Abstract
In the twentieth century, the dominant model of sexual differentiation stated that genetic sex (XX versus XY) causes differentiation of the gonads, which then secrete gonadal hormones that act directly on tissues to induce sex differences in function. This serial model of sexual differentiation was simple, unifying and seductive. Recent evidence, however, indicates that the linear model is incorrect and that sex differences arise in response to diverse sex-specific signals originating from inherent differences in the genome and involve cellular mechanisms that are specific to individual tissues or brain regions. Moreover, sex-specific effects of the environment reciprocally affect biology, sometimes profoundly, and must therefore be integrated into a realistic model of sexual differentiation. A more appropriate model is a parallel-interactive model that encompasses the roles of multiple molecular signals and pathways that differentiate males and females, including synergistic and compensatory interactions among pathways and an important role for the environment.
The value of understanding sex differences in the brain is both self-evident and underappreciated. The effects of sex on neural phenotypes are often as large as the effects of other important variables, and conclusions based on the study of one sex have not always been found to hold in the other1,2. Moreover, susceptibility to disease or dysfunction and the effect of injury can be as much as 2–5-fold greater in one sex. These include higher rates of neuropsychiatric and learning disorders with developmental origins in males and higher rates of aging-related neurodegenerative diseases and mental health dysfunctions in females3–5. Heuristically, contrasting males and females has revealed previously unknown mechanisms of neural development that were not otherwise accessible6–8. However, most studies of the brain and other tissues continue to focus on one sex, usually males, or fail to report the sex of the animals9. Thus, the still widespread assumption that the influence of sex is negligible retards progress in our field10. Even more paradoxical is that factors present in one sex sometimes counteract other sex-specific factors to eliminate sex differences in phenotype11,12. Thus, sexual equality of phenotype does not imply sexual equality of physiology or development and, more importantly, sex differences are more pervasive than can be realized just from considering traits in which males differ from females.
The study of sexual differentiation of the brain has long focused on a few model cases of brain function (for example, sex behavior, control of ovulation) and brain regions that are predominantly involved in reproduction and therefore show large sex differences that are amenable to study. The repeated investigation of a relatively small number of sexual dimorphisms may have contributed to the false impression that a few discrete male or female circuits sit in an otherwise sexually monomorphic brain. The notion that for specific behaviors there is a discrete male neural circuit versus a discrete female neural circuit remains widely held despite a lack of empirical evidence of the existence of either. Preconceived notions, stemming from the hormonally controlled elimination or retention of female (Müllerian) and male (Wollfian) reproductive tracts, may have contributed to the view of a similar system in the brain. Moreover, studies of only a few robustly dimorphic brain structures have contributed to the perception that sex differences in brain function are controlled by a unitary program: genetic sex determines gonadal sex and gonadal hormones determine brain sex. Gonadal steroids were believed to act via common mechanisms on a restricted group of brain regions to cause sex differences, promoting the formation of male circuits in males and female circuits in females. This traditional view, seductive in its simplicity, must now be replaced. Sufficient new evidence has accumulated to warrant a shift away from the old serial model and toward a more complex and nuanced model in which numerous sex-specific factors, hormonal, genetic and epigenetic, act in parallel to cause or eliminate sex differences in the brain and other tissues, by mechanisms that frequently are region specific and heterogeneous in terms of their intracellular mechanisms and mode of cell-to-cell communication. The modern view emphasizes a diversity of proximate mechanisms and an interaction of multiple sex-specific factors in many brain regions.
Biological theories of sexual differentiation have largely under-emphasized or even excluded the differential effect of sex-specific environments. The environment has far-reaching influences on self-concept and gendered behavior of humans and is poorly modeled by studies of rodents. Sex differences in the environment likely have major effects on brain biology, as has been suggested by recent studies of the importance of environmentally triggered epigenetic changes in the brain13. The effect of environment is rarely controlled for or empirically tested, but environmental and biological factors likely interact in complex ways to sculpt the female and male phenotype. The goal of this review is to present the historic serial model of sexual differentiation of the brain and propose its replacement by a parallel model that incorporates all of the variables relevant to brain development in the sexes, including the environment.
