Abstract
Quantitative analysis of the 16-mercaptohexadecanoic acid self-assembled monolayer (C16 COOH-SAM) layer thickness on gold nanoparticles (AuNPs) was performed using simulation of electron spectra for surface analysis (SESSA) software and x-ray photoelectron spectroscopy (XPS) experimental measurements. XPS measurements of C16 COOH SAMs on flat gold surfaces were made at 9 different photoelectron emission angles (5° to 85° in 10° increments), corrected using geometric weighting factors and then summed together to approximate spherical AuNPs. The SAM thickness and relative surface roughness (RSA) in SESSA were optimized to determine the best agreement between simulated and experimental surface composition. Based on the glancing-angle results, it was found that inclusion of a hydrocarbon contamination layer on top the C16 COOH-SAM was necessary to improve the agreement between the SESSA and XPS results. For the 16 COOH-SAMs on flat Au surfaces, using a SAM thickness of 1.1Å/CH2 group, an RSA of 1.05, and a 1.5Å CH2-contamination overlayer (total film thickness = 21.5Å) for the SESSA calculations provided the best agreement with the experimental XPS data. After applying the appropriate geometric corrections and summing the SESSA flat-surface compositions, the best fit results for the 16 COOH-SAM thickness and surface roughness on the AuNPs indicated a slightly thinner overlayer with parameters of 0.9Å/CH2 group in the SAM, a RSA of 1.06 RSA and a 1.5Å CH2-contamination overlayer (total film thickness = 18.5Å). The three angstrom difference in SAM thickness between the flat Au and AuNP surfaces suggests that the alkyl chains of the SAM are slightly more tilted or disordered on the AuNP surfaces.
Introduction
Self-assembled monolayers (SAMs) of alkanethiols on flat gold surfaces have been studied exhaustively and their properties are relatively well understood.1–13 They can exhibit surfaces with well-defined compositions and structures that allow control of chemical reactions, physical interactions and other phenomenon at their surfaces. Alkanethiol SAMs and their derivatives are used as model surfaces in biomaterial, catalyst, electrochemical, corrosion, etc. applications to develop fundamental understanding of surface and interface processes.6, 14–24 Unlike SAMs on flat Au surfaces, SAMs on gold nanoparticles (AuNPs) have a more complex structure of surface planes, edges and vertices due to the inherent NP curvature and faceting.25, 26 Molecular dynamics studies of SAMs on AuNPs have provided insight into the complex SAMs structure and ordering among other properties.27, 28 Studies have shown that the surface chemistries and structure of the AuNPs play significant roles in determining the overall properties of the AuNPs.26 However, the SAMs on AuNPs have not been subjected to the same level of fundamental surface characterization scrutiny as the SAMs on flat Au surfaces.29 Specifically, the overlayer structure and thickness are significantly less defined for AuNP-SAMs. Despite this lack of detailed understanding, even for the simplified AuNP-SAM model systems, AuNPs are widely utilized in wide array of studies and applications that involve very complex systems (e.g., multi-functionalized AuNPs in biological environments).
Although surface-sensitive techniques such as X-ray photoelectron spectroscopy (XPS) have been used often for qualitative characterization of NPs, quantitative characterization of functionalized AuNP surfaces with XPS is limited because it often requires sophisticated data analysis methods that are not routinely available.30 Typical XPS data analysis methods were developed for analysis of flat surfaces and not for analysis of curved surfaces, let alone NPs. Catalysis research is a notable exception where researchers have demonstrated that XPS data can be used to provide detailed, quantitative information about the structure of supported NPs.31 For example, Frydman et al. have developed mathematical formulas for evaluating XPS intensities from powdered catalysts particles to determine a structural model for the catalytic particles.32, 33 This model has recently been extended to determine the structure and composition of quantum dots.34 Recently, various groups have started using different approaches to determine overlayer structures and thicknesses from XPS analysis of surfaces with various morphologies. One approach to estimate the overlayer thickness of macroscopic and microscopic spheres and cylinders is through the use of a Topofactor, which corrects for the deviation of sample topography from a planar structure.35 In this method, the apparent overlayer thickness of the samples calculated from XPS measurements taken at the normal incidence to the analyzer is multiplied by the Topofactor, 0.67 for spheres, to find the correct overlayer thickness. A simple Topofactor analysis, however, is not available for nanoparticles when the electron inelastic mean free paths become comparable to the particle sizes. However, they provide analytical models for iterative calculation of overlayer thickness on small particles.35 Also, the topofactor approach is designed to determine the thickness of relatively simple coatings and does not deal with multilayered coatings that can be handled by other methods. Spectral modeling methods such as simulation of electron spectra for surface analysis (SESSA), quantitative analysis of surface by electron spectroscopy (QUASES) and XPS MultiQuant improve XPS data analysis through better depiction of the sample surface and allow determination of surface properties in addition to an average surface atomic composition. XPS MultiQuant analyzes the integrated intensity of the measured XPS peaks to determine surface information such as overlayer thickness and multilayer structures.36 It allows for correction of a contamination overlayer and surface nanostructure variations to improve the quantitative evaluation of XPS data. Unlike MultiQuant that uses only peak intensities, QUASES analyzes the XPS peak and background shape to determine surface information such as overlayer thickness and in-depth atomic distribution for various surface nanostructures.37 Specifically, QUASES uses the inelastic background shape and XPS peak intensity as the main source of information. Experimental spectra are uploaded into the program and compared with calculated results interactively using the QUASES graphical user interface (GUI) to facilitate the optimization process. SESSA generates spectra using a Monte Carlo algorithm to calculate partial intensities of photoelectrons and simulate their trajectories.38–41 In SESSA, as in several other modeling programs, the sample is modeled as a flat surface and can have multiple thin layers with various compositions and thicknesses. To a greater extent than QUASES, SESSA gives users control over various aspects of the input information for the sample, spectrometer settings, photoelectron phenomenona such as elastic and inelastic electron scattering data, and other parameters. This information is input with the aid of both a GUI and a command line interface (CLI).
