Abstract
Objective
To examine the effects of a community-based intervention on decisions about prostate-specific antigen (PSA) screening using multiple measures of informed decision making (IDM).
Data Sources/Study Setting
Nonequivalent control group time series design collecting primary data in late 2004 and 2005.
Study Design
We developed a multimodal intervention designed to convey the medical uncertainty about the benefits of PSA screening and early treatment and the limited predictive ability of both the PSA test and pathological specimens collected from prostate biopsy. We examined (1) patients' recognition that there is a decision to be made about PSA screening, (2) prostate cancer knowledge levels, (3) their preferred and actual levels of participation in decision making about screening at three points in time, and (4) screening decision.
Data Collection
Baseline data collection occurred in community-based organizations. These organizations served as recruiting sources and as sites for the intervention. We collected follow-up data by mail with telephone reminders.
Principal Findings
Our intervention was associated with greater recognition of the PSA test as a decision to be made, levels of knowledge, both preferred and actual levels of involvement in decision making, but did not have an impact on the screening decision.
Conclusions
Community-based interventions can influence key measures of IDM about PSA screening.
Keywords: Decision making, prostate cancer screening, level of involvement, patient–provider communication
BACKGROUND
In 2001–2002, the U.S. Preventive Services Task Force (USPSTF) conducted a systematic review of the evidence and concluded that it was insufficient to determine whether the benefits of prostate-specific antigen (PSA) screening outweigh the harms (Harris and Lohr 2002; USPSTF 2002;). According to a 2008 update of the evidence, whether PSA screening reduces mortality from prostate cancer still remains unclear (Andriole et al. 2009). PSA screening can trigger a rapid sequence of further testing and treatment that often produces significant negative consequences (urinary incontinence and erectile dysfunction in particular) (USPSTF 2002). Given these uncertainties, professional associations (Ferrini and Woolf 1998; American Urological Association 2000; Wolf et al. 2010;) widely recommend engaging men in informed decision making (IDM) for PSA screening decisions to ensure that they understand the uncertain benefits and potential harms associated with screening.
Despite the lack of clear evidence regarding the benefits of routine PSA screening, the practice is widespread in the United States; and testing frequently occurs without adequate discussion between patients and health professionals (Han et al. 2006; Guerra et al. 2007; Sirovich, Schwartz, and Woloshin 2007;). The 2000 National Health Interview Survey found physicians often initiate PSA screening without prior discussion with patients about its advantages and disadvantages (Han et al. 2006). Recipients of PSA screening are often unaware that they have been tested (Volk and Cass 2002; Chan et al. 2004;). Together, these findings suggest that men's opportunities to make informed decisions about PSA screening are constrained.
Because of the scientific uncertainty and general consensus that an IDM approach is needed, considerable work has gone into developing decision aids and other interventions to facilitate informed choices about PSA screening (Rimer et al. 2004; Evans et al. 2005; O'Connor et al. 2006;). IDM interventions for PSA screening increased knowledge, but their effects on PSA screening uptake are mixed (Rimer et al. 2004; Evans et al. 2005; O'Connor et al. 2006;). In one meta-analysis, use of PSA decision aids reduced screening uptake (Evans et al. 2005). Another meta-analysis (Volk et al. 2007) reported that PSA decision aids appeared to decrease interest in PSA testing and screening behavior among patients seeking routine care and had no impact on the screening behavior of patients seeking screening services. Evaluations of IDM interventions in general (not specific to prostate cancer) find that they increase knowledge and accuracy of risk perceptions, although effects are often not sustained over longer follow-up (Rimer et al. 2004; Evans et al. 2005;). IDM interventions also influence participants' preferences for an active role in decision making and increase satisfaction with the decision-making process (O'Connor et al. 2006).
Patient preferences for involvement in decision making vary along a spectrum from, at one end, a clinician-controlled (paternalistic) model in which the patient relies on the clinician to make decisions to, at the other, a preference for autonomous decision making (Robinson and Thompson 2001; Flynn, Smith, and Vanness 2006; Ryan and Sysko 2007; Thompson 2007;). Role preferences in decision making differ by the type and severity of the illness, patient characteristics, and patients' relationships with health professionals. A preference for greater autonomy in decision making is generally associated with younger age, higher educational attainment, and better health status (Benbassat, Pilpel, and Tidhar 1998; Robinson and Thompson 2001; Flynn, Smith, and Vanness 2006; Ryan and Sysko 2007;).
No single metric reflecting IDM exists. Most evaluations of IDM interventions have assessed knowledge and screening intention and behavior (Rimer et al. 2004; Mullen et al. 2006;). Few studies have examined a broader range of outcomes including participation in decision making at a level consistent with the patient's personal preference or satisfaction with the decision-making process (Rimer et al. 2004; Evans et al. 2005; O'Connor et al. 2006; Mullen et al. 2006;). To advance understanding of IDM in cancer screening, Mullen et al. (2006) recommend that studies use rigorous measures of (1) patients' participation in decision making at the level they desire, (2) consistency between personal values and screening decisions, and (3) satisfaction with the decision-making process and the decision made.
This is one of the first studies to implement a full range of IDM measures advocated by Mullen et al. (2006) along with other measures in an intervention study examining decision making about PSA screening. Specifically, we investigated the intervention's effects on men's beliefs that they have a decision to make about PSA screening (a necessary prerequisite to IDM), their knowledge levels, their preferred and actual levels of involvement in the decision of whether to have a PSA screening test (and concordance between the two), and their satisfaction with level of involvement and the decisions made. We used a community-based IDM intervention approach; it offers distinct advantages over clinic-based IDM promotion yet has rarely been implemented and evaluated.
STUDY DESIGN
We conducted an intervention study designed to promote IDM about PSA screening in three communities. The study received human subjects approval from the institutions involved.
Setting and Participants
We identified and defined the communities using an in-depth comparison process involving sociodemographic and economic comparability data (see Driscoll et al. 2008 for more information).