Classic model: hormone-mediated organization/activation
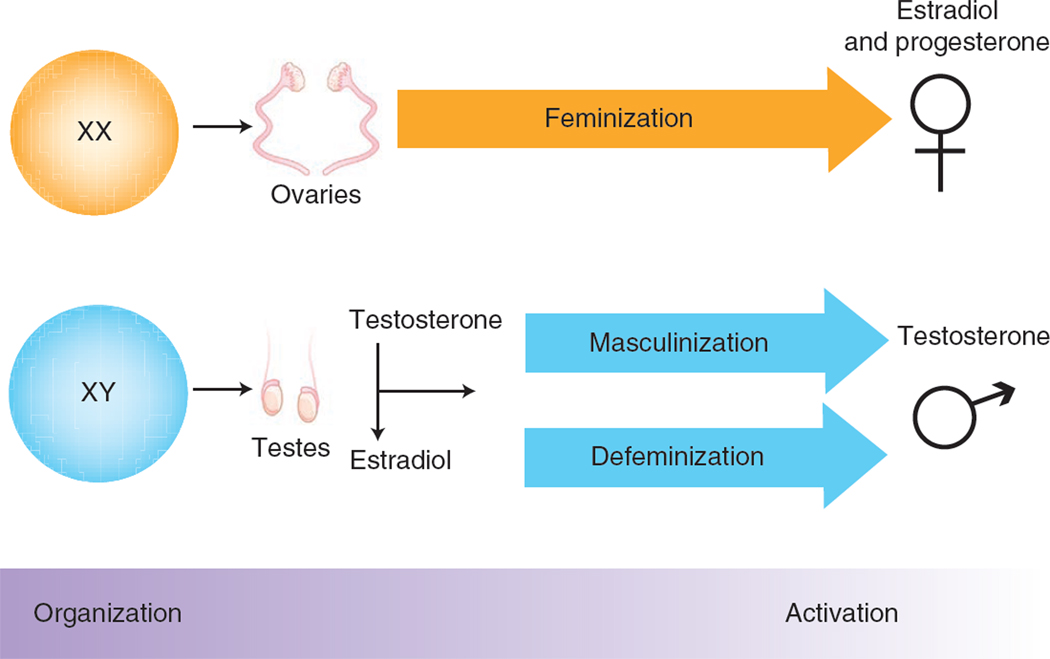
The concept that sex differences in adult brain and behavior are sexually differentiated during development by the action of gonadal hormones was first illustrated by the finding that female guinea pigs exposed to testosterone as fetuses have a permanent tendency to copulate like males rather than females14. This iconic study provided the conceptual framework for discriminating two types of sex-specific action of gonadal steroid hormones: organizational and activational15 (Fig. 1). The embryonic testes of mammalian species synthesize and release testosterone, which acts throughout the body to masculinize the genitalia, sperm ducts, brain and other tissues. These organizational effects are differentiating in the classic sense of developmental biology, in that the tissues permanently and irreversibly adopt a restricted fate (in this case, a sex-specific fate) with concomitant loss of cellular pluripotency. Contrasted with these are the reversible activational effects of gonadal hormones, which can occur at any time of life, but are predominantly studied in adults. As adults the male brain is exposed to testicular hormones and the female brain to ovarian hormones, resulting in sex differences that are abolished by gonadectomy of adults. In most instances, such as hormonal control of sexual behavior, activational effects of steroids are constrained by earlier organizational effects, including additional organization that occurs at puberty16. Thus, the dominant theory of mammalian sexual differentiation, championed even as recently as 2010 (ref. 17), is that the embryo is sexually indifferent until differentiation of the gonads and that all sex differences arise afterwards by differential action of gonadal hormones.
Figure 1.
Twentieth-century linear view of sexual differentiation. For the past 50 years, the prevailing view of sexual differentiation of the brain has been a linear model in which chromosomal sex determines gonadal sex, which determines brain sex. Feminization of the brain is the default process that occurs in the absence of high levels of gonadal steroids during a perinatal sensitive period. Masculinization and defeminization are separate hormonally driven processes that organize the neural substrate to promote male-typic behaviors while suppressing female-typic behaviors. The organized neural substrate is activated by adult gonadal steroids and required for sex-typic behaviors to be expressed. This iconic model based on the organizational/activational hypothesis14 has proved a sturdy framework for elucidating some, but not all, of the aspects of sexual differentiation of the brain.
Parallel model: multiple sex-specific signals and pathways
All sex differences must ultimately stem from the inherent imbalance of genes encoded by the sex chromosomes, which are the only factors thought to differ in the male and female zygote. By 1959, the mammalian Y chromosome was found to contain a dominant testis-determining gene that was later identified as Sry18, which initiates testis differentiation and is often placed at the top of the molecular cascade that differentiates testicular from ovarian development. The differentiation of gonads sets up sex differences in the level of gonadal hormones, which cause differences in male and female cells. Each XX and XY cell, however, inherently has a different complement of sex chromosomes. In brain and other tissues, XX and XY mice show differences in phenotype that are explained by differences in expression of X and Y genes. For example, Sry is expressed in the dopamine-containing cells of substantia nigra pars compacta (SNpc) that project to the striatum and has direct male-specific effects19. These neurons are the targets of Parkinson’s disease, which shows a 1.5-fold higher incidence in men. When the expression of Sry is temporarily reduced in adult rats, the expression of tyrosine hydroxylase in the SNpc and striatum are markedly reduced and motor performance declines. These results constitute one of the first demonstrations of a direct sex-specific effect on brain of an identified sex chromosome gene. One paradox is that, despite the sex differences in Parkinson’s disease, striatal function and SNpc neuron number19,20, the SNpc and striatum function quite well in both sexes so that a male-specific dynamic role for Sry is unexpected. The paradox raises the question of whether a female-specific factor maintains tyrosine hydroxylase expression in females and if another male-specific factor (testosterone?) has an undesirable effect in the SNpc that is counteracted by Sry expression. This case raises a common theme in the investigation of sex chromosome effects, that sex-specific factors do not always make males and females different, but sometimes counteract each other to make the sexes more similar11,12,21.
Another gene, Xist, is also encoded by the sex chromosomes and has sex-specific effects. Xist is expressed from one of the two X chromosomes in non-germline XX cells of eutherian mammals and initiates inactivation of that chromosome22. The ultimate effect of Xist is that only one X chromosome is transcriptionally active in females, and Xist is typically viewed as a factor that makes females more similar to males. As a result, Xist is rarely included on lists of genes that cause sexual differentiation. Its role in causing mosaicism of X gene effects in females, but not males, has, however, been emphasized23,24. But clearly, XX cells are different from XY cells precisely because they express Xist and engage a major epigenetic machinery that is not active in XY cells. However, we know little about the differentiating effects of Xist, perhaps because its role in compensating for sex differences has been emphasized.