The objective of the current study is to model the overlayer thickness on NPs using SESSA. Since the current version of SESSA only provides calculations for flat surfaces, the results from flat surface SESSA calculations at 9 photoelectron emission angles were summed (with appropriate geometric weighting) to approximate a spherical NP. 16-mercaptohexadecanoic acid SAMs (C16 COOH-SAMs), 14nm-diameter AuNPs and flat Au surfaces were used as the experimental system for this study.42 Angle-resolved XPS data measured on the flat Au-SAM surfaces were used as reference samples to optimize the SESSA parameters.
Experimental and Methods
Materials
The 14nm-diameter AuNPs used in this study were synthesized by the citrate reduction methods43, 44 using gold (III) chloride hydrate and trisodium citrate dihydrate. 16-mercaptohexadecanoic acid was used to functionalize the AuNPs as described previously.42 Flat Au surfaces used in this study were prepared by electron beam evaporation coating of cleaned silicon wafers primed with 10nm titanium films and then functionalized with either 16-mercaptohexadecanoic acid or 1-hexadecanethiol. Further details regarding the synthesis, functionalization of the AuNP and flat Au surfaces are provided in the supporting information.
X-ray photoelectron spectroscopy (XPS)
XPS measurements were performed on a Surface Science Instruments S-Probe XPS system (Mountain View, California) using a monochromatic Al Kα X-ray source, 12° analyzer aperture and an analyzer pass energy of 150 eV. For the flat Au surfaces, data were acquired at nine different average photoelectron emission angles ranging from 5° to 85°. The photoelectron emission angle is the angle between the sample substrate normal and the analyzer central axis. Surface elemental compositions were determined from scans of the C1s, Au4f, O1s and S2p peaks. To minimize any x-ray induced sample damage the XPS measurements were taken at 3 spots on each sample. At the first spot C1s, Au4f and O1s spectra were acquired at photoelectron emission angles of 5, 25, 45, 65, 85, and 5°. At the second spot C1s, Au4f and O1s spectra were acquired at photoelectron emission angles of 15, 35, 55, 75 and 15°. At the third spot S2p spectra were acquired at all photoelectron emission angles. The initial photoelectron emission angle at each spot was always measured again at the end of the data acquisition from that spot to check for any presence of sample damage. The AuNP samples, deposited onto a cleaned Si substrate, were only measured at photoelectron emission angles of 0, 45 and 55°. A Si 2p spectrum was included in the data acquisition for the AuNPs to check for the presence of any signal from the underlying Si substrate.42 The AuNP samples were run as electrical insulators and a low-energy flood gun was used for charge neutralization. The other XPS measurement conditions were kept the same for all AuNP and flat Au samples. Measurements were repeated on at least three samples. Data analysis to determine peak areas and surface elemental compositions was performed with the ESCA Analysis software. All binding energies were referenced to the hydrocarbon C 1s peak at 285 eV.
SESSA modeling for SAMs on flat Au surfaces
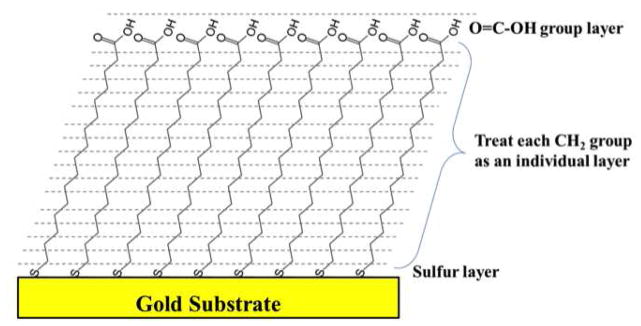
With SESSA spectra of multilayer flat surfaces can be simulated based on user-defined parameters that include layer compositions, thicknesses, density and surface roughness. The values for these parameters can be varied to match the measured experimental data. Spectrometer settings (photoelectron emission angle, photoelectron acceptance angle, etc.) for SESSA calculations were matched with the experimental settings. Simulations were performed for 9 photoelectron emission angles ranging from 5 to 85° in 10° increments to match the experimental measurements. After the spectrum is generated, the areas under the main photoelectron peaks are integrated using XPSPEAK freeware. Then, the areas are divided by the corresponding sensitivity factors and normalized to calculate the surface atomic compositions. The C16 COOH-SAM on the flat Au surface was modeled in SESSA as shown in Figure 1. Since the 14nm diameter of the AuNP is larger than the XPS sampling depth, only the outer SAM surface needs to be accounted for in the simulations.32, 33 The SAM was assumed to have 17 layers: S (bottom layer), CH2 (15 separate layers) and COOH (top layer). A SAM surface density of 21.4Å2 per chain9 was used to calculate the density of each sample layer in terms of number of atoms/cm3. The thickness of the CH2-layers and the relative surface area (RSA) of the Au surface were varied to determine the simulation conditions that provided the best agreement with the measured experimental data. To understand and optimize the SESSA results, various simulations were performed using the straight line approximation (no elastic scattering), the transport approximation for elastic scattering and the Mott cross sections for elastic scattering, where the elastic scattering cross-sections were calculated with a Dirac-Hartree-Fock potential (DHF) or a Thomas-Fermi-Dirac potential (TFD).38, 39
Figure 1.
Model for the carboxylic terminated SAM on a flat gold surface that was used in the SESSA calculations.
SESSA modeling for SAMs on AuNPs
TEM measurements showed the AuNPs had a spherical shape (Figure 1-SI in Supporting Information, also see reference 42). To model the AuNP shape, it was assumed that a sphere could be approximated as being constructed from 9 cylinders. The first cylinder is along the central axis of the sphere for emission angles between 0° and 10°, the second cylinder is for emission angles between 10° and 20°, etc., as shown in Figure 2. The end surface of each cylinder is taken as an angled, flat surface with an adsorbed SAM. The angled surfaces of each cylinder were then modeled as a SAM on an infinitely thick flat Au substrate that is measured at the photoelectron emission angle equal to the average angle of the corresponding cylinder. For example, the central cylinder between 0° and 10° had an average 5° photoelectron emission angle, and the outermost cylinder between 80° and 90° had an average 85° photoelectron emission angle. This type of modeling was previously reported in the XPS MultiQuant analysis of spherical samples.36
Figure 2.
