disease
Limitations and Arguments of 3DE Mitral Score
The 3D technology is not available in all cardiac centers and thus the lack of it is widespread. This is true; however, all new techniques passed the same stages and by time the new techniques gradually replace the old ones until the stage of standardization, availability, and widespread use is achieved.
The image acquisition, cut sections, and dealing with the analysis software are operator dependent to a high extent. However, the probe position and acquisition is easy for any echocardiographers. In addition, those dealing with the analysis software need training to be familiar and get the experience.
Most cardiologists are aware with the 2D images and interpretation of Wilkins score. The need for learning time to develop the experience with 3D technology is a must. The visual familiarity and mental conception of the cardiologists toward the 2D images are very helpful to build the experience with 3D.
The available 3D score is more complex and time consuming. This is due to many anatomical and morphological components included to achieve a detailed assessment. Rapid technical improvements will overcome such a limitation, and future studies may simplify this score.
The score is highly selective for the optimal result of BMV. This means that a considerable percentage of patients are grouped as nonfavorable or less favorable for BMV and will be excluded. Therefore, more referral and exposure to surgical intervention are needed. The decision to perform BMV is not solely dependent on the MV score but the clinical judgment is sharing. For example, in patients with an unacceptable high risk for heart surgery, BMV can be preferred even with high MV score with the need of patient and physician awareness regarding the higher risk, the lower success rate, and suboptimal long-term outcome of BMV. In general, the selection of therapeutic modality to patient is always dependent on risk/benefit ratio. Other factors may be considered (e.g., age, functional class, patient preference, cost and simplicity of procedure, associated other valve lesions, and associated comorbidities) [22].
Personal Perspectives/Conclusions
Due to higher resolution and better image quality, RT3D-TEE will save the time and minimize the cost to do many echo studies (2D-TTE, 2D-TEE, and RT3D-TTE) for a detailed assessment of MV morphology before BMV. The application of the 3D scoring system will be obtainable in all patients regardless of their transthoracic image quality and underlying heart rhythm.
At the start of learning, both the new RT3DE score and Wilkins score can be used complementary to each other for evaluation before BMV. This will facilitate future standardization of RT3DE for both quantitative and qualitative assessment of the MV.
Large-scale studies from multiple centers are needed to improve the applicability of 3D scoring. With the large number of patients, BMV outcome may direct the investigators to modify the available 3D score by adding or omitting some of the score components. With this article, we hope to encourage investigators and stimulate them to develop scientific studies to achieve establishment and standardization of the ideal 3D score. We expect that in the near future, assessment of MV by the ideal 3D score will be widely used as a stand-alone modality.
Acknowledgments
Disclosure
No potential conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance •• Of major importance
- 1.Karthikeyan G, Mayosi BM. Is primary prevention of rheumatic fever the missing link in the control of rheumatic heart disease in africa? Circulation. 2009;120(8):709–713. doi: 10.1161/CIRCULATIONAHA.108.836510. [DOI] [PubMed] [Google Scholar]
- 2.Inoue K, Owaki T, Nakamura T, et al. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87(3):394–402. [PubMed] [Google Scholar]