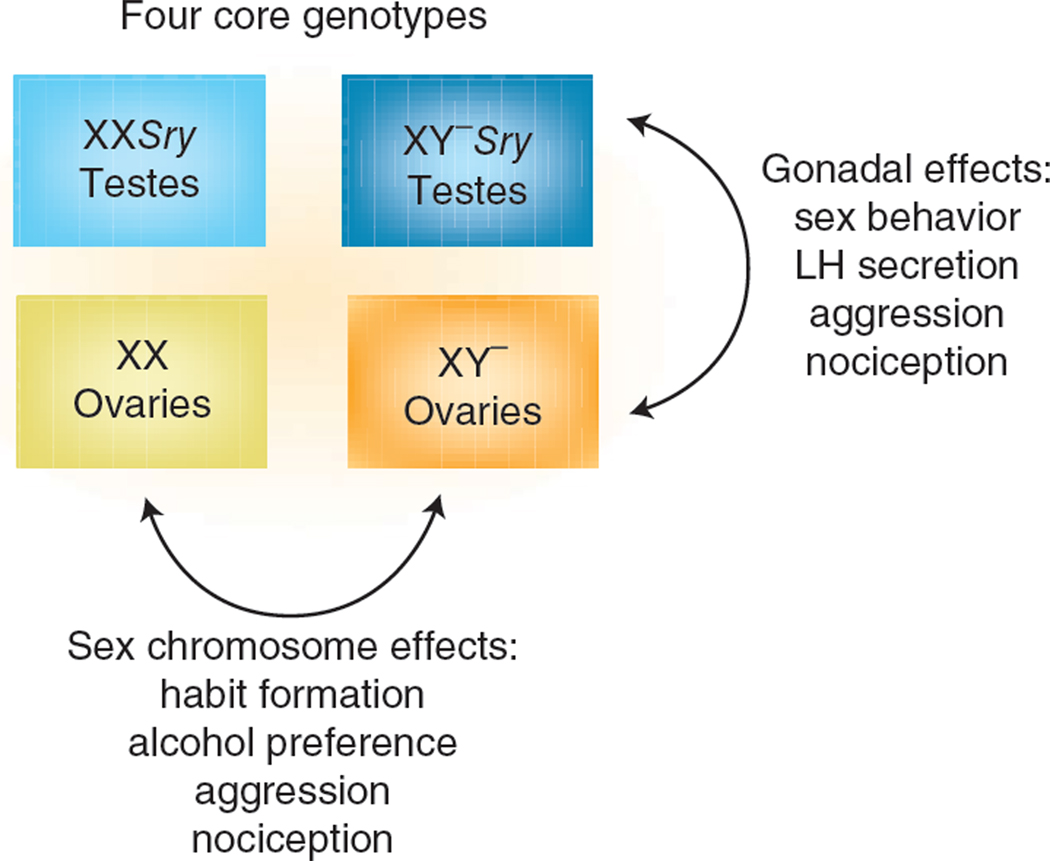
The direct roles of sex chromosome genes on sex differences are not well studied (and therefore probably are underestimated), not only because of the dominance of the hormonal theory of sexual differentiation, but also because few animal models have allowed manipulation of the sex chromosomes without also causing large changes in hormone levels25. To date, most models for studying direct sex chromosome effects are available in mice in which the sex chromosomes can be engineered26. The best studied is the four core genotypes (FCG) model, in which the complement of sex chromosomes (XX versus XY) is made independent of gonadal sex12,27,28. This is possible because the Sry gene is ‘moved’ to an autosome and the Y chromosome is therefore no longer testis-determining. The model produces four types of offspring in a 2×2 pattern: they are either XX or XY and they either do (producing gonadal males, XXM or XYM) or do not (producing gonadal females, XXF or XYF) possess the Sry transgene. The model allows simultaneous appreciation of the effects of gonadal sex (comparing the phenotypes of gonadal males and females) and of sex chromosome complement (comparing XX and XY mice of either gonadal sex; Fig. 2). The results available to date from analysis of FCG mice confirm the importance of hormones, but undermine their hegemony. Numerous sex differences in neural and behavioral phenotypes, such as sex behavior and the neural underpinnings of ovulation, are largely, if not entirely, sexually differentiated by perinatal gonadal steroid hormones. However, a number of variables that differ in males and females are robustly influenced by sex chromosome complement, with genetic sex exerting influences sometimes as large as those exerted by gonadal hormones. These include sex differences in vasopressin innervation of the lateral septum27, aggressive and parenting behavior29, nociception30,31, formation of habits32, alcohol abuse33, susceptibility to neural disease34,35, social behaviors36,37, and gene expression38–40. Notably, some sex differences stem from direct effects of X genes that are present in two copies in females and one copy in males34,38, which result in constitutive sex differences in the dose of X genes or their parent of origin12,15,41. Thus at the genetic level, Sry is not the only gene causing sex differences in brain phenotype (although this gene acts both indirectly by virtue of its effects on hormone levels and directly because of its expression in brain cells). Rather, various genes encoded on the X or Y chromosome have sex-specific effects, including Sry and Xist and other yet to be identified X and/or Y genes. Often the phenotypes differentiated by direct sex chromosome effects are also influenced by gonadal hormones, so there is a great need to understand the interaction of sex-specific hormonal and sex chromosome effects. Moreover, these results support the conclusion that every cell in the brain of males may differ from those in females, by virtue of differences in their sex chromosome complement, as well as in response to the important hormonal effects discussed next. Thus, sex differences are likely pervasive in the brain and not limited to a few sexually dimorphic regions. This view is further supported by recent evidence for a substantial sex-specific parental bias in gene expression across brain regions42, although the ramifications and importance of sex differences in imprinting are not yet understood.
