Abstract
Objective
Low-dose methotrexate [MTX] is an effective therapy for rheumatoid arthritis yet its mechanism of action is incompletely understood. Here, we explored induction of apoptosis by MTX.
Methods
We employed flow cytometry to assess changes in levels of intracellular proteins, reactive oxygen species [ROS], and apoptosis.Quantitative polymerase chain reaction was usedtoassess changes in transcript levels of select target genes in response to MTX.
Results
MTX does not directly induce apoptosis but rather ‘primes’ cells for markedly increased sensitivity to apoptosis via either mitochondrial or death receptor pathways by a Jun N-terminal kinase [JNK]-dependent mechanism. Increased sensitivity to apoptosis is mediated, at least in part, by MTX-dependent production of reactive oxygen species, JNK activation and JNK-dependent induction of genes whose protein products promote apoptosis. Supplementation with tetrahydrobiopterin blocks these methotrexate-induced effects. Subjects with rheumatoid arthritis on low-dose MTX therapy express elevated levels of the JNK-target gene, JUN.
Conclusions
Our results support a model whereby methotrexate inhibits reduction of dihydrobiopterin to tetrahydrobiopterin resulting in increased production of ROS, increased JNK activity and increased sensitivity to apoptosis. The finding of increased JUN levels in subjects with RA taking low-dose MTX supports the notion that this pathway is activated by MTX, in vivo, and may contribute to efficacy of MTX in inflammatory disease.
INTRODUCTION
The folic acid antagonist, methotrexate [MTX], was initially developed during the 1940s to inhibit dihydrofolate reductase [DHFR] for treatment of malignancies [1-3]. The clinical potential of MTX in treating rheumatoid arthritis [RA] was initially suggested by Gubner in 1951, after studying the effects of MTX in six patients diagnosed with RA and was confirmed by further studiesconducted during the 1980s [4-8]. These studies had durations of 12 to 18 weeks, and dosages varied from 7.5 to 25 milligrams per week. MTX possessed anti-inflammatory effects in RA patients, as trial subjects demonstrated improved function, global assessments, joint scores and marked decreases in pain. Use of weekly low doses of MTX is not limited to RA therapy. Over the years, this treatment option has expanded to include additional inflammatory and autoimmune diseases [9-12].
In contrast to treatment of malignancy, much lower and more infrequent doses of MTX are used to treat inflammatory disease and basic mechanisms may differ greatly from those targeting cancer. Exact mechanisms underlying anti-inflammatory actions of MTX remain inconclusive in spite of its widespread application [13]. MTX, like natural folates, is polyglutamated once taken up by cells. MTX-polyglutamates, found in red blood cells, neutrophils, and mononuclear cells after oral administration, are believed to represent the active form of the drug, and levels of MTX-polyglutamates correlate with clinical efficacy in patients with RA[14, 15]. MTX stimulated synthesis of adenosine and its release by cells and subsequent activation of adenosine receptors may be one mediating factor contributing to anti-inflammatory actions of MTX [13, 16, 17]. MTX also inhibits T cell activation, induces T cell apoptosis, and alters expression of T cell cytokines and adhesion molecules [18-21]. Such actions may be partly mediated by synthesis and release of adenosine. MTX action may also be dependent, in part, upon its ability to stimulate production of reactive oxygen species [ROS] [22].
From these studies, underlying mechanisms behind induction of apoptosis by low concentrations of MTX are not immediately apparent. In general, cells undergo apoptosis via their ability to activate intrinsic [mitochondrial] or extrinsic [death receptor] pathways [23-29]. For example, the stress-inducible p53 protein plays a central role in transducing DNA damage signals to cause cellular apoptosis. Death receptors, such as Fas or the family of TNF receptors transmit signals through adapter molecules such as Fas-associated death domain, TNFR-associated death domain or death domain-associated protein. These adaptor proteins activate death caspases causing apoptotic cell death. Both intrinsic and extrinsic apoptosis pathways are thought to involve JNK signaling [29]. Here, we sought to investigate induction of apoptosis by low concentrations of MTX in a transformed human T cell line. We find that low concentrations of MTX do not directly induce apoptosis. Rather, MTX markedly increases sensitivity of cells to apoptosis mediated via either death receptor or mitochondrial pathways, in part, by increasing expression of genes whose protein products play key roles in induction of apoptosis. This alteration in the transcriptional profile of cells treated with MTX is dependent upon induction and activation of JNK by ROS. Apoptosis sensitivity and ROS production are prevented by supplementation with tetrahydrobiopterin [BH4] suggesting that inhibition of reduction of dihydrobiopterin [BH2] by DHFR initiates this pathway. Studies of subjects with RA who are on current MTX therapy support the notion that these pathways may be activated, in vivo, by MTX and contribute to therapeutic efficacy of MTX.
MATERIALS AND METHODS
Drugs and Reagents
MTX, adenosine, caffeine, theophylline, NAC, H2O2, and BI-78D3 were from Sigma. PepJIP1 was from Enzo Life Sciences. Anti-Fas antibody was obtained from Medical and Biological Laboratories, LTD. Caspase 3 activity was determined by enzymatic assay from Promega [CaspACE Colorimetric Assay System]. 384-well apoptosis TLDA plates were from Applied Biosystems. Plasmids containing JNK1 [MAPK8] and JNK2 [MAPK9] dominant-negative [DN] mutants were obtained from Addgene.