AuNP modeled as multi-concentric cylinders with widths defined by angle increments of θ, where each cylinder surface has an average photoelectron emission angle of αi (10° ≤ θi ≤ 90° (hemisphere) and 5° ≤ αi ≤ 85°). The XPS detector is positioned at 0° from the central axis of the AuNP. (a) The sphere is divided into 9 concentric cylinders, (b) the end of each cylinder is modeled as a flat surface with infinite thickness of gold, and (c) the surface composition of each flat Au sample is weighted by its geometric factor and then summed together to find the AuNP surface composition.
After performing the flat Au-SAM simulations for all 9 photoelectron emission angles, the atomic composition for each photoelectron emission angle was multiplied by a geometric weighting factor to account for the different areas of each cylinder. The products were then summed together to obtain an atomic composition that could be compared to the atomic composition of the SAM on AuNPs (see Supporting Information for the geometric weighting factor equation).
Results and Discussion
Modeling the C16 COOH-SAM on the flat Au surface
The surface atomic composition of C16 COOH-SAM on flat Au was measured by XPS at 9 different photoelectron emission angles ranging from 5° to 85° in 10° increments. The left hand columns of Table 1 show the average XPS-determined atomic compositions with standard deviations based on measurements taken from three samples. Only C, Au, O and S were detected, as expected. The oxygen is located at the outermost surface of the carboxylic SAM. The oxygen intensity was constant, within experimental error, at photoelectron emission angles from 5 to 25° and then increased as the photoelectron emission angle increased from 25° to 85° since the XPS sampling depth decreases with increasing photoelectron emission angle. The Au substrate signal followed an opposite trend. The carbon signal increased with increasing photoelectron emission angle. Because there is only one S atom per chain and it is attached to the AuNP surface, the S2p intensity had larger errors and did not exhibit a clear trend. The S2p signal was not detectable at the 85° photoelectron emission angle. The SESSA calculated compositions shown in Table 1 exhibited trends similar to those observed in the experimental results.
Table 1.
Surface compositions of the C16 COOH-SAM on the flat Au surface measured or simulated at nine different emission angles ranging from 5 to 85° in 10° increments. The simulated compositions were based on 1.2Å per CH2 group, RSA = 1.04, and with or without a 1.5Å CH2-contamination layer. (Standard deviations are shown in parentheses)
| Photoelectron Take-off Angles (°) | XPS (atomic %) | SESSA: 1.2Å per CH2; RSA–1.04 No contamination (atomic %) |
SESSA: 1.2Å per CH2; RSA–1.04 Contamination = 1.5Å CH2 (atomic %) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C 1s | Au 4f | O 1s | S 2p | C 1s | Au 4f | O 1s | S 2p | C 1s | Au 4f | O 1s | S 2p | |
| 5 | 54.0 (2.8) | 33.6 (1.5) | 10.7 (1.5) | 1.7 (0.2) | 52.9 | 34.5 | 10.2 | 2.4 | 56.3 | 32.6 | 9.0 | 2.1 |
|
|
||||||||||||
| 15 | 54.7 (2.3) | 32.6 (1.1) | 10.9 (0.8) | 1.8 (0.4) | 54.4 | 33.8 | 9.6 | 2.3 | 55.1 | 33.9 | 8.8 | 2.2 |
|
|
||||||||||||
| 25 | 56.7 (2.9) | 30.0 (1.5) | 11.1 (0.4) | 2.3 (1.0) | 55.9 | 30.7 | 10.8 | 2.6 | 55.6 | 32.2 | 10.0 | 2.2 |
|
|
||||||||||||
| 35 | 58.3 (0.7) | 27.3 (0.5) | 12.6 (0.6) | 1.8 (0.6) | 57.6 | 29.1 | 11.1 | 2.3 | 60.1 | 25.9 | 11.9 | 2.1 |
|
|
||||||||||||
| 45 | 60.3 (1.5) | 24.9 (1.1) | 13.2 (0.8) | 1.8 (0.3) | 58.1 | 24.7 | 14.9 | 2.3 | 62.4 | 23.6 | 11.9 | 2.1 |
|
|
||||||||||||
| 55 | 64.9 (0.4) | 19.4 (0.6) | 13.9 (1.0) | 1.9 (0.1) | 61.7 | 17.3 | 19.2 | 1.8 | 67.4 | 16.2 | 14.4 | 2.0 |
|
|
||||||||||||
| 65 | 68.2 (1.0) | 14.0 (0.9) | 16.0 (0.3) | 1.9 (0.5) | 62.2 | 10.5 | 26.1 | 1.3 | 71.7 | 10.7 | 16.3 | 1.4 |
|
|
||||||||||||
| 75 | 72.2 (2.1) | 9.1 (1.8) | 17.8 (1.3) | 0.8 (0.5) | 57.8 | 6.9 | 34.3 | 1.0 | 74.0 | 6.9 | 18.0 | 1.0 |
|
|
||||||||||||
| 85 | 74.6 (2.2) | 5.1 (0.9) | 20.3 (1.3) | nd | 55.0 | 4.9 | 38.9 | 1.2 | 74.3 | 5.2 | 19.9 | 0.7 |
nd = not detected
Performing the SESSA calculations at various combinations of relative surface area (RSA) and SAM thickness values, it was noticed that these two parameters tend to compensate one another. As seen in Figure 3-SI, for photoelectron emission angles ranging from 5 to 55°, the combinations of RSA and SAM thickness that provided the best agreement with the experimental XPS data fell on the diagonal line from the larger-RSA/smaller-thickness combination to smaller-RSA/larger-thickness combination. The accuracy of the derived overlayer thickness is limited by knowledge of the surface roughness (and vice-versa).
The C16 COOH-SAM SESSA simulation with RSA =1.04 and a CH2 layer thickness of 1.2Å at the different photoelectron emission angles (Table 1, middle columns) showed reasonably similar surface compositions as the XPS experimental results, especially for photoelectron emission angles from 5 to 55°. Note that 1.2Å per CH2-group corresponded to an overall C16 COOH-SAM thickness of 21.5Å when the COOH and S groups were included. The XPS and SESSA results differed at the glancing angles, especially for the oxygen and carbon concentrations (compare left and middle columns in Table 1). At the glancing angles, the SESSA results showed a steeper increase in the O1s concentration and a decrease in the C1s concentration compared to the XPS results. To quantify these differences, we used the relative sum-of-squares difference, ΣXr2, between the SESSA and XPS determined atomic compositions. Equations defining ΣXr2 are provided in the Supporting Information.