Figure 2.
Genetics matter. The ability to distinguish the contributing role of genes versus gonads was markedly advanced by the development of the four core genotypes model of mice. These mice bear a Y chromosome from which the Sry gene has been deleted (denoted Y−) and carry Sry on an autosome, allowing the development of XX individuals with testes and XY individuals with ovaries. Analysis of this model supports the view that sexual differentiation of reproductive endpoints is largely driven by the testicular hormone testosterone or estradiol synthesized in the developing nervous system from this testosterone, consistent with the organizational/activational hypothesis. Conversely, many nonreproductive endpoints involve direct genetic contributions to variability between males and females.
Parallel hormonal effects on diverse pathways and circuits
The permanent differentiating (organizational) effects of testosterone in the pre- or postnatal brain are often caused by estradiol, a major brain metabolite of testosterone (for a review, see ref. 43). Estrogens act on estrogen receptors to masculinize (enhance behaviors and functions typical of males) and defeminize (suppress behaviors and functions typical of females). Advances in understanding of steroid-mediated brain differentiation are occurring on two fronts: elucidation of cellular mechanisms of steroid action and downstream effects, and characterization of behavioral and neuronal phenotypes of genetically modified mice. For instance, loss of the estrogen receptor alpha (Esr1) results in males with greatly reduced sex behavior, although they retain simple mounting behavior44. Knockout of estrogen receptor beta (Esr2) alone has no effect on male sex behavior, but when both estrogen receptors are dysfunctional male sex behavior is lost completely45. Esr2 is specifically implicated in the suppression of female sex behavior (defeminization) in males46. The importance of estradiol, as opposed to the estrogen receptor, for masculinizing sex behavior is confirmed by the disruption of the gene coding for aromatase, the enzyme required for estradiol synthesis from testosterone47. Notably, experiments in aromatase knockout mice show that there is a requirement for estradiol in normal female brain development48. Estradiol is unlikely to masculinize males and feminize females at the same site and time; thus, sexual differentiation involves temporally and/or spatially separate estradiol-induced patterns in the two sexes49. Disrupting the androgen receptor also predictably impairs male sexual behavior50,51. Thus, the study of mice bearing null mutations for steroid receptors mediating sexual differentiation largely confirms the major conclusions of earlier studies using manipulations of steroid levels or steroid receptors52, but also reveals multiple molecular pathways responding to estrogens and androgens in males and females.
The study of mice has been highly informative in parsing out the mechanisms of a major contributor to sex differences in the brain, differential cell death. Many regions and subnuclei in the brain are larger in one sex than in the other. In mammalian males, the spinal cord nucleus, SNB, which contains motoneurons controlling striated muscles of the penis, is larger in males, as are several nuclei in or directly associated with the medial preoptic area of the hypothalamus (MPOA), a major brain region controlling male sexual behavior53. In each instance this is a result of a greater number of neurons surviving through the perinatal period of hormonal sensitivity in males as opposed to high rates of cell death in females. Treatment of females with estrogens or androgens during the sensitive period will rescue the cells from death and result in a permanently masculinized, larger nucleus. Examination of mice lacking the cell death gene, Bax, confirms that the higher rate of cell death in several brain nuclei in females results from apoptosis54. Conversely, a preoptic ventral forebrain nucleus, the AVPV, is larger in females than in males and is a critical node in the neural circuit controlling ovulation. In this case, estradiol actively promotes cell death in males via a complex mix of classic caspase-3–mediated cell death of the tyrosine hydroxylase–expressing population55, combined with an independent program of death in the GABAergic cells mediated by downregulation of TNFα56. This orchestrated killing of discrete populations of neurons in the male AVPV is mediated by the estrogen receptor57 and appears to occur in response to estradiol only in cells expressing estrogen receptor. These studies indicate that the sex-specific cellular responses to the same gonadal hormones are different in specific CNS regions (SNB versus AVPV) and involve different molecular pathways in specific cell populations of a single brain region (AVPV).
Studies beyond steroid receptor null mutant mice are needed to elucidate the cellular events downstream of the nuclear steroid receptors, which involve active organization of neural elements and the neuropil. This is illustrated in part by emerging evidence of sex differences in cell proliferation in the rat hippocampus, which is in marked contrast to the well-documented sex differences in cell death seen in reproductively relevant brain regions. Measures of cell birth indicate that twice as many new cells are born in the male rat hippocampus during the perinatal sensitive period than in the female58, and this sex difference is a product of higher endogenous estradiol action in males stimulating neurogenesis59. The majority of cells born in the first few days of life will endure until at least the juvenile stage and differentiate into neurons. The overall hippocampal volume is only modestly larger in male rats than in females60, suggesting that the enhanced neurogenesis in males may serve purposes other than contributing to increased volume.
In marked contrast to the male bias in neurogenesis in the hippocampus, more new cells are born in the developing amygdala of female rats and, in this instance, those that survive to adulthood largely differentiate into astrocytes. Moreover, sex differences in endocannabinoids mediate the higher rate of cell genesis in females61. These provocative findings of a role for differential cell birth in brain regions outside those directly involved in reproduction further emphasize the potential for widespread, but region-specific, sex differences.
A third strategy for increasing the size of specific brain region is differentiation of more cells into the phenotype by which that region is defined. The male bed nucleus of the stria terminalis contains more vasopressin-expressing cells than the female nucleus and is therefore larger. Null mutation of Bax and overexpression of the anti–cell death gene, Bcl2, reveal that, although cell death regulates the overall number of vasopressin neurons in this nucleus, it does not regulate the sex difference. Taken together, these observations suggest, but do not prove, that sex differences in the rate of phenotypic differentiation is the most likely underlying mechanism62.