The baseline data collection took place within community-based organizations (e.g., senior, faith-based, fraternal, fitness, and recreational organizations) in two North Carolina communities; a third North Carolina community served as a control population. The community-based organizations served both as recruiting sources and as physical sites for delivering the intervention. Organizations advertised the intervention and data collection sessions to their members and others in the community primarily using flyers and word of mouth.
Intervention
The study team delivered a total of 20 intervention sessions, with 10–30 male participants per session, between September 2004 and February 2005. Our multicomponent intervention comprised an oral scripted presentation by a community physician followed by a question-and-answer session, a 20-minute video, a website, and print materials, including a trifold brochure, a 4 × 6-in. poster, and a shirt-pocket card decision aid (see Soloe et al. 2009 for a detailed description of the intervention rationale, theoretical foundation, images of the materials, formative research process including iterative development using multiple rounds of pretesting). The key intervention messages were as follows:
there are two types of prostate cancers: slow growing and fast growing;
a problem with the PSA test is that it leads some men with slow-growing prostate cancer to get treatment they do not need;
about half of all men who get treatment for prostate cancer will have permanent side effects; and
men should decide whether they feel the PSA test is right for them and talk with their physicians.
The final messages do not focus on the potential benefits of PSA screening. We originally presented equal amounts of information about the pros and cons of screening. However, extensive formative message testing of the original messages revealed cognitive dissonance for men associated with a message that (1) promotes IDM while (2) also conveying the potential (not the certainty) that screening might reduce risk of death (Driscoll and Harris-Kojetin 2002; Soloe et al. 2009;). That kind of complex message was not well understood because of the challenge associated with explaining medical uncertainty to a lay audience. The final intervention materials also contain information about risk factors for prostate cancer and different treatment options, and they encourage men to think about what choices are right for them based on their preferences and values. We developed two versions of the intervention materials: Prostate Only (PO) and Men's Health (MH). The MH version gave additional information about other common preventive health screening for men and highlighted the certainty in benefit from these screening tests. The materials contained no restrictions or guidance regarding which physicians study participants could or should see for their health care. Study participants could not be linked to provider-level data.
Data Collection Procedures
Only men between 40 and 80 years of age and not diagnosed previously with prostate cancer were invited to participate in the study. In the intervention communities, men attending the intervention sessions completed a self-administered baseline survey immediately before the intervention session. After the intervention, which lasted 45 minutes on average, men completed a shorter, immediate posttest survey intended to capture their impressions of the intervention. Approximately 6 and 12 months following the intervention, we conducted follow-up surveys by mail. Follow-up data collection was staggered over several months because of the schedules of the community-based organizations. In the control community, we conducted the baseline survey in-person and the remaining surveys by mail following the same schedule as in the intervention communities. Study participants received U.S.$10 for their time after completing each follow-up survey (see Driscoll et al. 2008 for additional information on data collection procedures).
Key Measures and Hypotheses
We tested whether the intervention affected men's belief that they have a decision to make about PSA screening, thus establishing the groundwork for making informed decisions. We hypothesized that the intervention would increase men's knowledge levels and their preferred and actual levels of involvement in the screening decision and raise the concordance between the preferred and actual levels. We also examined possible sociodemographic differences and psychosocial correlates of the study outcomes.
Main Outcomes Measures on the Survey
Belief That PSA Screening Is a Decision
We asked for men's level of agreement with a series of four statements to assess the extent to which they believed that PSA screening is a decision to be made. Three items were in the baseline and 6-month surveys. For the 12-month survey, we added a fourth item (a man between the ages of 50 and 70 in average health who decides not to have an annual PSA test is irresponsible) to reflect a similar measure in a contemporaneous national survey (Schwartz et al. 2004). We created the “PSA is a Decision” composite score based on responses to three of the items (Cronbach's α = 0.76).
Knowledge of Prostate Cancer Screening and Treatment
The survey included 10 knowledge questions in all three surveys designed to assess respondents' understanding of the intervention content; they were based partly on work by Radosevich et al. (2004) (e.g., possible reasons for a high PSA test, which type of treatment works best, common side effects from treatment). We coded responses as correct or incorrect (“don't know” as incorrect) and computed a knowledge index score (range 0–10; Cronbach's α = 0.70).
Preferred Level of Involvement in PSA Decision
To assess men's preferences related to participation in the PSA screening decision and changes over time, we adapted a validated survey item from Degner, Sloan, and Venkatesh (1997). We grouped the five response options below as indicated for a logistic regression analyses:
 |
Actual Level of Involvement in PSA Decision
We asked a parallel question in the 6- and 12-month follow-up surveys to assess men's actual involvement in the PSA decision. However, at 6 months, this question was asked only of men who had discussed PSA screening with their provider. The percentage of men who had a discussion with their provider at 6 months was 55 percent (and 59 percent at 12 months).
Concordance between Preferred and Actual Levels of Involvement in the PSA Decision
We created a “concordance” (or “preference match”; Kiesler and Auerbach 2006) measure based on the match between participants' preferred and actual levels of involvement in the PSA decision at the same measurement points (i.e., match between preferred and actual levels of involvement at 6- or 12-month follow-up). We classified participants who selected parallel response options for both questions as concordant. At 6 months, we applied the concordance measure only to men who had discussed PSA screening with their physician because only these men had been asked about their actual level of involvement at that point.
Satisfaction with Involvement in Decision Making and Decision Made
At 12 months, we asked respondents about their screening decision, how satisfied they were with their actual level of involvement, and how satisfied they were with their decision.
Other Measures
Sociodemographic and health variables were measured at baseline. Because we anticipated that the intervention might affect other variables, we used men's responses to all other measures at the 12-month follow-up to examine the effects of recent experience and of knowledge on the outcomes of interest.
Self-Efficacy for Communicating with a Physician
We developed a composite self-efficacy score based on the mean responses to three questions about how well respondents felt their physician answered all their questions, listened carefully to them, and explained things in a way they could understand (Cronbach's α = 0.89).