The ΣXr2(F) values for SESSA compositions calculated using RSA =1.04 and a thickness =1.2Å/CH2 layer (21.5Å total SAM thickness) for the 9 photoelectron emission angles are shown in Figure 4-SI in the Supporting Information. This figure also includes the ΣXr2(F) values for all the atoms except O and ΣXr2(F) values for just O. Figure 4-SI and Table 1 show there is a large difference between the SESSA and XPS results at the glancing photoelectron emission angles and that differences in the oxygen concentrations accounts for most of this difference.
Differences between the SESSA and XPS results at glancing angles
In addition to relative surface roughness and SAM thickness, other simulation conditions, including the presence of elastic scattering, the method used to calculate elastic cross-section, the number of inelastic collisions and the SAM model structure, were investigated to optimize the simulation results. However, varying these parameters did not significantly improve the agreement between the simulated and the experimental data at the glancing photoelectron emission angles (see Figure 5-SI). To examine if the elastic scattering was accurately accounted for at the glancing emission angles and if there was any other unaccounted error with the simulation, SESSA and XPS results from a methyl-terminated SAM prepared from 1-hexadecane thiol (C16 CH3-SAM) on flat Au were compared. The simulation and the experimental results matched well for the C16 CH3-SAM at all photoelectron emission angles (Figure 5-SI). Since long X-ray exposure could result in sample damage and decrease the O1s signal in the experimental data due to loss of the carboxyl functionality, a sample damage study was performed for C16 COOH-SAM on the flat Au surface. No significant change in oxygen concentration was detected for X-ray exposures up to 60 minutes (see Figure 6-SI), which is longer than the X-ray exposure times used to collect the XPS data for comparison with the SESSA results. Thus, X-ray induced damage in the sample cannot explain the difference between the SESSA and XPS results at the glancing photoelectron emission angles.
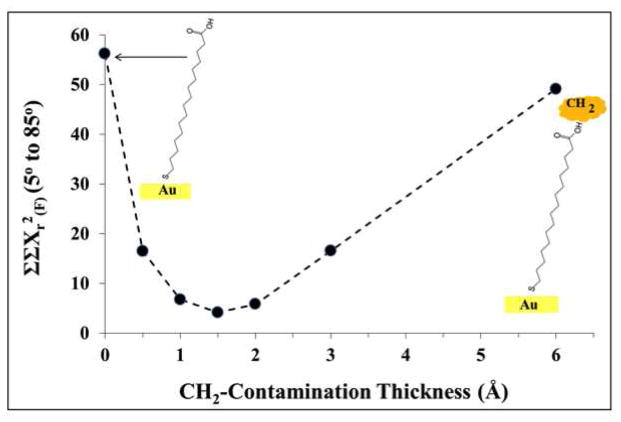
Another factor that could result in lower oxygen and higher carbon concentrations in the experimental data, especially at the glancing photoelectron emission angles, would be the presence of hydrocarbon contamination on the sample surface. A thin hydrocarbon layer on top of the carboxylic groups would contribute higher carbon signals in the XPS measurements. Since the oxygen would be covered by this contamination layer, its measured XPS concentration would be lower than the oxygen concentration predicted at the glancing photoelectron emission angles by SESSA. To investigate this possibility, various thicknesses of a hydrocarbon contamination overlayer (input as CH2) were included in the SESSA calculations using a sample roughness of RSA=1.04 and a thickness of 1.2Å/CH2-group. Figure 3 shows the ΣΣXr2(F)(5° to 85°), as defined by Equation 3 in the Supporting Information, for various effective thicknesses of the hydrocarbon contamination layer. The agreement between the SESSA and the XPS results improved significantly when a hydrocarbon contamination layer with effective thickness between 1 and 2 Å was included in the SESSA calculations. Figure 7-SI clearly shows the improvement in ΣXr2(F) across all photoelectron emission angles when a 1.5Å hydrocarbon contamination overlayer is included in the SESSA calculations. The surface atomic compositions for C16 COOH-SAM on flat Au with the hydrocarbon contamination overlayer are given in the right-hand columns of Table 1 for comparison with the XPS results and the corresponding SESSA results without the contamination layer.
Figure 3.
(a) Comparison between XPS and SESSA results for the C16 COOH-SAM on a flat Au surface, where the simulation sample includes a contamination layer overlayer. The sample RSA and the CH2 layer thickness were set at 1.04 and 1.2Å, respectively.