- 3.Arora R, Kalra GS, Murty GS, et al. Percutaneous transatrial mitral commissurotomy: immediate and intermediate results. J Am Coll Cardiol. 1994;23(6):1327–1332. doi: 10.1016/0735-1097(94)90374-3. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz CE, Zhang HP, Macaya C, et al. Comparison of inoue single-balloon versus double-balloon technique for percutaneous mitral valvotomy. Am Hear J. 1992;123(4 Pt 1):942–947. doi: 10.1016/0002-8703(92)90700-6. [DOI] [PubMed] [Google Scholar]
- 5.Hung JS, Lau KW, Lo PH, et al. Complications of inoue balloon mitral commissurotomy: impact of operator experience and evolving technique. Am Hear J. 1999;138(1 Pt 1):114–121. doi: 10.1016/S0002-8703(99)70255-3. [DOI] [PubMed] [Google Scholar]
- 6.Turi ZG, Reyes VP, Raju BS, et al. Percutaneous balloon versus surgical closed commissurotomy for mitral stenosis. A prospective, randomized trial. Circulation. 1991;83(4):1179–1185. doi: 10.1161/01.cir.83.4.1179. [DOI] [PubMed] [Google Scholar]
- 7.Ben Farhat M, Ayari M, Maatouk F, et al. Percutaneous balloon versus surgical closed and open mitral commissurotomy: seven-year follow-up results of a randomized trial. Circulation. 1998;97(3):245–250. doi: 10.1161/01.cir.97.3.245. [DOI] [PubMed] [Google Scholar]
- 8.Iung B, Garbarz E, Michaud P, et al. Immediate and mid-term results of repeat percutaneous mitral commissurotomy for restenosis following earlier percutaneous mitral commissurotomy. Eur Hear J. 2000;21(20):1683–1689. doi: 10.1053/euhj.1999.1992. [DOI] [PubMed] [Google Scholar]
- 9.Vahanian A, Michel PL, Cormier B, et al. Immediate and mid-term results of percutaneous mitral commissurotomy. Eur Hear J. 1991;12(Suppl B):84–89. doi: 10.1093/eurheartj/12.suppl_b.84. [DOI] [PubMed] [Google Scholar]
- 10.Hung JS, Chern MS, Wu JJ, et al. Short- and long-term results of catheter balloon percutaneous transvenous mitral commissurotomy. Am J Cardiol. 1991;67(9):854–862. doi: 10.1016/0002-9149(91)90619-V. [DOI] [PubMed] [Google Scholar]
- 11.Pan M, Medina A, Suarez de Lezo J, et al. Factors determining late success after mitral balloon valvulotomy. Am J Cardiol. 1993;71(13):1181–1185. doi: 10.1016/0002-9149(93)90643-Q. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr: The Journal of the Working Group on Echocardiography of the European Society of Cardiology. 2010;11(10):i3–9. doi: 10.1093/ejechocard/jeq153. [DOI] [PubMed] [Google Scholar]
- 13.Ho SY. Anatomy of the mitral valve. Heart. 2002;88(Suppl 4):iv5–10. doi: 10.1136/heart.88.suppl_4.iv5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Mieghem NM, Piazza N, Anderson RH, et al. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol. 2010;56(8):617–626. doi: 10.1016/j.jacc.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Silbiger JJ, Bazaz R. Contemporary insights into the functional anatomy of the mitral valve. Am Hear J. 2009;158(6):887–895. doi: 10.1016/j.ahj.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL. Pathogenesis of acute rheumatic fever and rheumatic heart disease: evasive after half a century of clinical, epidemiological, and laboratory investigation. Heart. 2005;91(1):3–4. doi: 10.1136/hrt.2004.034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung JS, Lau KW. Pitfalls and tips in inoue balloon mitral commissurotomy. Cathet Cardiovasc Diagn. 1996;37(2):188–199. doi: 10.1002/(SICI)1097-0304(199602)37:2<188::AID-CCD19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Iung B, Garbarz E, Michaud P, et al. Late results of percutaneous mitral commissurotomy in a series of 1024 patients. Analysis of late clinical deterioration: frequency, anatomic findings, and predictive factors. Circulation. 1999;99(25):3272–3278. doi: 10.1161/01.cir.99.25.3272. [DOI] [PubMed] [Google Scholar]
- 19.Song JK, Kim MJ, Yun SC, et al. Long-term outcomes of percutaneous mitral balloon valvuloplasty versus open cardiac surgery. J Thorac Cardiovasc Surg. 2010;139(1):103–110. doi: 10.1016/j.jtcvs.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Carabello BA. Modern management of mitral stenosis. Circulation. 2005;112(3):432–437. doi: 10.1161/CIRCULATIONAHA.104.532498. [DOI] [PubMed] [Google Scholar]