Cell Preparation and Culture
The human Jurkat T cell leukemia line was from ATCC. PBMC were purified by Ficoll-Hypaque gradient centrifugation. Cells were cultured in RPMI media, which contains 1 μg/ml folic acid, with 10% FCS, Penn-Strep, and L-glutamine in a 37°C atmosphere of 5% CO2 in air. Cells were plated at 0.8 × 106 cells per ml in 5 ml cultures. Cell viability and numbers were determined microscopically after staining with trypan blue.
Concentrations of MTX employed in the different experiments ranged between 0.01-1 μM [see figures and figure legends] and culture periods ranged from 24-72 hr. of continuous exposure to MTX where indicated. Pharmacokinetic analysis indicates that ingestion of a 20 mg tablet of MTX yields a concentration of MTX in plasma of approximately 0.5 μM in plasma after 1 hr. and a concentration of MTX in plasma of approximately 0.1 μM in plasma after 10 hr.[30].
Cell Transfection
Cells were transfected using the Amaxa Cell Line Nucleofector Kit V [Amaxa, Koeln, Germany]. Briefly, 1×106 Jurkat T cells were suspended in 100uL Cell Line Nucleofector Solution V containing 2μg of plasmid DNA and 2μg pmaxGFP vector. The cell suspension was transferred to the provided cuvette and nucleofected using program X-001 on the Amaxa Nucleofector apparatus [Amaxa]. Cells were incubated at room temperature for 10 minutes then transferred to pre-warmed culture medium in 6-well plates.
Quantitative RT-PCR
Total RNA was purified from blood collected in PAXgene tubes according to manufacturer's instructions [Qiagen] or from cell cultures using Tri-Reagent and quantified using a NanoDrop-1000 spectrophotometer. Five μg total RNA was used for cDNA synthesis [SuperScript III First-Strand Synthesis Kit, Invitrogen] with Oligo dT as the primer. RT-PCR reactions were prepared in duplicate in reaction volumes of 25 μl with 50 ng cDNA, TaqMan assay mix, and TaqMan gene expression assay. GAPDH was used as a housekeeping gene and control. RT-PCR was performed using the ABI-7300 Real Time PCR System [Applied Biosystems].
Western Blotting
Whole cell lysates were prepared in PBS containing 1% NP-40 [Igepal CA 630], 50mm Tris HCl, 150mm NaCl, 2mm EDTA, 0.1% SDS, plus a cocktail of protease inhibitors [Roche] and sodium orthovanadate. Equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. Membranes were blocked in 5% nonfat milk, 0.1% Tween-20 in PBS. Rabbit polyclonal antibodies to JNK1 [ab10664] and phosphorylated JNK1 [cross-reactive with P-JNK2; P-JNK 1/2, ab4821] were from Abcam. The ECL Plus Chemiluminescent Kit [Applied Biosystems] was used to visualize protein bands.
Flow Cytometry
For apoptosis determinations, cells were labeled with the PE Annexin-V Apoptosis Detection Kit I from BD-Pharmingen. For intracellular protein determinations,cells were fixed [paraformaldehyde], permeabilized [triton X-100 and NP-40], and labeled with primary antibodies for 24 hr. at 0-4° C as described in the text followed by incubation with fluorescent-labeled secondary antibodies for 1 hr. at 0-4°C. The following antibodies were employed: primary antibodies, rabbit anti-JNK [Santa Cruz, sc-571], polyclonal rabbit anti-p-JNK [pT183/pY185] [BD Pharmingen 558268], rabbit monoclonal to PUMA [abcam 33906], c-JUN [abcam, ChIP Grade [ab31419], TRAILR1 [DR4] [abcam 18362], and c-Fos antibody [abcam 7963]. FITC goat anti-rabbit Ig [BD Pharmingen, 554020] was used as the secondary. Cells were analyzed using the 3-laser BD LSRII flow cytometer.
Patient Populations
The study group was composed of 36 control subjects who had no current chronic or acute infections and no family history of autoimmune diseases and 50 subjects meeting the American College of Rheumatology clinical criteria for RA, 28 RA subjects were on current methotrexate therapy and 22 RA subjects were not on current methotrexate therapy. No other exclusion or inclusion criteria were employed except for the ability to provide informed consent. The Committees for the Protection of Human Subjects of Vanderbilt University and UT Southwestern Medical Center approved these studies. The approximate female-to-male ratio in all study groups was 3:1. Age ranges [36-58 years] and racial distributions in all groups were similar. Current therapies were determined by questionnaire and confirmed by chart review. Patients on MTX therapy were receiving doses of 15-25 mg per week.
Statistics
Statistical significance was determined by the unpaired T test with Welch's correction. P < .05 was considered significant.