Decisional Uncertainty
We administered an adaptation of the decisional uncertainty subscale from the Decisional Conflict Scale (O'Connor 1995) (Cronbach's α = 0.76).
Exposure to PSA Information
We asked men how much they had heard, read, or seen about prostate cancer over the past year.
PSA Discussion and Screening Decision
We asked men whether they had discussed PSA screening with a health professional in the past year and about their screening behavior 12 months after exposure to the intervention.
Statistical Analyses
To test the study hypotheses, first, we conducted regression analyses to test for differences between the intervention and control groups in the 12-month outcomes. We used linear regression models for the “3-item PSA is a decision” composite score and satisfaction with level of involvement, and logistic regression models for the preferred and actual levels of involvement and concordance between the two. Each regression model included the sociodemographic and psychosocial variables described above. We used multiple-imputation techniques outlined by Schafer (1997) to impute missing data for predictor variables to ensure proper inference to all members of the target population (missing data for most items were 5 percent or less). Missing values for outcome measures were not imputed.
We also examined patterns of change in the outcome variables across the entire study period (baseline to 12 months) for the two groups (intervention and control). To analyze the repeated measurements across the multiple time points (baseline, 6, and 12 months), we used generalized estimating equations (GEE) to account for the potential correlation between observations within communities and over time (Liang and Zeger 1986). For the GEE models, we included a time, group (intervention versus control), and time-by-group interaction to allow us to determine whether the two groups differed in patterns of change over time. Because one item in this “PSA is a decision” composite score appeared only in the 12-month survey, we fit GEE models for individual items rather than for the composite score. However, we used the composite score for the linear regression model at 12 months as noted above.
RESULTS
Participant Characteristics
As shown in Figure 1, a total of 584 men completed the baseline survey (n = 361 intervention; n = 223 control). For the 6-month survey, 107 of the 125 PO baseline participants completed it (response rate [RR]=86 percent); 167 of the 236 MH baseline participants completed it (RR=71 percent), and 118 of the 223 control baseline group participants completed it (RR=53 percent). At 12 months, we sent the follow-up survey to all 584 baseline participants. RRs were as follows: PO=89 men (RR=71 percent); MH=165 (RR=70 percent); and control=122 (RR=55 percent).
Figure 1.
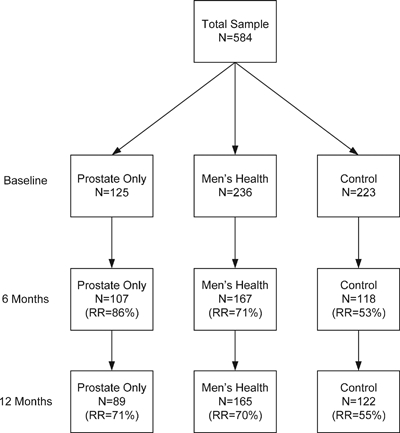
Sample Distribution by Intervention Group and Time Point
Notes. Baseline numbers are the denominators in all cases.RR, response rate.
This paper focuses primarily on 12-month outcomes, which include men who completed the baseline and 12-month surveys. We compared the characteristics of men who completed the 12-month survey with those of men lost to follow-up at this point. In the intervention and control groups, men who dropped out were more likely to be black, less educated, and younger (p<.05). Baseline characteristics of the final (12 month) sample are presented in Table 1. The intervention and control groups differed significantly in terms of age and race. The MH group had significantly lower knowledge than the PO group and control group and higher income than the control group, and they were significantly less likely to have a personal doctor than the PO group. The PO group had a PSA test more recently than the control group.
Table 1.
Baseline Demographic Characteristics of Participants by Study Group (N = 376)
| Prostate Only (PO) (N = 89) | Men's Health (MH) (N = 165) | Control (C) (N = 122) | ||
|---|---|---|---|---|
| Characteristic | N (%) | N (%) | N (%) | Significant Difference p<0.05 |
| Age, mean (SD) | 69 (8) | 63 (11) | 57 (11) | PO-MH, PO-C, MH-C |
| Black race | 8 (9) | 53 (32) | 62 (51) | PO-MH, PO-C, MH-C |
| Married | 68 (76) | 135 (82) | 98 (80) | |
| Education | ||||
| College or more | 53 (60) | 95 (58) | 82 (67) | |
| Some college | 21 (24) | 31 (19) | 26 (21) | |
| High school or less | 8 (9) | 28 (17) | 12 (10) | |
| Annual household income | ||||
| U.S.$60,000 or more | 37 (42) | 76 (46) | 16 (13) | MH-C |
| U.S.$40,000–U.S.$59,999 | 20 (22) | 37 (22) | 23 (19) | |
| <U.S.$39,999 | 16 (18) | 37 (22) | 76 (62) | |
| Health status | ||||
| Excellent/very good | 55 (62) | 92 (56) | 72 (59) | |
| Good | 21 (24) | 54 (33) | 41 (34) | |
| Fair/poor | 9 (10) | 17 (10) | 8 (7) | |
| Have a personal physician | 77 (87) | 128 (78) | 107 (88) | PO-MH |
| Most recent PSA test | ||||
| >1 year | 14 (16) | 27 (16) | 26 (21) | PO-C |
| 6 months–1 year | 32 (36) | 51 (31) | 39 (32) | |
| < 6 months | 29 (33) | 49 (30) | 27 (22) | |
| Never | 8 (9) | 25 (15) | 27 (22) | |
| Have had cancer other than prostate cancer | 19 (21) | 31 (19) | 16 (13) | |
| Knowledge of prostate cancer, mean (SD) | 4 (2) | 3 (2) | 4 (2) | PO-MH, MH-C |
| Perceived risk of prostate cancer | ||||
| Very/somewhat high | 19 (21) | 26 (16) | 25 (20) | |
| Moderate | 31 (35) | 63 (38) | 42 (34) | |
| Very/somewhat low | 22 (25) | 31 (19) | 39 (32) | |
| Self-efficacy, mean (SD) | 3 (1) | 3 (1) | 3 (1) | |
Belief That PSA Screening Is a Decision
Based on a multiple linear regression model of continuous scores on the “belief that PSA screening is a decision” scale, participants in both the PO and MH groups had significantly greater beliefs that PSA screening is a decision at 12 months than control group respondents (p<.001; Table 2). The results also suggest that black men were less likely than white men to believe screening is a decision. Higher income, higher knowledge, and either never having a PSA test or having a PSA test more than a year ago were associated with greater belief that PSA screening is a decision. Men who had discussed the PSA with a health professional in the past year, those who had seen a lot of information about prostate cancer, and those with higher self-efficacy for communication with a physician were less likely to believe that PSA screening is a decision.