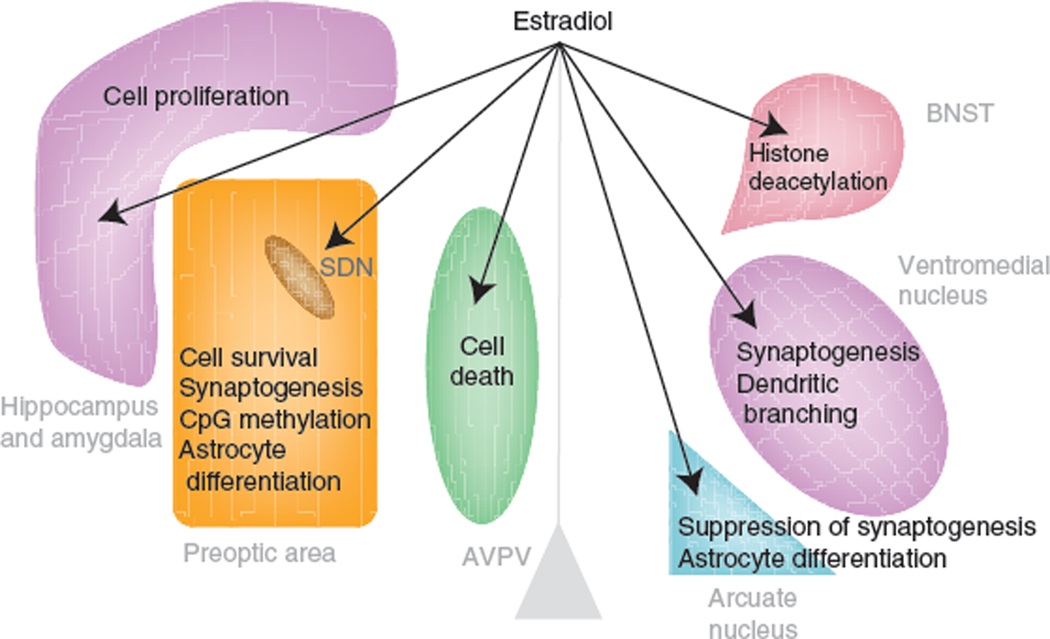
Diverse mechanisms also mediate the estradiol-induced sexual differentiation of synaptic patterning in specific brain regions (Fig. 3). The higher level of estradiol during the perinatal period in males organizes the number and/or density of dendritic spine versus axosomatic synapses, resulting in 2–3-fold differences between males and females in specific nuclei. These are particularly well characterized in preoptic and hypothalamic nuclei such as the MPOA, ventromedial nucleus of the hypothalamus (VMN) and arcuate nucleus52. The regional specificity of response to estradiol is evident by comparing the MPOA, where male preoptic neurons have a 2–3-fold greater density of dendritc spine synapses than females6, the hypothalamic arcuate nucleus, where female neurons have twice the density of dendritic spine synapses as males63, and the VMN, where males have more overall dendritic spine synapses secondary to the longer and more highly branched dendrites of male VMN neurons64. The cellular mechanism organizing the synaptic pattern is also distinct for each nucleus, with estrogen receptor activation invoking unique strategies in each case. In the MPOA, estradiol upregulates the cyclooxygenases genes COX1 and COX2 to increase prostaglandin synthesis, activating EP2 and EP4 receptors linked to adenlyl cyclase, and activating PKA, which promotes stabilization and membrane insertion of AMPA glutamate receptors65,66. In the arcuate nucleus, estradiol upregulates GAD and GABA production, which act on and differentiate neighboring astrocytes67, and estradiol rapidly and nongenomically activates PI3 kinase in the VMN and promotes presynaptic glutamate release, activating postsynaptic AMPA and NMDA receptors7,68.
Figure 3.
Multiple mechanisms of estradiol-induced differentiation. In the rodent, estradiol is a masculinizing hormone, but it exerts multiple region-specific effects via distinct cellular mechanisms. Thus, during a perinatal sensitive period, the same hormone, estradiol, promotes cell survival, cell death and cell proliferation in separate brain regions. Estradiol also promotes the formation of new dendritic spine synapses in some brain regions while suppressing them in others. The enduring consequences of the organizational effects of estradiol may be mediated in part via epigenetic changes to the DNA and chromatin in processes that are region-specific, but are still poorly understood.
Although these mechanisms downstream of estradiol have no observable overlap, a common theme is that releasable factors and cell-to-cell communication are important. Estradiol-induced glutamate release alters the synaptic profile of the downstream neuron, with no requirement for estrogen receptor in that neuron. Similarly, GABA released from neurons permanently alters the morphology of neighboring astrocytes with no requirement for estrogen receptor in those cells. The consequence of emancipation from expression of estrogen receptor to be organized by estrogen receptor is that many more cells are affected both locally and in a domino fashion as one brain region projects to and alters the sexual differentiation of others. Similar cross-cellular effects are seen in the song control circuit in birds, where implants of estradiol near nucleus HVC masculinize nucleus RA downstream of HVC, and lesions of HVC block estradiol’s masculinizing effect on nucleus RA69,70. Put more simply, steroid-mediated sexual differentiation of neural circuits is not limited to direct targets of the hormone. Just as every brain cell has a genetic sex, many cell types in specific regions are organized during development by virtue of interactions with other cells in its milieu, so that any information coming into that region is integrated in the context of its sex. This concept argues against the idea that a few steroid-response neurons sit in an otherwise sexually monomorphic brain.