RESULTS
MTX ‘primes’ cells for apoptosis via death receptor and mitochondrial pathways
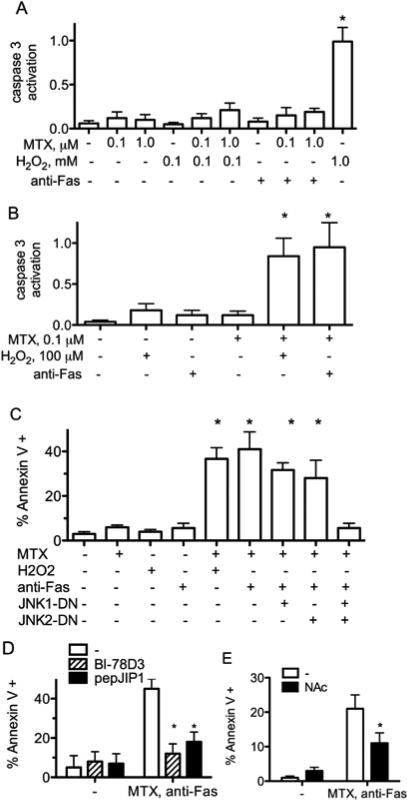
Various studies demonstrate the ability of MTX to induce apoptosis or alter cell viability. We cultured Jurkat T cells with MTX and monitored apoptosis by measuring activity ofcaspase 3. Jurkat cells cultured with MTX, low concentrations of either H2O2or anti-Fas for 24 hr., or combinations of MTX and H2O2 or MTX and anti-Fas exhibited minimal activation of caspase 3 relative to cells cultured with high concentrations of H2O2 [Figure 1A]. Second, we cultured cells for 48 hr. with MTX and then exposed them to either anti-Fas antibody or H2O2 for an additional 24 hr. to activate death receptor or mitochondrial apoptosis pathways, respectively. Pre-treatment of Jurkat cells with MTX at concentrations of 0.1-1.0 μM resulted in a marked increase in activity of caspase 3 after subsequent treatments with either H2O2 or anti-Fas [Figure 1B]. As a second measure of apoptosis we investigated changes in annexin V labeling by flow cytometry. We used JNK1-DN and JNK2DN mutants to assess relative contributions of JNK1 and JNK2 to increased apoptosis sensitivity. Jurkat cells, either untreated or cultured with MTX for 48 hours exhibited low percentages of annexin V positive cells. Treatment with H2O2 or anti-Fas only slightly increased percentages of annexin V positive cells. However, treatment of MTX-cultured cells with H2O2 or anti-Fas resulted in a marked increase in the percentage of annexin V positive cells [Figure 1C]. This increase in apoptosis [annexin V positive cells] was slightly abrogated by the presence of either JNK1 or JNK2 DN mutants but was substantially abrogated by the presence of both JNK1 and JNK2 DN mutants. We also tested the ability of specific JNK inhibitors, BI-78D3 and pepJIP1 that target JNK-JIP [JNK-interacting protein 1] interaction sites, to prevent apoptosis[31-33]. Both inhibitors blocked MTX-dependent increases in sensitivity to apoptosis [Figure 1D]. N-acetyl cysteine [NAc], a free radical scavenger and reducing agent, also blocked MTX-dependent apoptosis [Figure 1E]. We conclude that MTX ‘primes’ cells for markedly increased sensitivity to apoptosis via death receptor and mitochondrial pathways, which is dependent upon JNK1 and JNK2 enzymes. Additionally, ROS may contribute to increased apoptosis sensitivity.
Figure 1.
MTX ‘primes’ Jurkat cells for increased sensitivity to apoptosis. Jurkat cells,A, were cultured for 48 hr. with MTX, H2O2, or 50 ng/ml anti-Fas and levels of caspase 3 activity determined. B, were cultured for 48 hr. with the indicated concentrations of MTX, followed by culture with H2O2 or anti-Fas for 24 hours before determination of caspase 3 activities. C,JNK1-DN, JNK2-DN, or ‘empty vector’ plasmids with a GFP plasmid were introduced by transient transfection. Cells were cultured for 48 hr. with or without MTX, 10-7M, and treated with anti-Fas for an additional 6 hr. Percent Annexin V positive cells were determined by flow cytometry after gating on GFP-positive and GFP-negative cells ± S.D. D, were cultured for 48 hr. with MTX, 10-7 M, in the presence or absence of BI-78D3 or pepJIP1, followed by culture with anti-Fas for 6 hours.Percent Annexin V positive cells were determined by flow cytometry ± S.D. E, were cultured for 48 hr. with MTX, 10-7 M, in the presence or absence NAc, followed by culture with anti-Fas for 6 hrs.Percent Annexin V positive cells were determined by flow cytometry ± S.D.* P < 0.05 relative to protein levels in untreated Jurkat cells.
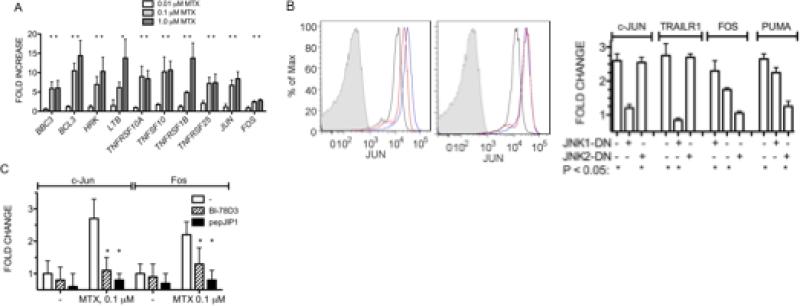
We next asked if MTX treatment of Jurkat cells altered expression levels of a panel of genes whose protein products are known to have activating or inhibitory effects upon apoptosis. Jurkat cells were cultured with MTX for 48 hrs.prior to RNA isolation, cDNA synthesis, and analysis by quantitative PCR. While we did not find reduced expression of genes, such as BCL2, whose protein products protect cells from apoptosis [Supplemental Figure 1], we found increased expression of a number of genes, BBC3 [PUMA, BCL2 binding component 3], BCL3 [B-cell CLL/lymphoma 3], HRK [hara-kiri], LTB [lymphotoxin beta], TNFRSF10A [cytotoxic TRAIL receptor, TRAILR1], TNFRSF1B [tumor necrosis factor receptor 2], TNFRSF25 [TRAMP, APO-3], and TNFSF10 [TRAIL, APO2L], whose protein products are known to play critical roles in promoting sensitivity to apoptosis [Figure 2A]. We also investigated changes in expression of the prototypical JNK target genes, JUN and FOS. These genes also increased expression levels following MTX treatment. To assess changes in protein levels of target genes and to assess relative contributions of JNK1 and JNK2 to their changes, we measured intracellular protein levels by flow cytometry with specific antibodies and employed JNK1 or JNK2 DN mutants to specifically inhibit JNK1 or JNK2. MTX-treatment resulted in increased protein expression of the known JNK target, c-Jun, and this increase was inhibited by the JNK1 DN mutant but not the JNK2 DN mutant [Figure 2B]. We also determined changes in c-Fos, PUMA, and TRAILR1 protein levels after MTX treatment in the presence of either JNK1 or JNK2 DN mutants by flow cytometry. Induction of TRAILR1 by MTX was also abrogated by the JNK1 DN mutant whereas c-Fos and PUMA induction was relatively unaffected by the JNK1 DN mutant but almost completely abrogated by the JNK2 DN mutant [Figure 2B]. JNK specific inhibitors, BI-78D3 or pepJIP1, also inhibited induction of c-Jun and c-Fos expression by MTX [Figure 2C]. Taken together, these results argue that MTX increased sensitivity to apoptosis, at least in part, by inducing expression of genes whose protein products promote apoptosis. Results with JNK DN mutants implicated both JNK1 and JNK2 as regulators of this gene family.