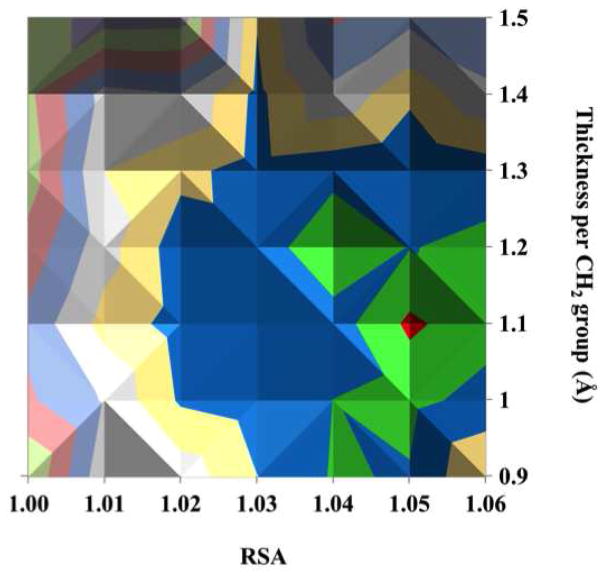
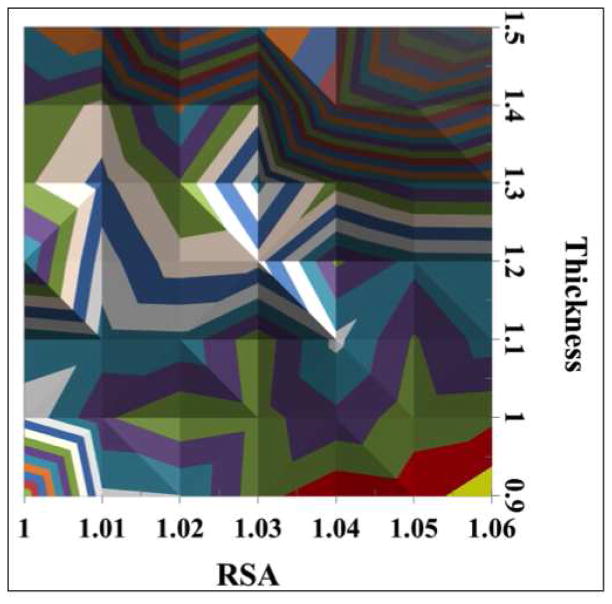
The results of simulations with various combinations of RSA and SAM thickness for C16 COOH-SAM on flat Au with a 1.5Å hydrocarbon contamination overlayer are shown in Figure 4. There is a general trend that the larger RSA values combined with smaller SAM thicknesses provide better agreement between the SESSA and XPS results (blue, green and red colors in the figure represent the smallest difference between the two). The simulation based on a combination of RSA of 1.05 and thickness of 1.1Å/CH2-group (corresponding to a total SAM thickness of 20Å) in the presence of 1.5Å CH2-contamination layer gave the smallest difference, ΣΣXr2(F) (5° to 85°) = 3.6, between the SESSA and XPS results. The 1.1 Å/CH2 value (total SAM + contamination film thickness = 21.5Å) compares favorably to the previous value of 1.16Å/CH2 (total film thickness = 22.2Å) reported by Bain et al. from ellipsometry studies of COOH-SAMs on Au surfaces, where ellipsometric film thickness(Å)=1.16n + 4.8 with n equal to the number of CH2 groups in COOH-(CH2)n-SH.1 Note that the ellipsometry results would include the thicknesses of both the SAM and any contamination present on the SAM.
Figure 4.
Comparison of XPS and SESSA results based on ΣΣXr2(F) (5° to 85°) for surface compositions of the C16 COOH-SAM on a flat Au surface at various combinations of RSA and thickness per CH2 group. The bottom figure is the top view.
Modeling C16 COOH-SAM on AuNPs
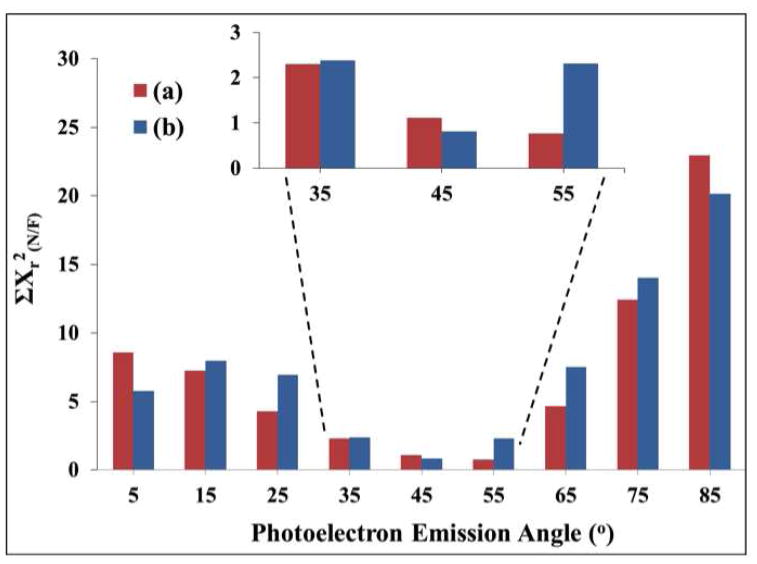
From the TEM measurements, Figure 1-SI and reference 42, the AuNPs had an average diameter of 14nm ± 0.9nm and a circularity of 1.09±0.06, where the circularity here is defined as the ratio of major axis to minor axis. The surface compositions of the C16 COOH-SAM covered 14nm AuNPs measured with XPS at three different macroscopic photoelectron emission angles (0, 45 and 55°) are shown in Table 2. The surface composition dependence on the emission angle was negligible, as expected since spherical NPs should sample the full range of photoelectron emissions angles regardless of the nominal angle the substrate is placed with respect to the XPS analyzer. The C16 COOH-SAM AuNP results at the 0° macroscopic photoelectron emission angle were compared with SESSA and XPS results of the flat surfaces at the nine different photoelectron emission angles (for SESSA using RSA = 1.05, thickness = 1.1Å/CH2-groups and hydrocarbon contamination thickness = 1.5Å). It was found that the XPS experimental measurements on a flat Au surface at 55 ° and the SESSA calculations on a flat Au surface at 45° photoelectron emission angles gave the best agreement with the measured XPS AuNP-SAM results (see Figure 5). Hence, these results indicate the effective or “magic” photoelectron emission angle for the 14nm AuNPs is between 45 and 55°. Kappen et al.45 have previously suggested using a magic photoelectron emission angle of approximately 55° for spherical samples. However, the exact value of the magic angle will vary with the structure and composition of the spherical particle.32
Table 2.
XPS determined surface compositions of the C16 COOH-SAM on the 14nm AuNPs (with standard deviations in parentheses) at three macroscopic photoelectron emission angles.
| XPS (atomic %) | |||
|---|---|---|---|
| Photoelectron Line | Photoelectron Emission Angle (°) | ||
| 0 | 45 | 55 | |
| C 1s | 64.9 (1.1) | 66.1 (0.7) | 66.8 (0.7) |
| Au 4f | 21.6 (0.4) | 21.4 (1.7) | 21.4 (0.3) |
| O 1s | 11.4 (1.0) | 10.6 (1.3) | 9.8 (0.9) |
| S 2p | 2.1 (0.3) | 1.9 (0.4) | 2.0 (0.6) |
Figure 5.
ΣXr2(N/F) showing comparisons of the XPS determined surface compositions of C16 COOH-SAMs on 14nm AuNP at 0° macroscopic photoelectron emission angle with (a) the SAM on flat Au measured at various photoelectron emission angles ranging from 5 to 85°; (b) the SAM on flat Au simulated at various photoelectron emission angles ranging from 5 to 85° using 1.1Å per CH2, RSA=1.05 and CH2-contamination thickness=1.5Å.