Table 2.
Regression Models of Belief That PSA Testing Is a Decision Scale and Shared or Autonomous PSA Decision Making (Preferred and Actual), at 12 Months
| Belief That PSA Testing Is a Decision Scale (N = 373) | Preferred Level of Involvement (N = 370)* | Actual Level of Involvement (N = 346)† | ||||
|---|---|---|---|---|---|---|
| Variable | Coefficient (SE) | p | OR (95% CI) | p | OR (95% CI) | p |
| Intervention group | ||||||
| Prostate Only | 0.55 (0.12) | <.001 | 6.35 (1.88, 21.41) | .003 | 2.29 (0.95, 5.51) | .064 |
| Men's Health | 0.44 (0.10) | <.001 | 2.40 (1.01, 5.70) | .048 | 2.04 (1.02, 4.11) | .045 |
| Age | 0.00 (0.01) | .414 | 0.94 (0.90, 0.99) | .011 | 0.98 (0.95, 1.01) | .206 |
| Black race | −0.26 (0.11) | .022 | 0.48 (0.17, 1.36) | .167 | 1.51 (0.63, 3.64) | .357 |
| Married | −0.16 (0.11) | .174 | 0.97 (0.37, 2.56) | .950 | 0.86 (0.42, 1.78) | .686 |
| Education | ||||||
| College or more | 0.08 (0.14) | .571 | 0.56 (0.17, 1.85) | .344 | 1.03 (0.45, 2.39) | .939 |
| Some college | −0.18 (0.14) | .201 | 0.76 (0.22, 2.66) | .669 | 1.70 (0.64, 4.51) | .286 |
| Income | ||||||
| U.S.$60,000 or more | 0.08 (0.11) | .477 | 1.07 (0.34, 3.33) | .912 | 2.21 (0.92, 5.30) | .077 |
| U.S.$40,000–U.S.$59,999 | 0.26 (0.13) | .047 | 0.82 (0.28, 2.37) | .713 | 1.16 (0.49, 2.73) | .734 |
| Health status | ||||||
| Excellent/very good | 0.10 (0.17) | .569 | 0.24 (0.04, 1.53) | .131 | 1.47 (0.53, 4.08) | .463 |
| Good | 0.04 (0.17) | .834 | 0.27 (0.04, 1.74) | .166 | 1.55 (0.54, 4.44) | .409 |
| Have a personal physician | −0.07 (0.13) | .591 | 1.01 (0.31, 3.24) | .988 | 1.18 (0.45, 3.07) | .732 |
| Most recent PSA test | ||||||
| >1 year | 0.30 (0.13) | .023 | 1.28 (0.42, 3.93) | .666 | 1.14 (0.47, 2.73) | .773 |
| 6 months–1 year | 0.10 (0.11) | .378 | 1.25 (0.49, 3.21) | .641 | 0.85 (0.40, 1.80) | .663 |
| Never | 0.55 (0.18) | .002 | 1.47 (0.27, 8.01) | .654 | 1.51 (0.48, 4.74) | .477 |
| Have had cancer other than prostate cancer | −0.02 (0.10) | .824 | 0.64 (0.29, 1.21) | .654 | 1.44 (0.69, 3.04) | .333 |
| Knowledge of prostate cancer | 0.11 (0.02) | <.001 | 1.19 (0.99, 1.42) | .059 | 0.99 (0.87, 1.12) | .819 |
| Perceived risk of prostate cancer | ||||||
| Very/somewhat high | 0.00 (0.12) | .995 | 1.27 (0.37, 4.30) | .699 | 0.95 (0.38, 2.35) | .904 |
| Moderate | −0.14 (0.10) | .157 | 0.62 (0.24, 1.61) | .327 | 0.87 (0.44, 1.73) | .693 |
| Self-efficacy | −0.16 (0.06) | .016 | 0.45 (0.22, 0.90) | .024 | 0.53 (0.32, 0.88) | .015 |
| Had a PSA discussion with doctor | −0.36 (0.11) | .001 | 2.57 (1.06, 6.26) | .037 | 3.31 (1.66, 6.59) | <.001 |
| Amount of prostate cancer information seen or heard in past year | ||||||
| A lot | −0.27 (0.13) | .043 | 2.81 (0.80, 9.86) | .106 | 1.28 (0.51, 3.21) | .592 |
| Some | −0.11 (0.10) | .278 | 1.55 (0.70, 3.43) | .283 | 0.95 (0.48, 1.89) | .892 |
| Decisional uncertainty | – | – | 1.34 (0.85, 2.10) | .202 | 1.66 (1.13, 2.44) | .010 |
Note. Linear regression model was used for continuous belief that PSA testing is a decision scale scores and logistic regression models were used for categorical responses concerning preferred and actual involvement in decision. Reference categories are control group, white race, not married, high school or less education, less than $40,000 income, fair/poor health, no personal doctor, PSA test less than 6 months ago, no cancer, very/somewhat low risk, no discussion, and none/a little information.
Prefer shared responsibility or to make decision themselves (“I prefer that my doctor and I share responsibility for the decision,”“I prefer to make the final decision after seriously considering my doctor's opinion,” or “I prefer to make the final decision”) versus prefer doctor make decision (“I prefer that my doctor make the final decision but seriously consider my opinion” or “I prefer to leave all decisions to my doctor”).