Figure 2.
MTX induces expression of genes whose protein products promote apoptosis. A, Jurkat cells were cultured with MTXfor 48 hr., transcript levels of genes whose protein products are known to influence apoptosis were determined [Sup. Figure 1]. Results are expressed as fold increase in transcript levels over untreated cells after normalization to GAPDH. B, JNK1-DN, JNK2-DN or ‘empty vector’ plasmids with a GFP expression plasmid were introduced into Jurkat cells by nucleofection. Left panel: c-Jun levels in, black line, untreated cells, blue line, MTX treated cells, red line, MTX treated cells transfected with JNK1-DN. Middle panel: c-Jun levels in, black line, untreated cells, blue line, MTX treated cells, red line, MTX treated cells transfected with JNK2-DN. Right panel: Mean changes in expression levels of c-Jun, Fos, PUMA and TRAILR1 in MTX treated Jurkat cells determined by flow cytometry expressed as fold change relative to untreated Jurkat cells ± S.D. C, Inhibition of MTX-mediated increases in c-Jun and Fos protein levels by specific JNK inhibitors, BI-78D3 or pepJIP1 relative to untreated Jurkat cells ± S.D. * P < 0.05 relative to protein levels in MTX-treated Jurkat cells.
Induction of JUN and FOS by MTX
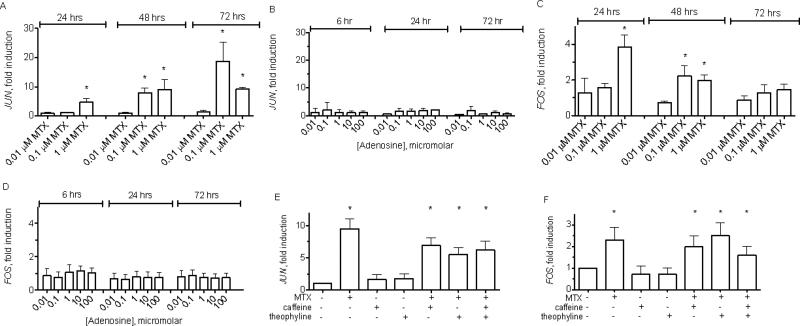
JUN and FOS are prototypical JNK target genes [34, 35]. Thus, we further explored induction of these genes by MTX. We also examined effects of adenosine because MTX is known to induce adenosine production [13]. MTX, at concentrations of 0.1-1.0 μM, stimulated a gradual increase in JUN levels over the course of 24-72 hours [Figure 3A]. In contrast, adenosine at concentrations between 0.01-100 μM failed to induce changes in JUN expression levels over this time period [Figure 3B]. This range of adenosine concentrations should be sufficient to activate known human adenosine receptors, which have EC50s for adenosine from ~0.3 to 30 μM [36-38]. We also determined expression levels of JUN family members, JUNB and JUND. Treatment of Jurkat cells with MTX or adenosine did not change expression levels of these JUN family members. Culture with MTX or adenosine did not affect cell numbers or viability as determined microscopically. Thus, MTX, but not adenosine, directly stimulated increased JUN expression levels in a uniform T cell population but not other Jun family members.
Figure 3.
Induction of JUN and FOS expression by MTX in Jurkat T cells. Jurkat T cells were cultured with the indicated concentrations of MTX A, C, or adenosine B, D. At the indicated times, cell cultures were harvested, total RNA purified and cDNA synthesized. Relative expression of JUNA, B, and FOSC, D, were normalized to GAPDH levels and are expressed as fold induction relative to untreated cultures. Jurkat cells were also cultured with MTX and adenosine receptor antagonists, caffeine [20 μM] and/or theophylline [20 μM] for 48 hr. E, F. Expression levels of JUNE, and FOSF, were determined by PCR. Results are expressed as fold induction ± S.D. * P< 0.05 relative to unstimulated cultures.
Since the AP-1 transcription factor is a heterodimer composed of a Jun family member and a Fos family member [39], we determined the impact of MTX treatment on levels of Fos family member transcripts. Similar to JUN, FOS mRNA expression levels increased in Jurkat cells after stimulation by MTX [Figure 3C]. In contrast, stimulation by adenosine did not alterFOS mRNA expression [Figure 3D]. We examined changes in mRNA levels of additional Fos family members, FOSL1 and FOSB. We did not detect changes in transcript levels of these two genes following exposure to MTX or adenosine. Kinetics of FOS induction were different from kinetics of JUN inductionfollowing MTX stimulation. FOS mRNA levels reached a peak after 24-48 hours depending upon concentration of MTX and declined by 72 hours, whereas JUN mRNA levels reached its peak at 72 hours. Taken together, these results demonstrate that MTX induces increased expression of both JUN and FOS components of the AP-1 transcriptional complex in Jurkat cells. They also show that MTX specifically induces JUN and FOS mRNA but not other Jun and Fos family members.