As described earlier, the curved AuNP surface was assumed to be a series of flat surfaces that encompass the entire range of emission angles when viewed from the XPS analyzer window. That is, the surface composition of C16 COOH-SAM on AuNP can be modeled by geometrically correcting and combining the surface compositions of the C16 COOH-SAM on flat Au measured under similar instrument settings at the 9 photoelectron emission angles. The geometric weighting method used to combine the data from the 9 photoelectron emission angles is described in the Supporting Information. The SESSA surface atomic compositions from various combinations of surface roughness and SAM thickness for the C16 COOH-SAM on flat Au (for each cylinder end) with and without a 1.5Å CH2-contamination layer were weighted by the geometric factors and compared with the experimental surface atomic compositions of the C16 COOH-SAM on the 14nm AuNPs. Including the contamination layer produced better agreement between the SESSA and XPS results, as shown in Table 3. Assuming that the roughness and CH2 layer thickness values apply equally well to the NPs produced reasonably consistent results. Both the summation of the weighted flat surface XPS experimental data (ΣXr2(N/F) = 0.64) and the summed weighted SESSA model of the layers show good agreement with the AuNP data. This result suggests that both approaches can be used to model NP data and determine the thickness of both a simple single-layer or complex multi-layer samples. However, the optimum SAM thickness and RSA combination values, which produced the best agreement between the SESSA calculations and the measured AuNP-SAM surface compositions, occurred for slightly larger RSA and lower CH2 layer thickness values compared to the flat film results. SESSA calculations with RSA=1.06 and CH2 layer thickness = 0.9Å/CH2 with a 1.5Å CH2-contamination overlayer gave the best matching with ΣXr2(N) = 0.03, as shown in Figure 6 and Table 3. This combination of SAM thickness and RSA values was at the limit of the range of values considered for these parameters in our study. However, considering the very small ΣXr2(N) values relative to the standard deviation values of the experimental results, further simulations beyond this limit are not expected to produce a significant improvement in the agreement. The SESSA calculations with the parameters that provided the best result for the flat Au surfaces (RSA=1.05, CH2 layer thickness = 1.1Å/CH2 and a 1.5Å CH2-contamination overlayer) gave a ΣXr2(N) = 0.29, as shown in Table 3. These results indicate that the C16 COOH-SAM had different thicknesses on the flat Au (= 20Å corresponding to 1.1Å/CH2-group) and AuNP surfaces (= 17Å corresponding to 0.9Å/CH2-group). In both cases, the COOH and S groups were set to have a total thickness of 3.5Å in SESSA. This difference could result from alkyl chains in the C16 COOH-SAM being, on average, more tilted from the surface normal on the AuNPs compared to these alkyl chains on flat Au surfaces. The three angstrom change in thickness would correspond to an approximately 13° additional tilt to the ~30° tilt found on flat Au4, 46–48. Ghorai et al.27 used molecular dynamics simulations to show that the tilt angle of the chains in a SAM is greater on AuNPs compared to flat Au. They observed that a C16 CH3-SAM on 5nm diameter AuNPs had a tilt angle of ~41° at 300K. They also observed that increasing the diameter of the AuNP decreased the tilt angle. For example, C13 CH3-SAMs had tilt angles of ~39°, ~37° and ~34° on AuNPs with a diameters of 5, 7 and 11nm, respectively, at 300K.27 The tilt angle they observed for the C13 CH3-SAM on flat Au was 24±5° at 300K. Thus, the molecular dynamics simulations of Ghorai et al.27 are qualitatively consistent with increased SAM tilt angles on AuNPs compared to flat Au. Disorder induced by the presence of surface defects, step edges, etc. on the AuNP surface could also account for some of the difference from the flat Au surface. Sum frequency generation vibrational spectroscopy studies by Weeraman et al.49 have shown that the number of gauche defects in SAM alkyl chains increases as the diameter of the AuNP decreases.
Table 3.
Summary of the atomic compositions for 14nm AuNP-C16 COOH-SAM measured from XPS and calculated from flat Au data of XPS and SESSA at various modeling conditions and photoelectron emission angles. Also shown is the ΣXr2(N*) when the standard deviations of the experimental measurements are included.
| XPS (atomic %) | SESSA (atomic %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AuNP | Flat Au | Flat Au | Flat Au | |||||||
| RSA = 1.05 1.1Å/CH2; layer |
RSA= 1.06 0.9Å/CH2; layer |
RSA = 1.03 0.9Å/CH2; layer |
||||||||
| Photoelectron Line | α=0° | α=55° | α=5° to 85° (geometric correction) | α=45° | α=5° to 85° (geometric correction) | |||||
| % | SD | % | SD | CH2-contamination = 1.5Å | No contamination | |||||
| C 1s | 64.9 | 1.1 | 64.9 | 0.4 | 62.1 | 61.2 | 63.1 | 64.7 | 58.2 | 59.1 |
| Au 4f | 21.6 | 0.4 | 19.9 | 0.6 | 22.6 | 25.2 | 22.1 | 21.5 | 23.5 | 24.4 |
| O 1s | 11.4 | 1.0 | 13.9 | 1.0 | 13.6 | 11.6 | 13.0 | 11.8 | 16.1 | 14.3 |
| S 2p | 2.1 | 0.3 | 1.9 | 0.1 | 1.7 | 2.0 | 1.9 | 1.9 | 2.1 | 2.2 |
| ΣXr2(N) | 0.0a | 0.76b | 0.64c | 0.81d | 0.29e | 0.03e | 2.14e | 1.62e | ||
| ΣXr2(N*) + (SD) | 0.15 | 0.59 | 0.52 | 0.91 | 0.25 | 0.14 | 2.80 | 1.24 | ||
| ΣXr2(N*) − (SD) | 0.15 | 1.30 | 1.13 | 1.02 | 0.67 | 0.22 | 3.93 | 2.39 | ||
= ΣXr2(N) comparing the average XPS AuNP composition to itself.
= ΣXr2(N) comparing the average XPS AuNP composition with the average XPS flat Au composition at a 55° photoelectron emission angle.
= ΣXr2(N) comparing the average XPS AuNP composition with the corrected XPS flat Au composition weighed and summed from the XPS compositions at 9 photoelectron emission angles.