Shared responsibility or made the decision themselves (“My doctor and I shared responsibility for the decision,”“I made the final decision after seriously considering my doctor's opinion,” and “I made the final decision”) versus doctor made decision (“My doctor made the final decision but seriously considered my opinion” or “I left the decision to my doctor”).
To examine changes in beliefs over time, we tested logistic GEE models for agreement with the three “PSA screening is a decision” items that were available at all three time points. The first model explored change in agreement with the statement “It is okay to decide not to have a PSA test after learning the facts.” The time-by-group interaction was significant (p<.001), indicating that agreement with this item changed differentially between the control and intervention groups. At baseline, the three groups had similar levels of agreement (p>.05). At 6 months, agreement with the statement among respondents in both intervention groups had risen substantially and it remained elevated at 12 months. In contrast, agreement levels decreased slightly over time in the control group and were significantly lower than those in either intervention group at both 6 and 12 months (p<.001). The two intervention groups did not differ significantly at any time point (p>.05) (Figure 2).
Figure 2.
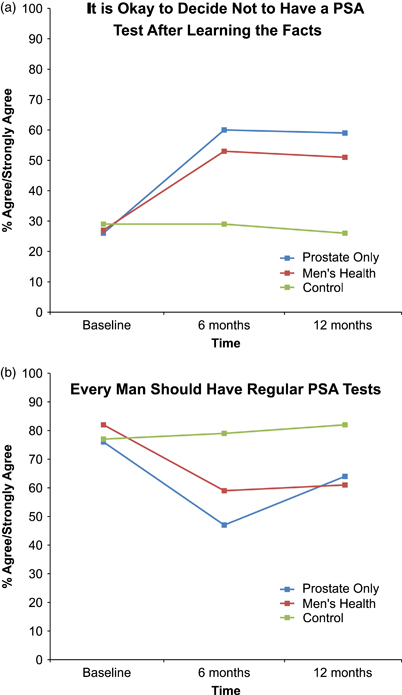
Belief That PSA Screening Is a Decision over Time, by Group
Notes. (a) Time × intervention group (p<.001) (N = 315).(b) Time × Intervention group (p = .006) (N = 315).
Second, we found a significant time-by-group interaction with respect to the statement “Every man should have a regular PSA test” (p = .006). Intervention and control groups' beliefs were similar at baseline (p>.05). At 6 months, participants' agreement that “every man should have a regular PSA test” had declined among both intervention groups, whereas the control groups' agreement was unchanged. The control group had significantly greater agreement with this item at 6 months than the PO (p<.001) and MH (p = .002) groups; the PO group had slightly lower agreement than the MH group (p = .053). The difference between the intervention and control groups remained significant at 12 months (PO versus control [p = .004] and MH [p<.001]); however, the difference between the two intervention groups disappeared (p = .523).
Finally, we found no significant time-by-group interaction for the final item (“Every man should decide whether or not a PSA test is right for him”; p = .579) (not shown). Therefore, we removed the interaction and reran the model. The results indicated that the PO and MH groups had greater agreement than the control group (p = .001), and the levels of agreement rose over time (p<.001). Agreement ranged from 52 percent (baseline) to 78 percent (12 months) for the PO group, 53–75 percent for the MH group, and 43–57 percent for the control group.
Change in Knowledge after Exposure to the Intervention
For both intervention groups, knowledge increased significantly from baseline to 12 months for all but one of the 10 items (not shown). For the control group, knowledge did not change significantly except for a significant decrease in awareness that men are most likely to die from heart attack and stroke. On all except the item indicating that men can have cancer even with a normal PSA test, change in knowledge differed between the intervention groups and the control group (elsewhere, we report that our intervention resulted in significant and sustained increases in prostate cancer knowledge levels over the 12-month study period using multivariate analysis; McCormack et al. 2009).
Preferred and Actual Levels of Involvement in the PSA Decision
Figure 3 depicts men's preferred level of involvement in the PSA screening decision at baseline and 12 months. Both intervention groups changed significantly over time in terms of preferred level of involvement (PO: p = .009; MH: p = .002). At 12 months, a higher percentage of men than at baseline preferred sharing responsibility or making the decision themselves (PO group, 81 percent at baseline and 94 percent at follow-up; MH, 76 and 88 percent; controls, 75 and 80 percent). The control group differences over time were not significant. Figure 3 also shows (right-hand bars) the proportion of each group who engaged in shared or autonomous decision making at 12 months.
Figure 3.
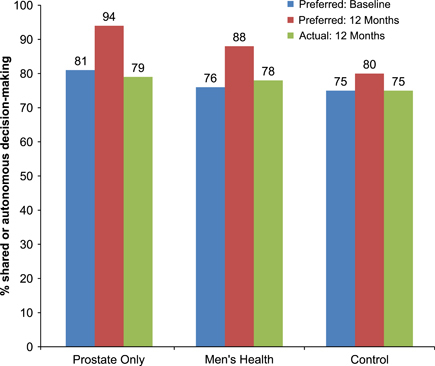
Preferred and Actual Levels of Involvement in PSA Decision by Study Group at Baseline and 12 Months
Note. N = 370 for preferred level of involvement and N = 346 for actual level of involvement. Comparisons of preferred involvement at baseline versus 12 months: Prostate Only (p = .009), Men's Health (p = .002), and control (p = .265).
Results from logistic regression analyses of 12-month preferred and actual levels of involvement appear in Table 2. At 12 months, participants in both intervention groups were more likely than the control group to prefer more active involvement in decision making about PSA screening. Men who received the PO intervention materials were six times more likely than control group participants to prefer active involvement. Other significant correlates of a preference for more active involvement were younger age, lower self-efficacy, and having a PSA discussion with a physician.
At 12 months, those in the MH intervention group were significantly more likely than control group participants to report actual involvement in the PSA decision (Table 2). The PO group tended toward greater involvement than the control group, but this difference was not statistically significant (p = .064). Other predictors of actual involvement in decision making were having discussed PSA screening with a physician, greater decisional uncertainty, and lower self-efficacy.