As an alternate approach, we asked if the adenosine receptor antagonists with broad specificity, caffeine and/or theophylline, interfered with induction of JUN and FOS mRNA. Caffeine, theophylline, or their combination, at pharmacologically active concentrations, did not interfere with induction of JUN and FOS mRNA by MTX [Figure 3E and F]. Taken together, these results do not argue against the idea that anti-inflammatory effects of MTX may be mediated, in part, via stimulation of adenosine release and activation of adenosine receptors. Rather, results presented here clearly demonstrate that adenosine, alone, is not sufficient to increase expression of FOS and JUN mRNA in T cells and that adenosine receptor antagonists with broad specificity do not interfere with induction of FOS and JUN mRNA by MTX.
Free radical scavengers and tetrahydrobiopterin [BH4] supplementation block MTX-induced apoptosis
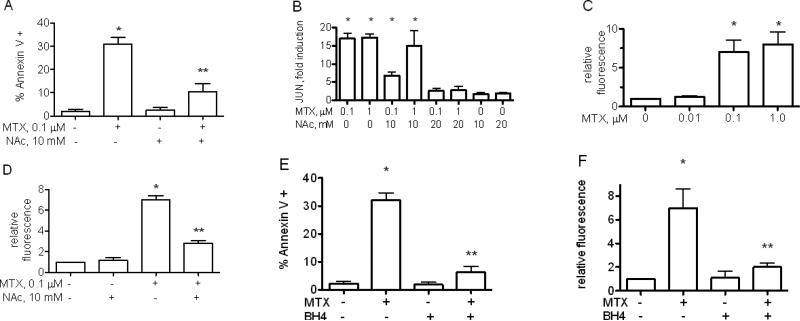
MTX, at concentrations similar to those inducing JUN and FOS expression,leads to production of ROS and priming for apoptosis [22]. Further, ROS are known to activate JNK leading to increased JUN expression [29, 40-42]. We cultured Jurkat cells with MTX and the free radical scavenger, NAc, and examinedchanges in apoptosis sensitivityand JUNexpression levels. NAc addition inhibited MTX-mediated changes in apoptosis sensitivity [Figure 4A] and MTX-mediated increases in JUN expression in Jurkat cells but did not alter baseline JUN levels [Figure 4B]. Therefore, we determined if MTX increased production of ROS by flow cytometry after labeling cells with CM-H2DCFDA. MTX caused a marked increase in ROS production [Figure 4C] that was effectively reduced by NAc [Figure 4D]. Our conclusion is that JNK-dependent increases in apoptosis sensitivity and JUN transcript levels induced by MTX are mediated, at least in part, through ROS production.
Figure 4.
Free radical scavengers or BH4 supplementation block MTX-mediated increases in apoptosis sensitivity.A, Jurkat cells were cultured for 48 hr. with MTX and/or the free radical scavenger, NAc, and stimulated with anti-Fas for 5 hr. Results are expressed as % Annexin V+ cells ± S.D. B, as in [A] except cultures were harvested after 48 hrs., processed, and JUN transcript levels determined. Results are expressed as fold induction ± S.D.C, Jurkat cells were treated with MTX for 48 hrs. ROS production was determined by labeling cells with CMH2DCFDA.D, Jurkat cells were treated with MTX for 48 hrs in the presence or absence of NAc. ROS production was determined as in C. Jurkat cells were cultured for 48 with MTX, 0.1 μM, with or without BH4 supplementation, 30 μM. E, % Annexin V+ cells ± S.D. were determined after anti-Fas treatment by flow cytometry. F, ROS levels, expressed as fold increase relative to untreated cells ± S.D., were determined after loading Jurkat cells with CM-H2DCFDA.* P< 0.05 relative to unstimulated cultures. ** P< 0.05 relative to MTX stimulated Jurkat cells.
MTX inhibits reduction of dihydrofolate to tetrahydrofolate and reduction of dihydrobiopterin [BH2] to BH4 catalyzed by DHFR [43-46]. BH4 is a necessary cofactor for nitric oxide synthases [NOS] including endothelial NOS [eNOS], also expressed in T lymphocytes [47, 48]. Loss of BH4 uncouples eNOS from NO synthesis leading to excess production of ROS. Thus, we tested whether supplementation with BH4 reversed MTX-mediated increased apoptosis and ROS production. We found that BH4 supplementation effectively reduced MTX-dependent apoptosis [Figure 4E] and MTX-dependent increased ROS production [Figure 4F]. These results are consistent with a model whereby inhibition of DHFR by MTX reduces intracellular levels of BH4 resulting in NOS catalyzed ROS overproduction leading to activation of JNK and increased apoptosis sensitivity.