The idea that there are sex-specific circuits may stem in part from the existence of numerous robust and reliable anatomical sex differences in brain regions critical for the expression of sex behavior43,53,57. But consider the nature of the differences. One of the most celebrated, the sexually dimorphic nucleus of the preoptic area (POA), is 3–5-fold larger in males, but is nevertheless present in females, the only difference being that it is smaller than the sexually dimorphic nucleus in males. Similarly, males have 2–3-fold more dendritic spines on POA and VMN neurons, but again, females have plenty of dendritic spines and attendant synapses, just not as many as males. Thus, instead of two distinct neural circuits, it is equally likely that there is only one neural network and that it is differentially weighted toward sex-specific responses as a function of early organization and adult context and hormonal activation.
Recent studies have also found previously unappreciated diversity in the cellular mechanisms of steroid hormone action in the brain. According to the serial model of sexual differentiation of the last century, steroids were viewed as being synthesized by the gonads and acting at sites far from their origin. Steroid receptors such as androgen receptor, estrogen receptor and progesterone receptor are nuclear transcription factor receptors that are capable of directly interacting with DNA at specific response elements and modulating gene transcription71,72. Induction of transcription, translation and the construction of new biologically active proteins require time. Combined, these characteristics contributed to the view of steroids as slow mediators of distant cellular processes with long onset and offset. Although that view still has validity, it is equally true that steroids act rapidly on membrane bound receptors, activating signal transduction pathways associated with dynamic changes in cell physiology, including excitability73. Moreover, we now know that steroids, including estradiol, can be synthesized locally, quickly and on demand by neural cells. This is a shift from the concept of steroids as humoral signaling molecules and has led to speculation that estrogens can function in a manner akin to neurotransmitters74,75. The fact that steroids are not stored distinguishes them from neurotransmitters, but places them in a category similar to that of endocannabinoids and gaseous messengers, such as nitrous oxide, that are synthesized on demand76. Finely tuned changes in the rate of synthesis and degradation of these signaling molecules regulates their ‘tone’ and thereby their effect on neural functioning. Responses to external stimuli that induce internal changes, such as activating the stress axis, often involve changes in neuromodulatory tone77 and this potential may also exist for steroids. However, the process of steroid-mediated permanent sexual differentiation of the brain was not expected to be modulated by rapidly initiated signaling cascades. That expectation was proved wrong by the discovery of rapid membrane effects of estradiol leading to permanent organization of dendritic morphology in the mediobasal hypothalamus7. The precise nature of membrane receptors for estradiol is still being debated, but their distribution both in and between cells appears to be far greater than that of the classic nuclear transcription factor, in part because estradiol can be synthesized in glia and likely has effects on non-neuronal cells that interact with neurons78–80. These findings further underscore the diversity of cellular responses to steroids and, because of the possibility that these effects are more distributed than effects mediated by nuclear steroid receptors, belie the notion of a limited set of hormonally responsive neurons that are dedicated to the control of sex-specific functions. Nevertheless, there are quite likely some nodes in the brain that are more critical than others in the regulation of sexually dimorphic physiology and behavior, such as the POA for sexual behavior and the AVPV for control of gonadotropin secretion57.
Experience matters
The biological sex of a child immediately influences its social and physical environment, even before birth. Our gendered place in society strongly conditions our life history, concept of self, and reaction to social and nonsocial events81. Parents and teachers create robust sex-specific expectations for children, fostering gendered behavioral development82. The different environments for boys and girls contribute to strong sex differences in choice of occupation and other gender-specific environmental stratification83, no doubt contributing to life-long sex differences in social roles, stress and disease. The sex-specific effects of these differentiated social environments, no doubt pervasive and profound, have long been the purview of social psychology and not a topic integral to the study of brain sexual differentiation. In part, this deficit stems from the difficulty of modeling human social environments in animal models. Studies on humans are also problematic because of confounding sex-specific biological and environmental factors, which makes it impossible to disentangle the effects of the two.
This situation is likely to change soon as a result of the emerging discovery of epigenetic modifications of the genome caused by specific environments, which can be measured in model animals. A salient example is the rat dams’ differential response to male and female pups. The anogenital grooming of newborn pups is a critical component of maternal care, and variation in the amount of attention a dam gives her offspring has enduring consequences for adult behavior, an effect that is mediated, at least in part, by epigenetic changes. Lasting changes in the responsiveness of the stress axis and behavioral strategies for coping with fear and novelty are correlated with the degree of CpG methylation in the promoter region of the genes coding the gluccocorticoid receptor in the hippocampus and estrogen receptor in the POA84. Dams distinguish between their male and female pups by providing more anogenital grooming of males than is required for their survival. This not only provides critical somatosensory stimulation needed for normal development of the nerves controlling the penis85,86, but also produces a sex difference in the percentage of CpG methylation of the Esr1 promoter in the preoptic area, which is correlated with a sex difference in Esr1 expression later in development87.
Another example of sex-specific epigenetic programming is that estradiol aromatized from testicular androgens during the perinatal sensitive period influences the degree of CpG methylation of the Esr1 promoter88, as well as that of Esr2 and Pgr89, revealing a potential for steroid hormone feedback on its own sensitivity throughout the lifespan. Sex differences in the epigenetic modulation of histones in specific brain regions90 and evidence for an epigenetic underpinning to the differential cell death observed in at least one brain region, the bed nucleus of the stria terminalis91, further emphasizes the potential for enduring and widespread effects of early hormone exposure via epigenetic modulation. These recent findings foreshadow extensive future work to measure the epigenetic effects of sex-specific environments and biological signals, which likely interact with the hormonal and sex chromosomal control of sexual differentiation. We are only beginning to be able to frame this question and little is known in this area92. The combination of experience, steroid hormonal milieu and epigenetic changes represents a convergence point of hormonal and genetic influences on sex differences in the brain that are further modified by individual experience.