Concordance between Preferred and Actual Levels of Involvement in PSA Decision
We explored concordance between men's preferred and actual levels of involvement in the PSA decision at 6 and 12 months. At 6 months, we examined concordance only among participants who had had a discussion with their doctor about PSA screening (given the skip pattern in the survey); the 12-month analyses included all respondents who answered the baseline and 12-month surveys. At 6 months, levels of concordance by group were as follows: PO, 73 percent; MH, 71 percent; and control, 59 percent. At 12 months, these values were, respectively, 56, 51, and 51 percent.
A logistic regression analysis showed that, after controlling for other factors, participation in the intervention groups was a significant predictor of concordance at 6 months (not shown). Relative to the control group, men in the PO group were more likely to be concordant (OR [95 percent CI]=7.81 [1.78, 34.23], p = .007); the same was true for those in the MH group (OR [95 percent CI]=4.41 [1.38, 14.07], p = .012). However, at 12 months, participation in the interventions was no longer a significant predictor of concordance, based on analyses using the full sample.
Actual Screening Decision and Satisfaction Levels
At 12 months, all participants were asked if they had a PSA test in the last year (including those who had a discussion with their provider as well as those who did not). The percentages of men receiving a PSA test during the prior 12 months (i.e., the study period) were PO, 71 percent (n = 60); MH, 61 percent (n = 93); and control, 64 percent (n = 76). Using a logistic regression model (n = 355, not shown), we predicted the probability of receiving a PSA test in the prior 12 months based on intervention group, actual involvement in PSA screening decision, and the other factors shown in Table 2. The intervention groups did not differ significantly relative to the control group in their likelihood of getting a PSA test. The following factors were associated with greater odds of having a PSA test: older age (p<.001), excellent/very good or good health status (versus fair/poor) (p = .033 and .013, respectively), greater prostate cancer knowledge (p = .041), having a discussion with doctor (p<.001), and greater decisional conflict (p = .019). Level of actual involvement in the PSA screening decision at 12 months was not a significant predictor, but the results suggest a general trend: those for whom the doctor made the decision or who reported that they engaged in shared decision making were more likely to receive a PSA test than those making the decision themselves (p = .068 and .052, respectively).
Based on descriptive statistics, the intervention and control groups did not differ in terms of satisfaction with their testing decision at 12 months (the only point in time this question was asked). Using a logistic regression analysis (n = 338, not shown), we then found that neither participation in the intervention nor concordance between preferred and actual levels of involvement predicted being very satisfied with level of involvement in the decision.
CONCLUSIONS
Our IDM intervention was associated with greater recognition of PSA testing as a decision to be made, levels of knowledge, and both preferred and actual levels of involvement in decision making, but had no impact on screening decisions. Ensuring that patients and their families are involved in the care process is a key aspect of patient-centered communication, as advocated by the Institute of Medicine (2001). Through this involvement and communication process, personal values and preferences can be considered and taken into account when medical decisions are made. Considering personal preferences and values is particularly important for decision making when physicians and patients face uncertainty about the benefits and risks of medical interventions and decisions are therefore “preference sensitive” (O'Connor, Légaré, and Stacey 2003).
The study has some limitations. First, we had some baseline differences between the intervention and control groups. We also experienced differential attrition from the study; more control group members and certain subgroups were lost to follow-up. A possible implication of losing younger and less educated men over time is a lower level of actual level of involvement in decision making, because these groups tend to be less autonomous in this process. Given that this was a community-based study, the IDM measures were not assessed immediately following the intervention, and the data are self-reported.
IDM will become even more critical than in the past as reviews of clinical evidence and comparative effectiveness initiatives emerge, giving patients more responsibility for decisions related to screening and therapeutic options. For example, the USPSTF now makes the following recommendation regarding breast cancer screening: “that physicians and patients discuss the potential harms and benefits when making the individual, personalized decision about when to start screening” (Petitti and Calogne 2010). However, patients must first recognize that they have decisions to make, whether about cancer screening or other clinical issues. Making this point was a key goal of our intervention.
Promoting IDM about cancer screening is challenging given the broad support for such screening in general in the United States. A national survey found widespread belief that routine cancer screening is “almost always a good idea”; recognition of the risks associated with overtesting and overtreating is limited (Schwartz et al. 2004). Thus, our intervention's message that screening may not necessarily be the right choice in all circumstances is counterintuitive. In our study, formative research participants often did not comprehend the potential downsides of testing (only the potential advantages). This phenomenon is likely a result, in part, of the plethora of nonevidence-based messages to which the public is exposed. Despite the somewhat unbalanced presentation of the message, changing actual behaviors is typically difficult.
Credible health communication messages are needed to help consumers understand that medicine is imperfect and that they have a role to play in making health-related decisions. More attention is needed to help the general public understand prevention and clinical preventive services and the role of evidence in developing recommendations. Our findings have important implications for how to convey information about topics to which the USPSTF has given an “I” Recommendation grade (Insufficient Evidence to Make a Recommendation) and, possibly, “C” Recommendations (Recommendations against Screening) as well. Given the general proscreening climate, IDM messages for “I” and “C” grades need to achieve the appropriate balance of information about the potential advantages and disadvantages of screening. These findings are particularly timely given the late 2009 controversy regarding the release of breast cancer guidelines to the public and the ensuing public confusion.
Finding that men who discussed PSA screening with their physician (relative to those who did not) were less likely to believe that PSA screening is a decision raises intriguing questions about the current state of patient–provider communication. It may be that doctors present PSA screening as standard procedure rather than as a choice, which goes against USPSTF recommendations. Understandably, clinicians face challenges in promoting IDM: time constraints, conflicting guidelines, and uncertainty about the best way to present complex medical information to patients (Guerra et al. 2007). Future research could explore physician attitudes about USPSTF recommendations—particularly those that recommend IDM—and the association between physician attitudes and barriers with the recommendations they make to patients.