MTX increases JNK expression and activity
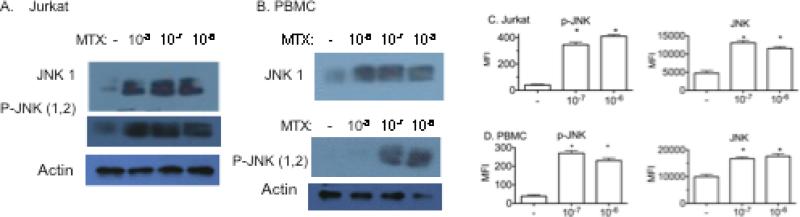
Inhibition of MTX-mediated increased JUN expression, priming for apoptosis, and expression of genes whose protein products are proapoptotic, by JNK inhibitors and by JNK1 and JNK2 DN mutants suggests that MTX may increase JNK expression or activity. The JNK protein kinase is a member of the MAP kinase family and is activated by phosphorylation on threonine and tyrosine residues by JNK kinases, also termed MAP kinase kinases, in response to an array of stimuli [49, 50]. There are three JNK genes that produce multiple mRNA and protein isoforms. We used western blotting to determine if stimulation by MTX changed JNK1 total protein levels and phosphorylated JNK1/2 levels. Jurkat cells were cultured with different concentrations of MTX for 2 days. Whole cell lysates were prepared and analyzed by SDS-PAGE and western blotting with antibodies specific for JNK1 total protein or antibodies specific for the phosphorylated forms of JNK1 and JNK2. Culturing cells with MTX resulted in increased in total JNK1 protein levels and levels of phosphorylated JNK1/2 [Figure 5A]. We also cultured human PBMC with MTX for 2 days and analyzed whole cell lysates by SDS-PAGE and western blotting. Similar to what was observed in Jurkat cells, culturing PBMC with MTX resulted in concentration-dependent increases in total JNK1 protein and phosphorylated JNK1/2 [Figure 5B]. We also examined changes in total phosphorylated JNK and total JNK levels by flow cytometry. These experiments confirmed changes in phosphorylated JNK and total JNK levels in Jurkat cells [Figure 5C] and PBMC [Figure 5D] following MTX treatment. We conclude that MTX induces a rise in cellular levels of phosphorylated JNK protein and total JNK protein in both Jurkat cells and human PBMC.
Figure 5.
MTX induces increased levels of JNK and phosphorylated [P]-JNK. Jurkat T cells A, or human PBMC B, were treated with the indicated concentrations of MTX. After 48 hrs, cells were harvested, whole cell lysates prepared and JNK 1 and P-JNK levels determined by western blotting with specific antibodies for either JNK 1 or P-JNK [1/2]. C, Jurkat cells and D, PBMC were treated with or without MTX, labeled with specific antibodies for P-JNK or JNK and analyzed by flow cytometry. Results are expressed as the mean fluorescence intensity above isotype control ± S.D. * P < 0.05 relative to untreated Jurkat cells or PBMC.
Therapeutic doses of MTX in RA increase JUN expression levels
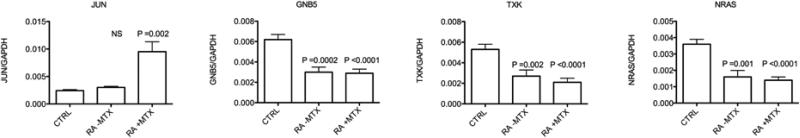
Given that MTX increased P-JNK levels in both Jurkat cells and PBMC and expression of the known JNK target gene, JUN, we sought evidence for similar effects of MTX, in vivo. To test this hypothesis, we determined expression levels of JUN in control subjects relative to RA subjects who were or were not on current low dose weekly MTX therapy. Blood samples were collected in PAXgene tubes. Expression levels of target genes were determined as the ratio to GAPDH for normalization. We found increased expression of JUN in blood from RA subjects on current MTX therapy relative to RA subjects not on MTX therapy or control subjects [Figure 6]. In contrast, expression levels of three other genes, NRAS, TXK, and GNB5, were reduced in subjects with RA compared to control subjects independent of MTX therapy [or other therapies]. We conclude that low-dose MTX therapy increased expression levels of JUN in whole blood while expression levels of other target genes, which are reduced in subjects with RA, did not change in response to MTX therapy.
Figure 6.
Expression levels of the JNK target gene, JUN, in vivo, in RA subjects as a function of current MTX therapy.Blood was obtained in PAXgene tubes from control subjects [CTRL, N=36], subjects with RA not on MTX [RA-MTX, N=28], and subjects with RA on MTX [RA+MTX, N=22]. Relative gene expression levels are expressed relative to GAPDH ± S.D. P values are relative to control groups.
DISCUSSION
MTX was developed in the 1940's as an antagonist of DHFR and since that time, therapeutic efficacy of MTX has been attributed to inhibiting reduction of dihydrofolate to tetrahydrofolate resulting in inhibition of purine synthesis necessary for DNA and RNA synthesis [7, 8]. However, folate supplementation does not interfere with anti-inflammatory effects of MTX suggesting the presence of additional mechanisms of action of low-dose MTX for treatment of this class of disease [51]. In the 1990's, it was proposed that production of adenosine, as a byproduct of DHFR inhibition, simulates adenosine receptors, thus contributing to the anti-inflammatory effects of MTX [13, 16]. An additional possible mechanism by which MTX may exert its anti-inflammatory effects is by induction of apoptosis of inflammatory cells, such as lymphocytes, yet it is not immediately apparent how known biochemical pathways activated or inhibited by MTX may induce apoptosis. Our new model proposes that MTX inhibits DHFR catalyzedBH2 reduction to BH4. Downstream biochemical effector pathways stimulated by loss of BH4 lead to altered cell sensitivity to apoptosis thus shifting the decades-long focus of MTX action away from inhibition of dihydrofolate reduction to inhibition of BH2 reduction to BH4 [Supplemental Figure 2]. Our results support a model whereby sub-micromolar concentrations of methotrexate stimulate increased sensitivity to apoptosis via death receptor and mitochondrial pathways. Increased apoptosis sensitivity depends upon increased expression and activity of JNK and subsequent increased expression of JNK target genes. JNK target genes include those whose protein products increase sensitivity of cells to apoptosis. JNK activation is mediated in part by ROS, which we propose is induced by MTX-dependent depletion of BH4 levels uncoupling eNOS from NO synthesis resulting in over-production of ROS. Specific JNK inhibitors prevent both MTX-mediated effects upon gene and protein induction and changes in sensitivity to apoptosis. Both JNK1-DN and JNK2-DN mutants interfere with these MTX-mediated effects. Thus, both JNK enzymes contribute to MTX mediated changes in the transcriptional program in Jurkat cells and to alterations in sensitivity to apoptosis by regulating expression of different target genes. Further, subjects with RA taking low-dose weekly MTX exhibit increased JUN expression, the prototypical JNK target gene thus supporting the notion that this pathway is activated by MTX, in vivo, and may contribute to efficacy of MTX in inflammatory disease.