Summary: reframed view of brain sexual differentiation
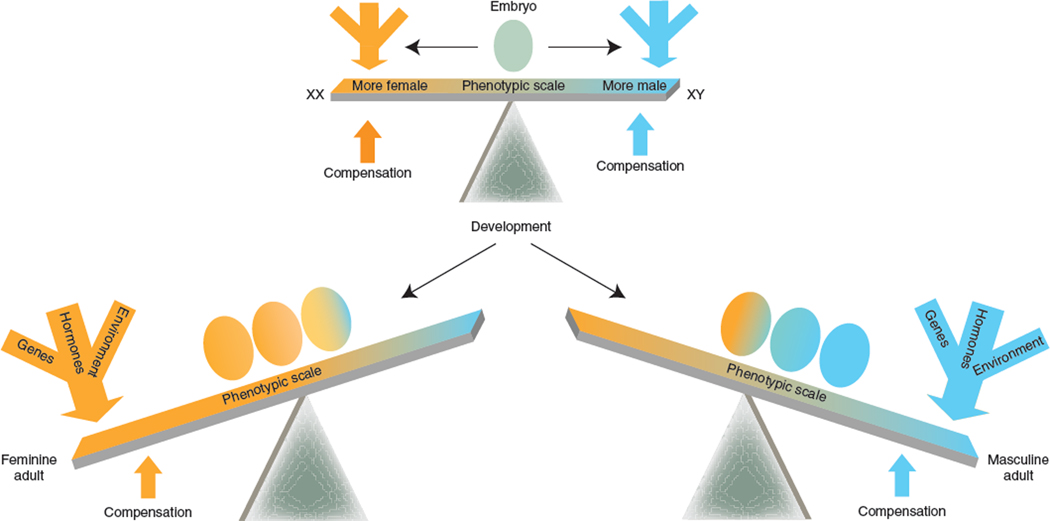
This review presents a new framework for integrating the multiple factors affecting developing brains of males and females (Fig. 4) and highlights several important conclusions drawn from recent studies that alter our concept of sexual differentiation of the brain. First, the sex chromosomes, both X and Y, harbor multiple genes, not just Sry, that initiate sexual differentiation. At the genetic level, these are the factors that are the root cause of all sex differences in phenotype. Second, the proximate signals that act directly on brain cells to cause sexual differentiation are not just gonadal hormones, but include other factors, such as those encoded by the sex chromosomes and non-gonadal gene products downstream from sex chromosome genes. Third, different brain regions have different programs of response to the sex-specific signals, involving regional cell type–specific responses, cell-to-cell communication, effects mediated by membrane and nuclear hormone receptors, local steroid synthesis, and compensatory sex-specific effects that antagonize each other and reduce sex differences in phenotype. Changes in gonadal hormone levels over the lifespan and other dynamic changes likely condition sex differences and enhance or suppress them over time. Finally, sex differences in the environment have an enormous effect on gender in humans and are arguably more potent in sculpting the gender-based social phenotype of humans. There is virtually nothing known about the biological basis for these environmental effects, but the rodent literature (epigenetic effects of early stress, differences in maternal behavior) hints at epigenetic mechanisms that could mediate environmental effects on the brain, throughout the lifespan. We conclude that the long established and mature field of study of sex differences in the brain is as vibrant and dynamic as ever, with many valuable lessons left to be learned.
Figure 4.
Redefining sexual differentiation. In a twenty-first-century view of sexual differentiation of the brain, the importance of genetics and environment are incorporated along with the effects of hormones to provide a more nuanced portrayal of the types of variables that cause sex differences. Included in this view are the principles that hormones, sex chromosome genes and sex-specific environments have independent parallel differentiating effects that can interact with each other, often synergistically, to cause sex differences in the brain. However, there are also compensatory sex-specific variables that act to reduce sex differences rather than induce them. The result is that some aspects of male and female brain, behavior and physiology are unique from each other, whereas others are highly similar. Two important aspects of the redefined view are not illustrated here: sex differences are pervasive throughout the brain and not restricted to reproductively relevant neural circuits, and variability in the degree to which brain regions are masculinized or feminized in one individual results in a mosaic of relative maleness or femaleness and thereby greatly increases the variance between individuals of the same sex in a population.