In this study, men with greater decisional uncertainty were more likely to report greater levels of involvement in decision making and also greater satisfaction with their level of involvement. One explanation may be that men who are less certain about the decision deliberate more about their values and preferences and talk more to their clinician and, ultimately, are more actively involved in the decision and more satisfied with their level of involvement. This notion is consistent with prior research (O'Connor et al. 2006). Thus, some level of uncertainty may be beneficial to promoting informed decisions, as long as the uncertainty is managed appropriately.
The IDM intervention in this study increased concordance between preferred and actual levels of participation among the subset of participants who discussed PSA screening with their physician at the 6-month follow-up. However, this finding did not hold at 12 months when looking at a larger sample, including both men who did and did not have a discussion. We cannot be sure based on this study, but having a discussion may well influence men's perceptions of their actual level of participation. Additional research in both community and clinical settings—including studies of patient–provider interactions and specifically how decisions are being made—is needed to better understand the communication interaction (Epstein and Street 2007). Such research, which should include multiple methods (e.g., observation, interviews, surveys), may also reveal barriers to concordance between patients' preferred and actual levels of involvement in decision making and shed light on how decision aids can most effectively enhance communication.
When measuring patient-centered communication, some have argued that the quality of the patient–provider interaction and the decision itself deserves greater focus (Sepucha, Fowler, and Mulley 2004); level of involvement may warrant relatively less attention in the future. Addressing “quality” and “satisfaction” questions more fully may be appropriate given that people's preferences have been shown to change over time for various reasons (Slovic 1995). Some people simply prefer to be less involved (Woolf et al. 2005).
Community-based interventions can inform and educate men about issues surrounding the PSA decision and promote IDM before they face the screening decision in the clinical setting. Communicating health-related information in a setting that people trust and when they have time to process the information is a key benefit of community-based health interventions (Driscoll et al. 2008). Reaching individuals who are unlikely to participate in clinical IDM interventions because of barriers related to individual socioeconomic status (Isaacs and Schroeder 2004), lower health literacy (Braveman et al. 2005), and fewer problem-solving skills (Ross and Wu 1995) is an additional benefit. To facilitate adoption, the intervention we developed was low cost and fairly easy to implement. A two-pronged approach that combines clinician training in IDM principles with patient education and empowerment techniques may have even greater impact on IDM adoption than focusing on only one or the other. Health information technology and other tools (e.g., decision aids) offer great promise in facilitating this two-pronged approach.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We would like to thank Brandon Welch for his programming assistance.
Disclosures: None.
Disclaimers: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- American Urological Association. Prostate-Specific Antigen (PSA) Best Practice Policy. Oncology (Williston Park) 2000;14:267–83. [PubMed] [Google Scholar]
- Andriole GL, Crawford ED, Grubb RL, III, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD. Mortality Results from a Randomized Prostate-Cancer Screening Trial. New England Journal of Medicine. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbassat J, Pilpel D, Tidhar M. Patients' Preferences for Participation in Clinical Decision Making: A Review of Published Surveys. Behavioral Medicine. 1998;24:81–8. doi: 10.1080/08964289809596384. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic Status in Health Research: One Size Does Not Fit All. Journal of the American Medical Association. 2005;294(22):2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Chan EC, Vernon SW, Ahn C, Greisinger A. Do Men Know That They Have Had a Prostate-Specific Antigen Test? Accuracy of Self-Reports of Testing at Two Sites. American Journal of Public Health. 2004;94:1336–8. doi: 10.2105/ajph.94.8.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Canadian Journal of Nursing Research. 1997;29:21–43. [PubMed] [Google Scholar]
- Driscoll DL, Harris-Kojetin L. 2002. Final Messages for Prostate-Specific Antigen Screening for Prostate Cancer. Medicare Screening Project, Final report submitted to AHRQ/CMS, Research Triangle Park, NC: RTI International.
- Driscoll DL, Rupert DJ, Golin CE, McCormack LA, Sheridan SL, Welch BM, Poehlman JA. Promoting PSA Informed Decision-Making: Evaluating Two Community-Level Interventions. American Journal of Preventive Medicine. 2008;35(2):87–94. doi: 10.1016/j.amepre.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Street RL., Jr. Patient-Centered Communication in Cancer Care: Promoting Health and Reducing Suffering. NIH Publication No. 07-6225. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- Evans R, Edwards A, Brett J, Bradburn M, Watson E, Austoker J, Elwyn G. Reduction in Uptake of PSA Tests Following Decision Aids: Systematic Review of Current Aids and Their Evaluations. Patient Education and Counseling. 2005;58(1):13–26. doi: 10.1016/j.pec.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Woolf SH. American College of Preventive Medicine Practice Policy: Screening for Prostate Cancer in American Men. American Journal of Preventive Medicine. 1998;15:81–4. doi: 10.1016/s0749-3797(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Flynn KE, Smith MA, Vanness D. A Typology of Preferences for Participation in Healthcare Decision Making. Social Science and Medicine. 2006;63:1158–69. doi: 10.1016/j.socscimed.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CE, Jacobs SE, Holmes JH, Shea JA. Are Physicians Discussing Prostate Cancer Screening with Their Patients and Why or Why Not? A Pilot Study. Journal of General Internal Medicine. 2007;22:901–7. doi: 10.1007/s11606-007-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PK, Coates RJ, Uhler RJ, Breen N. Decision Making in Prostate-Specific Antigen Screening National Health Interview Survey, 2000. American Journal of Preventive Medicine. 2006;30:394–404. doi: 10.1016/j.amepre.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Harris R, Lohr KN. Screening for Prostate Cancer: An Update of the Evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2002;137:917–29. doi: 10.7326/0003-4819-137-11-200212030-00014. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- Isaacs SL, Schroeder SA. Class: The Ignored Determinant of the Nation's Health. New England Journal of Medicine. 2004;351:1137–42. doi: 10.1056/NEJMsb040329. [DOI] [PubMed] [Google Scholar]