It is well established that JNK enzymes play both positive and negative roles in both death receptor and mitochondrial pathways of apoptosis [34]. These roles can be mediated via JNK translocation to the nucleus and phosphorylation of transcription factors. c-Jun is the prototypical transcription factor phosphorylated by JNK, but JNK also phosphorylates additional Jun family members, ATF and Elk family members, p53, c-Myc and others [52]. JNK can also translocate to mitochondria and regulate activity of proteins involved in apoptosis, such as BAD, Bim, and 14-3-3, via phosphorylation. In large part, these studies have examined roles of JNK in stimulating or preventing apoptosis in response to either external or internal stimuli. Our results support a new role for JNK in regulating apoptosis. Treatment of Jurkat T cells with MTX induces increased expression and activity of JNK, increased expression of JUN mRNA, and increased expression of a number of genes whose protein products are pro-apoptotic. However, MTX does not directly stimulate apoptosis under these conditions. Rather, MTX-treated Jurkat cells are ‘primed’ to exhibit markedly increased sensitivity to apoptosis when exposed to stimuli activating death receptor or mitochondrial pathways via a JNK-mediated pathway. Further experimentation will be required to address whether JNK also ‘primes’ Jurkat cells for apoptosis by modulating activity of pro- or anti-apoptotic proteins via direct phosphorylation.
In many cell types, activation of JNK is mediated by its phosphorylation in response to stimuli including cellular stress and inflammatory cytokines. In contrast, T lymphocytes exhibit a second level of regulation. Resting or naïve T lymphocytes express low quantities of JNK enzymes [53]. Activation via T cell receptor ligation leads to markedly increased expression of JNK genes and protein whereas JNK phosphorylation requires CD28-mediated co-stimulatory signals. Our results demonstrate that MTX treatment of Jurkat cells or human PBMC [70% T lymphocytes] leads to increased levels ofphosphorylated JNK and total JNK protein. Whether MTX treatment of non-T cells or non-lymphoid cells inducesincreased levels ofphosphorylated JNK and total JNK protein remains to be determined.
The potential of JNK inhibitors as therapeutics for inflammatory disease has also attracted considerable interest [34, 49, 54-58]. Modulation of JNK enzymes either by genetic means or with small molecule inhibitors interferes with a number of processes linked to inflammatory disease. For example, many genes linked to inflammation, such as those encoding TNF-α, matrix metalloproteinases, and adhesion molecules, possess AP-1-response elements in their promoters and are regulated by JNK enzymes through activation of AP-1 and ATF-2 transcription factors. Our results suggest a second paradigm shift. Our model suggests that correctly targeted activation of JNK, rather than its inhibition, represents a therapeutic goal for the treatment of inflammatory disease, especially RA. In lymphocytes, increased JNK activity and subsequent increased sensitivity to apoptosis may also have therapeutic benefits in inflammatory diseases that are dependent upon T lymphocyte function by eliminating self-reactive T cells. For example, activated T cells undergo apoptosis if exposed to secondary T cell receptor ligation in a process termed activation-induced cell death [23]. We speculate that MTX therapy may ‘prime’ self-reactive T cells to undergo increased activation-induced cell death in response to secondary exposure to self-antigen and thus produce its therapeutic benefit. Future studies will be required to determine if this novel MTX-induced pathway can be exploited for therapeutic benefit.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health [R42 AI53984, RO1 AI44924], the American College of Rheumatology ‘Within Our Reach’ grant's program and the McGee Foundation. The VMC Flow Cytometry Shared Resource is supported in part by the Vanderbilt Ingram Cancer Center [P30 CA68485] and the Vanderbilt Digestive Disease Research Center [DK058404].
Footnotes
Current address: ZTA; Washington School of Law, American University, Washington, D.C., JPA; Democratic Congressional Campaign Committee, Washington, D.C.
Conflict of interest statement
TMA and NJO are co-owners of ArthroChip. Other authors claim no conflicts of interest.