ACKNOWLEDGMENTS
Work on this manuscript was supported by grants MH52716 and NS050525 to M.M.M. and grants NS043196 and DC000217 to A.P.A.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 2.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Voskuhl R. Sex differences in autoimmune diseases. Biol. Sex Differ. 2011;2:1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaab DF, Hofman MA. Sexual differentiation of the human hypothalamus in relation to gender and sexual orientation. Trends Neurosci. 1995;18:264–270. [PubMed] [Google Scholar]
- 5.McCarthy M, De Vries G, Forger N. Ch. 17. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 3. Academic Press; 2009. pp. 1707–1744. [Google Scholar]
- 6.Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat. Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Bonthius PJ, Tsai HW, Bekiranov S, Rissman EF. Amyloid beta precursor protein regulates male sexual behavior. J. Neurosci. 2010;30:9967–9972. doi: 10.1523/JNEUROSCI.1988-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jazin E, Cahill L. Sex differences in molecular neuroscience: from fruit flies to humans. Nat. Rev. Neurosci. 2010;11:9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- 11.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 12.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ. Mol. Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 14.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 15.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhao D, et al. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464:237–242. doi: 10.1038/nature08852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annu. Rev. Genet. 1993;27:71–92. doi: 10.1146/annurev.ge.27.120193.000443. [DOI] [PubMed] [Google Scholar]
- 19.Dewing P, et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann. NY Acad. Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front. Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Chang SC, Tucker T, Thorogood NP, Brown CJ. Mechanisms of X-chromosome inactivation. Front. Biosci. 2006;11:852–866. doi: 10.2741/1842. [DOI] [PubMed] [Google Scholar]
- 23.Migeon BR. Females are Mosaic: X Inactivation and Sex Differences in Disease. Oxford University Press; 2007. [DOI] [PubMed] [Google Scholar]
- 24.Arnold AP. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 25.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J. Neuroendocrinol. 2009;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Front. Neuroendocrinol. 2011 February 19; doi: 10.1016/j.yfrne.2011.02.009. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vries GJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold AP, et al. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–1062. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- 29.Gatewood JD, et al. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. Sex chromosome complement affects nociception and analgesia in newborn mice. J. Pain. 2008;9:962–969. doi: 10.1016/j.jpain.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioiosa L, et al. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm. Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat. Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 33.Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J. Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, et al. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev. Neurobiol. 2008;68:265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- 35.Smith-Bouvier DL, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox KH, Rissman EF. Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes Brain Behav. 2011 March 17; doi: 10.1111/j.1601-183X.2011.00688.x. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm. Behav. 2008;54:565–570. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur. J. Neurosci. 2009;29:768–776. doi: 10.1111/j.1460-9568.2009.06610.x. [DOI] [PubMed] [Google Scholar]
- 39.van Nas A, et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology. 2009;150:1235–1249. doi: 10.1210/en.2008-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abel JM, Witt DM, Rissman EF. Sex differences in the cerebellum and frontal cortex: roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinology. 2011 February 16; doi: 10.1159/000324402. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies W, Isles AR, Burgoyne PS, Wilkinson LS. X-linked imprinting: effects on brain and behavior. Bioessays. 2006;28:35–44. doi: 10.1002/bies.20341. [DOI] [PubMed] [Google Scholar]
- 42.Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogawa S, et al. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa S, et al. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc. Natl. Acad. Sci. USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc. Natl. Acad. Sci. USA. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 48.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann. NY Acad. Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 49.Bakker J, Brock O. Early oestrogens in shaping reproductive networks: evidence for a potential organisational role of oestradiol in female brain development. J. Neuroendocrinol. 2010;22:728–735. doi: 10.1111/j.1365-2826.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- 50.Juntti SA, Coats JK, Shah NM. A genetic approach to dissect sexually dimorphic behaviors. Horm. Behav. 2008;53:627–637. doi: 10.1016/j.yhbeh.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juntti SA, et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto A. Sexual Differentiation of the Brain. CRC Press; 2000. [Google Scholar]
- 53.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 54.Forger NG, et al. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc. Natl. Acad. Sci. USA. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J. Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc. Natl. Acad. Sci. USA. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J-M, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur. J. Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowers J, Waddell J, McCarthy M. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous estradiol. Biol. Sex Differ. 2010;1:8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm. Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- 61.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc. Natl. Acad. Sci. USA. 2010;107:20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Vries GJ, et al. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J. Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABAA receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev. Neurobiol. 2007;67:304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- 65.Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev. Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate and metabotropic glutamate receptor signaling. J. Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J. Neuroendocrinol. 2002;14:45–55. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 68.Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm. Behav. 2008;54:662–668. doi: 10.1016/j.yhbeh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrmann K, Arnold AP. Lesions of HVc block the developmental masculinizing effects of estradiol in the female zebra finch song system. J. Neurobiol. 1991;22:29–39. doi: 10.1002/neu.480220104. [DOI] [PubMed] [Google Scholar]
- 70.Grisham W, Mathews GA, Arnold AP. Local intracerebral implants of estrogen masculinize some aspects of the zebra finch song system. J. Neurobiol. 1994;25:185–196. doi: 10.1002/neu.480250209. [DOI] [PubMed] [Google Scholar]
- 71.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 72.Beato M, Klug J. Steroid hormone receptors: an update. Hum. Reprod. Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 73.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 74.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr. Opin. Investig. Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- 77.Hill MN, et al. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signaling in reproduction. J. Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcia-Segura LM, Lorenz B, DonCarlos LL. The role of glia in the hypothalamus: implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction. 2008;135:419–429. doi: 10.1530/REP-07-0540. [DOI] [PubMed] [Google Scholar]
- 80.London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front. Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fausto-Sterling A. Sexing the Body: Gender Politics and the Construction of Sexuality. Basic Books; 2000. [Google Scholar]
- 82.Frome PM, Eccles JS. Parents’ influence on children’s achievement-related perceptions. J. Pers. Soc. Psychol. 1998;74:435–452. doi: 10.1037//0022-3514.74.2.435. [DOI] [PubMed] [Google Scholar]
- 83.Lippa RA. Sex differences in personality traits and gender-related occupational preferences across 53 nations: testing evolutionary and social-environmental theories. Arch. Sex. Behav. 2010;39:619–636. doi: 10.1007/s10508-008-9380-7. [DOI] [PubMed] [Google Scholar]
- 84.Champagne FA. Epigenetic mechanisms and the transgernational effects of maternal care. Front. Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev. Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- 86.Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Res. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 87.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCarthy MM, et al. The epigenetics of sex differences in the brain. J. Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]