- Kiesler DJ, Auerbach SM. Optimal Matches of Patient Preferences for Information, Decision-Making and Interpersonal Behavior: Evidence, Models and Interventions. Patient Education and Counseling. 2006;61:319–41. doi: 10.1016/j.pec.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73:13–22. [Google Scholar]
- McCormack LA, Bann C, Williams-Piehota PA, Driscoll D, Soloe C, Poehlman J, Kuo TM, Lohr KN, Sheridan SL, Golin CE, Harris R, Cykert S. Communication Message Strategies for Increasing Knowledge about Prostate Cancer Screening. Journal of Cancer Education. 2009;24(3):238–43. doi: 10.1080/08858190902935498. [DOI] [PubMed] [Google Scholar]
- Mullen PD, Allen JD, Glanz K, Fernandez ME, Bowen DJ, Pruitt SL, Glenn BA, Pignone M. Measures Used in Studies of Informed Decision Making about Cancer Screening: A Systematic Review. Annals of Behavioral Medicine. 2006;32(3):188–201. doi: 10.1207/s15324796abm3203_4. [DOI] [PubMed] [Google Scholar]
- O'Connor AM. Validation of a Decisional Conflict Scale. Medical Decision Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- O'Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, Entwistle VA, Fiset V, Homes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner D. Decision Aids for People Facing Health Treatment or Screening Decisions (Review) Cochrane Collaboration. 2006;4 doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- O'Connor AM, Légaré F, Stacey D. Risk Communication in Practice: The Contribution of Decision Aids. British Medical Journal. 2003;327:736–40. doi: 10.1136/bmj.327.7417.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti D, Calogne N. Comments and Response on the USPSTF Recommendation on Screening for Breast Cancer. Annals of Internal Medicine. 2010;152(8):543–4. doi: 10.7326/0003-4819-152-8-201004200-00202. [accessed September 21, 2010]. Available at http://www.annals.org/content/152/8/543.full. [DOI] [PubMed] [Google Scholar]
- Radosevich DM, Partin MR, Nugent S, Nelson D, Flood AB, Holtzman J, Dillon N, Haas M, Wilt TJ. Measuring Patient Knowledge of the Risks and Benefits of Prostate Cancer Screening. Patient Education and Counseling. 2004;54(2):143–52. doi: 10.1016/S0738-3991(03)00207-6. [DOI] [PubMed] [Google Scholar]
- Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed Decision Making: What Is Its Role in Cancer Screening? Cancer. 2004;101(5, suppl):1214–28. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- Robinson A, Thompson R. Variability in Patient Preferences for Participating in Medical Decision Making: Implication for the Use of Decision Support Tools. Quality in Health Care. 2001;19(suppl 1):134–8. doi: 10.1136/qhc.0100034... [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CE, Wu C. The Links between Education and Health. American Sociological Review. 1995;60:719–45. [Google Scholar]
- Ryan J, Sysko J. The Contingency of Patient Preferences for Involvement in Health Decision Making. Health Care Management Revision. 2007;32:30–6. doi: 10.1097/00004010-200701000-00005. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman and Hall; 1997. [Google Scholar]
- Schwartz LM, Woloshin S, Fowler FJ, Jr., Welch HG. Enthusiasm for Cancer Screening in the United States. Journal of the American Medical Association. 2004;291:71–8. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- Sepucha K, Fowler F, Mulley A. Policy Support for Patient Centered Care: The Need for Measurable Improvements in Decision Quality. Health Affairs. 2004;23:54–62. doi: 10.1377/hlthaff.var.54. [DOI] [PubMed] [Google Scholar]
- Sirovich BE, Schwartz LM, Woloshin S. Screening Men for Prostate and Colorectal Cancer in the United States: Does Practice Reflect the Evidence? Journal of the American Medical Association. 2007;289:1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- Slovic P. The Construction of Preferences. American Psychologist. 1995;50:364–71. [Google Scholar]
- Soloe C, McCormack L, Treiman K, Driscoll D, Harris S. 2009. “Informed Decision Making about Prostate-Specific Antigen (PSA) Testing: Findings and Implications from Formative Testing of a Multimodal Intervention.” RTI Press publication No. RR-0006-0902. Research Triangle Park, NC: RTI International. [accessed on January 2, 2009]. Available at http://www.rti.org/rtipress.
- Thompson AG. The Meaning of Patient Involvement and Participation in Health Care Consultations: A Taxonomy. Social Science and Medicine. 2007;64:1297–310. doi: 10.1016/j.socscimed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force (USPSTF) Screening for Prostate Cancer: Recommendation and Rationale. Annals of Internal Medicine. 2002;137:915–6. doi: 10.7326/0003-4819-137-11-200212030-00013. [DOI] [PubMed] [Google Scholar]
- Volk RJ, Cass AR. The Accuracy of Primary Care Patients' Self-Reports of Prostate-Specific Antigen Testing. American Journal of Preventive Medicine. 2002;22:56–8. doi: 10.1016/s0749-3797(01)00397-x. [DOI] [PubMed] [Google Scholar]
- Volk RJ, Hawley ST, Kneuper S, Holden EW, Stroud LA, Cooper CP, Berkowitz JM, Scholl LE, Saraykar SS, Pavlik VN. Trials of Decision Aids for Prostate Cancer Screening: A Systematic Review. American Journal of Preventive Medicine. 2007;33(5):428–34. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, Brooks DD, Dash C, Guessous I, Andrews K, DeSantis C, Smith RA the American Cancer Society Prostate Cancer Advisory Committee. American Cancer Society Guideline for the Early Detection of Prostate Cancer: Update 2010. A Cancer Journal for Clinicians. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- Woolf SH, Krist AH, Johnson RE, Stenborg PS. Unwanted Control: How Patients in the Primary Care Setting Decide about Screening for Prostate Cancer. Patient Education and Counseling. 2005;56(1):116–24. doi: 10.1016/j.pec.2003.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