- 21.Cribier A, Eltchaninoff H, Koning R, et al. Percutaneous mechanical mitral commissurotomy with a newly designed metallic valvulotome: immediate results of the initial experience in 153 patients. Circulation. 1999;99(6):793–799. doi: 10.1161/01.cir.99.6.793. [DOI] [PubMed] [Google Scholar]
- 22.Prendergast BD, Shaw TR, Iung B, et al. Contemporary criteria for the selection of patients for percutaneous balloon mitral valvuloplasty. Heart. 2002;87(5):401–404. doi: 10.1136/heart.87.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Hear J. 1988;60(4):299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CG, Wang X, Wang Y, et al. Value of two-dimensional echocardiography in selecting patients and balloon sizes for percutaneous balloon mitral valvuloplasty. J Am Coll Cardiol. 1989;14(7):1651–1658. doi: 10.1016/0735-1097(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 25.Reid CL, Chandraratna PA, Kawanishi DT, et al. Influence of mitral valve morphology on double-balloon catheter balloon valvuloplasty in patients with mitral stenosis. Analysis of factors predicting immediate and 3-month results. Circulation. 1989;80(3):515–524. doi: 10.1161/01.CIR.80.3.515. [DOI] [PubMed] [Google Scholar]
- 26.Nobuyoshi M, Hamasaki N, Kimura T, et al. Indications, complications, and short-term clinical outcome of percutaneous transvenous mitral commissurotomy. Circulation. 1989;80(4):782–792. doi: 10.1161/01.CIR.80.4.782. [DOI] [PubMed] [Google Scholar]
- 27.Iung B, Cormier B, Ducimetiere P, et al. Immediate results of percutaneous mitral commissurotomy. A predictive model on a series of 1514 patients. Circulation. 1996;94(9):2124–2130. doi: 10.1161/01.cir.94.9.2124. [DOI] [PubMed] [Google Scholar]
- 28.Vahanian A. Balloon valvuloplasty. Heart. 2001;85(2):223–228. doi: 10.1136/heart.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein SA, Lindsay J., Jr Do we need more echo scores for balloon mitral valvuloplasty? J Am Soc Echocardiogr: Official Publication of the American Society of Echocardiography. 2010;23(1):23–25. doi: 10.1016/j.echo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Sutaria N, Shaw TR, Prendergast B, et al. Transoesophageal echocardiographic assessment of mitral valve commissural morphology predicts outcome after balloon mitral valvotomy. Heart. 2006;92(1):52–57. doi: 10.1136/hrt.2004.058297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anwar AM, Attia WM, Nosir YF, et al. Validation of a new score for the assessment of mitral stenosis using real-time three-dimensional echocardiography. J Am Soc Echocardiogr: Official Publication of the American Society of Echocardiography. 2010;23(1):13–22. doi: 10.1016/j.echo.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Fatkin D, Roy P, Morgan JJ, et al. Percutaneous balloon mitral valvotomy with the inoue single-balloon catheter: commissural morphology as a determinant of outcome. J Am Coll Cardiol. 1993;21(2):390–397. doi: 10.1016/0735-1097(93)90680-Y. [DOI] [PubMed] [Google Scholar]
- 33.Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol. 1997;29(1):175–180. doi: 10.1016/S0735-1097(96)00422-6. [DOI] [PubMed] [Google Scholar]
- 34.Sutaria N, Northridge DB, Shaw TR. Significance of commissural calcification on outcome of mitral balloon valvotomy. Heart. 2000;84(4):398–402. doi: 10.1136/heart.84.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messika-Zeitoun D, Blanc J, Iung B, et al. Impact of degree of commissural opening after percutaneous mitral commissurotomy on long-term outcome. JACC Cardiovasc Imaging. 2009;2(1):1–7. doi: 10.1016/j.jcmg.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Turgeman Y, Atar S, Rosenfeld T. The subvalvular apparatus in rheumatic mitral stenosis: methods of assessment and therapeutic implications. Chest. 2003;124(5):1929–1936. doi: 10.1378/chest.124.5.1929. [DOI] [PubMed] [Google Scholar]