REFERENCES
- 1.Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–10. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 2.Seeger DR, Cosulich DB, Smith JM, Hultquist ME. Analogs of Pteroylglutamic Acid. III. 4-Amino Derivatives. J Am Chem Soc. 1949;71:1753–8. [Google Scholar]
- 3.Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff JA. Temporary remissions in acute leukemia in children produced by folic antagonist, 4-aminopteroylglutamic acid (aminopterin). N Engl J Med. 1948;238:787–93. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- 4.Williams HJ, Willkens RF, Samuelson COJ, Alarcón GS, Guttadauria M, Yarboro C, et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum. 1985;28:721–30. doi: 10.1002/art.1780280702. [DOI] [PubMed] [Google Scholar]
- 5.Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;28:818–22. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- 6.Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- 7.Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity: II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am J Med Sci. 1951;221:176–82. [PubMed] [Google Scholar]
- 8.Gubner R. Therapeutic suppression of tissue reactivity: I. Comparison of the effects of cortisone and aminopterin. Am J Med Sci. 1951;221:169–75. doi: 10.1097/00000441-195102000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–85. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 10.van Dieren JM, Kuipers EJ, Samsom JN, Nieuwenhuis EE, van der Woude CJ. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: their mechanisms of action and role in the treatment of IBD. Inflamm Bowel Dis. 2006;12:311–27. doi: 10.1097/01.MIB.0000209787.19952.53. [DOI] [PubMed] [Google Scholar]
- 11.Alarcón GS. Methotrexate use in rheumatoid arthritis. A clinician's perspective Immunopharmacology. 2000;47:259–71. doi: 10.1016/s0162-3109(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 12.Braun J, Rau R. An update on methotrexate. Curr Opin Rheumatol. 2009;21:216–23. doi: 10.1097/BOR.0b013e328329c79d. [DOI] [PubMed] [Google Scholar]
- 13.Chan SLC, Cronstein BN. Methotrexate-how does it really work? NatRev Rheum. 2010;6:175–8. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 14.Stamp LK, O'Donnell JL, Chapman PT, Zhang M, Frampton C, James J, Barclay ML. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum. 2009;60:2248–56. doi: 10.1002/art.24653. [DOI] [PubMed] [Google Scholar]
- 15.Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis. 2005;64:1180–5. doi: 10.1136/ard.2004.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–73. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003;48:240–7. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- 18.Wessels JAM, Huizinga TWJ, Guchelaar H- J. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology. 2008;47:249–55. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 19.Constantin A, Loubet-Lescoulié P, Lambert N, Yassine-Diab B, Abbal MM, B., de Préval C, et al. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Paillot R, Genestier L, Fournel S, Ferraro C, Miossec P, Revillard JP. Activation-dependent lymphocyte apoptosis induced by methotrexate. Transplant Proc. 1998;30:2348–50. doi: 10.1016/s0041-1345(98)00648-4. [DOI] [PubMed] [Google Scholar]
- 21.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips DC, Woollard KJ, Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol. 2003;138:501–11. doi: 10.1038/sj.bjp.0705054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 24.Kitson J, Raven T, Jiang YP, Goeddel DV, Giles KM, Pun KT, et al. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;6607:372–5. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 25.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;5684:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 26.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;6513:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 27.Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53 target genes. Nature. 1994;6486:220–3. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- 28.Ashkenazi A, Dixit VM. Death receptors: signalling and modulation. Science. 1998;5381:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 29.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combe B, Edno L, Lafforgue P, Bologna C, Bernard JC, Acquaviva P, et al. Total and free methotrexate pharmacokinetics, with and without piroxicam, in rheumatoid arthritis patients. Br J Rheumatol. 1995;34:421–8. doi: 10.1093/rheumatology/34.5.421. [DOI] [PubMed] [Google Scholar]
- 31.Stebbins JL, De SK, Machleidt T, Becattini B, Vazquez J, Kuntzen C, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci U S A. 2008;105:16809–13. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–6. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- 34.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 35.Das M, Sabio G, Jiang F, Rincón M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:49–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacology & Therapeutics. 2007;114:208–21. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Cristalli G, Lambertucci C, Marucci G, Volpini R, Dal Ben D. A2A adenosine receptor and its modulators: overview on a druggable GPCR and on structure-activity relationship analysis and binding requirements of agonists and antagonists. Cur. Pharm. Design. 2008;14:1525–52. doi: 10.2174/138161208784480081. [DOI] [PubMed] [Google Scholar]
- 39.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–6. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 40.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 41.Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Com Signal. 2009;7:9. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–7. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- 43.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–61. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–44. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 46.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284:28128–36. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibiza S, Pérez-Rodríguez A, Ortega A, Martínez-Ruiz A, Barreiro O, García-Domínguez CA, et al. Endothelial nitric oxide synthase regulates N-Ras activation on the Golgi complex of antigen-stimulated T cells. Proc Natl Acad Sci U S A. 2008;105:10507–12. doi: 10.1073/pnas.0711062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibiza S, Víctor VM, Boscá I, Ortega A, Urzainqui A, O'Connor JE, et al. Endothelial nitric oxide synthase regulates T cell receptor signaling at the immunological synapse. Immunity. 2006;24:753–65. doi: 10.1016/j.immuni.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–65. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 51.Morgan SL, Baggott JE, Vaughn WH, Austin JS, Veitch TA, Lee JY, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med. 1994;121:833–41. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- 52.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–70. [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss L, Whitmarsh AJ, Yang DD, Rincón M, Davis RJ, Flavell RA. Regulation of c-Jun NH(2)-terminal kinase (Jnk) gene expression during T cell activation. J Exp Med. 2000;191:139–46. doi: 10.1084/jem.191.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 55.Han Z, Chang L, Yamanishi Y, Karin M, Firestein GS. Joint damage and inflammation in c-Jun N-terminal kinase 2 knockout mice with passive murine collagen-induced arthritis. Arthritis Rheum. 2002;46:818–23. doi: 10.1002/art.10104. [DOI] [PubMed] [Google Scholar]
- 56.Köller M, Hayer S, Redlich K, Ricci R, David JP, Steiner G, et al. JNK1 is not essential for TNF-mediated joint disease. Arthritis Res Ther. 2005;7:166–73. doi: 10.1186/ar1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rincón M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–24. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 58.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.