= ΣXr2(N) comparing the average XPS AuNP composition with the SESSA flat Au composition at a 45° photoelectron emission angle (RSA=1.05, 1.1Å/CH2 layer, and 1.5Å CH2-contamination).
= ΣXr2(N) comparing the average XPS AuNP composition with the SESSA flat Au composition weighed and summed from the SESSA compositions at 9 photoelectron emission angles (RSA=1.05, 1.06 or 1.03; CH2-group thickness = 1.1 or 0.9Å; and with or without 1.5Å CH2-contamination).
The values for ΣXr2(N*) compare the AuNP’s average XPS% plus its standard deviation (ΣXr2(N*)+(SD)) or minus its standard deviation (ΣXr2(N*)−(SD)) with the other composition values described above (a through e).
Figure 6.
ΣXr2(N) comparison of XPS% with the SESSA% using Equation 2. The XPS data is from the C16 COOH-SAM on 14nm AuNPs. The SESSA data is from the C16 COOH-SAM on a flat Au surface, summed across all nine photoelectron emission angles using the geometric weighting factors. For the SESSA calculations the RSA and CH2-group thickness were varied and a 1.5Å thick hydrocarbon contamination overlayer was included in all calculations.
To get an idea of the sensitivity of the ΣXr2(N), the standard deviations (SD) of the AuNP’s composition determined experimentally by XPS (XPS%) were included in the calculation of ΣXr2(N), as shown in the bottom rows of Table 3 (indicated as ΣXr2(N)+(SD) and ΣXr2(N)−(SD)). When comparing the AuNP’s average XPS% with the AuNP’s average XPS% ± SD, ΣXr2(N/N*) = 0.15, where (N/N) signifies that the comparison was made between AuNP XPS% values, and * signifies that the reference AuNP XPS% included its SD. And, ΣXr2(N)+(SD) and ΣXr2(N)−(SD) were 0.14 and 0.22, respectively, when the optimum simulation condition (RSA=1.06, thickness = 0.9Å/CH2 layer and a 1.5Å CH2-contamination layer) was compared with the average AuNP’s XPS% plus and minus the SD, respectively. Both values were similar to the measured AuNP-SAM’s ΣXr2 value of 0.15. When performing the same calculations but with RSA=1.05, thickness = 1.1Å/CH2 layer and a 1.5Å CH2-contamination layer, theΣXr2(N)+(SD) and ΣXr2(N)−(SD) values were 0.25 and 0.67, respectively. Although these values are slightly higher than the AuNP-SAM’s ΣXr2 value of 0.15, they do suggest that the optimum parameters obtained from comparing SESSA results (SESSA%) with the flat Au XPS experimental results also provide a reasonable agreement with the AuNP experimental XPS results. In contrast, when no contamination layer (RSA=1.06 and thickness = 0.9Å/CH2-group) was included in the SESSA calculations, the agreement was much worse (ΣXr2(N) =2.14, with that could be achieved without a ΣXr2(N)+(SD) = 2.8 and ΣXr2(N)−(SD) = 3.93). The minimum ΣXr2(N) contamination layer was 1.62 when RSA =1.03 and SAM thickness = 0.9Å/CH2 (also shown in Table 3). Thus, for both the flat Au and AuNPs the SESSA results indicate it is important to account for the presence of a hydrocarbon contamination layer.
Conclusions
The overlayer thicknesses and the relative surface roughness for a C16 COOH-SAM on a flat Au surface have been calculated using SESSA and compared to the corresponding XPS results. To obtain the best agreement between the SESSA and XPS determined compositions, the presence of a hydrocarbon contamination overlayer on the experimental sample had to be accounted for. A thickness of 1.1Å/CH2 group, a RSA of 1.05, and a 1.5Å thick hydrocarbon contamination overlayer (21.5Å thickness for SAM + hydrocarbon overlayer) produced the best agreement between the simulated and measured intensities This result is in reasonable agreement with previous studies. The flat Au-SAM results from SESSA were extended by geometric weighting to approximate a spherical nanoparticle and to calculate the overlayer thickness and RSA combination for the C16 COOH-SAM on AuNPs. It was found that on the 14 nm diameter AuNPs, the overall SAM thickness decreased by 3Å with SESSA parameters of 0.9Å/CH2 group, RSA of 1.06 and a 1.5Å thickness of CH2-contamintion providing the best agreement with the measured XPS compositions. This decreased SAM thickness on the AuNPs suggests that the alkyl chains may be slightly more tilted or disordered on the AuNP surfaces compared to the flat Au surfaces. The results from this study show that SESSA is an effective simulation method that improves the quantitative analysis of both flat and nanoparticle surfaces with XPS.
Supplementary Material
Acknowledgments
This research was supported by NIH grants GM-074511 and EB-002027 (NESAC/Bio). SDT thanks NSF for an IGERT fellowship and Intel for a fellowship. DRB acknowledges support for nanoparticle research from Office of Basic Energy Sciences of the US DOE and NIH grant U19 ES019544. Portions of this work were associated with the Environmental Molecular Sciences Laboratory (EMSL), a DOE user facility operated by Pacific Northwest National Laboratory for the Office of Biological and Environmental Research of the DOE. The authors gratefully acknowledge helpful discussions and encouragement from Dr. Cedric Powell of the National Institute of Standards and Technology.
Footnotes
Additional text and figures about the comparison of SESSA and XPS results, the variation of SESSA parameters, the x-ray degradation studies, and the TEM analysis, as well additional detail about the experimental methods are provided in the supporting information.
References
- 1.Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. J Am Chem Soc. 1989;111:321–335. [Google Scholar]
- 2.Bain CD, Evall J, Whitesides GM. J Am Chem Soc. 1989;111:7155–7164. [Google Scholar]
- 3.Castner DG, Hinds K, Grainger DW. Langmuir. 1996;12:5083–5086. [Google Scholar]
- 4.Dubois LH, Nuzzo RG. Annu Rev Phys Chem. 1992;43:437–463. [Google Scholar]
- 5.Graham DJ, Ratner BD. Langmuir. 2002;18:5861–5868. [Google Scholar]
- 6.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 7.Nuzzo RG, Allara DL. J Am Chem Soc. 1983;105:4481–4483. [Google Scholar]
- 8.Nuzzo RG, Fusco FA, Allara DL. J Am Chem Soc. 1987;109:2358–2368. [Google Scholar]
- 9.Strong L, Whitesides GM. Langmuir. 1988;4:546–558. [Google Scholar]
- 10.Tao F, Bernasek SL. Chem Rev. 2007;107:1408–1453. doi: 10.1021/cr050258d. [DOI] [PubMed] [Google Scholar]
- 11.Ulman A. An introduction to ultrathin organic films: from Langmuir-Blodgett to self-assembly. Academic Press; Boston: 1991. [Google Scholar]
- 12.Ulman A. Chem Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- 13.Pan S, Castner DG, Ratner BD. Langmuir. 1998;14:3545–3550. [Google Scholar]
- 14.Tidwell CD, Ertel SI, Ratner BD, Tarasevich B, Atre S, Allara DL. Langmuir. 1997;13:3404–3413. [Google Scholar]
- 15.Apte JS, Collier G, Latour RA, Gamble LJ, Castner DG. Langmuir. 2010;26:3423–3432. doi: 10.1021/la902888y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baio JE, Weidner T, Samuel NT, McCrea K, Baugh L, Stayton PS, Castner DG. J Vac Sci Technol, B: Nanotechnol Microelectron: Mater, Process, Meas, Phenom. 2010;28:C5D1–C5D8. doi: 10.1116/1.3456176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baugh L, Weidner T, Baio JE, Nguyen PCT, Gamble LJ, Stayton PS, Castner DG. Langmuir. 2010;26:16434–16441. doi: 10.1021/la1007389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng F, Gamble LJ, Grainger DW, Castner DG. Anal Chem. 2007;79:8781–8788. doi: 10.1021/ac0715423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finklea HO. Electroanal Chem. 1996;19:109–335. [Google Scholar]
- 20.Adams DM, Brus L, Chidsey CED, Creager S, Creutz C, Kagan CR, Kamat PV, Lieberman M, Lindsay S, Marcus RA, Metzger RM, Michel-Beyerle ME, Miller JR, Newton MD, Rolison DR, Sankey O, Schanze KS, Yardley J, Zhu X. J Phys Chem B. 2003;107:6668–6697. [Google Scholar]
- 21.Gooding JJ, Mearns F, Yang W, Liu J. Electroanalysis. 2003;15:81–96. [Google Scholar]
- 22.Burleigh TD, Gu Y, Donahey G, Vida M, Waldeck DH. Corrosion. 2001;57:1066–1074. [Google Scholar]
- 23.Aizenberg J. Dalton. 2000:3963–3968. [Google Scholar]
- 24.Ostuni E, Yan L, Whitesides GM. Colloids Surf, B. 1999;15:3–30. [Google Scholar]
- 25.Grainger DW, Castner DG. Adv Mater. 2008;20:867–877. [Google Scholar]
- 26.Daniel M-CAD. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 27.Ghorai PK, Glotzer SC. J Phys Chem C. 2007;111:15857–15862. [Google Scholar]
- 28.Luedtke WD, Landman U. J Phys Chem B. 1998;102:6566–6572. [Google Scholar]
- 29.Baer DR, Gaspar DJ, Nachimuthu P, Techane SD, Castner DG. Anal Bioanal Chem. 2010;396:983–1002. doi: 10.1007/s00216-009-3360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baer DR, Engelhard MH. J Electron Spectrosc Relat Phenom. 2010;178–179:415–432. [Google Scholar]
- 31.Venezia AM. Catal Today. 2003;77:359–370. [Google Scholar]
- 32.Frydman A, Castner DG, Schmal M, Campbell CT. J Catal. 1995;157:133–144. [Google Scholar]
- 33.Frydman A, Castner DG, Schmal M, Campbell CT. J Catal. 1995;152:164–178. [Google Scholar]
- 34.Zorn G, Dave SR, Gao X, Castner DG. Anal Chem. 2011;83:866–873. doi: 10.1021/ac102516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shard AG, Wang J, Spencer SJ. Surf Interface Anal. 2009;41:541–548. [Google Scholar]
- 36.Mohai M. XPS Multiquant. Budapest: 2008. http://www.chemres.hu/aki/XMQpages/XMQhome.htm. [Google Scholar]
- 37.Tougaard S. QUASES: Quantitative Analysis of Surfaces by Electron Spectroscopy. Denmark: 2004. http://www.quases.com. [Google Scholar]
- 38.Smekal W, Werner WSM, Powell CJ. Surf Interface Anal. 2005;37:1059–1067. [Google Scholar]
- 39.Werner WSM, Smekal W, Powell CJ. NIST Database for the Simulation of Electron Spectra for Surface Analysis, Version 1.0. National Institute of Standards and Technology; Gaithersburg, Maryland: 2010. [Google Scholar]
- 40.Werner WSM. Surf Interface Anal. 2005;37:846–860. [Google Scholar]
- 41.Powell CJ, Jablonski A, Werner WSM, Smekal W. Appl Surf Sci. 2005;239:470–480. [Google Scholar]
- 42.Techane SD, Gamble LJ, Castner DG. J Phys Chem C. 2011;115:9432–9441. doi: 10.1021/jp201213g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turkevich J, Stevenson PC, Hillier J. Discussions of the Faraday Society. 1951;11:55–75. [Google Scholar]
- 44.Frens G. Nature (London), Phys Sci. 1973;241:20–22. [Google Scholar]
- 45.Kappen P, Reihs K, Seidel C, Voetz M, Fuchs H. Surf Sci. 2000;465:40–50. [Google Scholar]
- 46.Bain CD, Whitesides George M. Angewandte Chemie International Edition. 1989;28:506–512. [Google Scholar]
- 47.Porter MD, Bright TB, Allara DL, Chidsey CED. J Am Chem Soc. 1987;109:3559–3568. [Google Scholar]
- 48.Bain CD, Whitesides George M. J Am Chem Soc. 1989;111:7164–7175. [Google Scholar]
- 49.Weeraman C, Yatawara AK, Borkenyuk AN, Benderskii AV. J Am Chem Soc. 2006;128:14244–14245. doi: 10.1021/ja065756y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.