Abstract
The gene for an essential protein subunit of nuclear RNase P from Saccharomyces cerevisiae has been cloned. The gene for this protein, RPP1, was identified by virtue of its homology with a human scleroderma autoimmune antigen, Rpp30, which copurifies with human RNase P. Epitope-tagged Rpp1 can be found in association with both RNase P RNA and a related endoribonuclease, RNase MRP RNA, in immunoprecipitates from crude extracts of cells. Depletion of Rpp1 in vivo leads to the accumulation of precursor tRNAs with unprocessed 5′ and 3′ termini and reveals rRNA processing defects that have not been described previously for proteins associated with RNase P or RNase MRP. Immunoprecipitated complexes cleave both yeast precursor tRNAs and precursor rRNAs.
Keywords: Rpp1, essential protein subunit, nuclear RNase P, S. cerevisiae, precursor tRNA, precursor rRNA
Ribonuclease P (RNase P) is a ubiquitous endoribonuclease that consists of protein and RNA subunits. It cleaves 5′-terminal leader sequences of precursor tRNAs (Darr et al. 1992; Altman et al. 1993). Escherichia coli RNase P is also known to process precursors of other small, metabiologically stable RNAs in vivo, such as 4.5S RNA (Bothwell et al. 1976), 10Sa RNA (Komine et al. 1994), the polycistronic mRNA from the histidine operon (Alifano et al. 1994), and some small RNAs encoded by bacteriophage (Bothwell et al. 1974; Hartmann et al. 1995). In eubacteria, the RNA component alone of RNase P is catalytic in vitro (Guerrier-Takada et al. 1983). The eubacterial protein subunit is a basic protein of ∼14 kD and serves as an essential cofactor in vivo by enhancing the catalytic efficiency and substrate range of the holoenzyme (Liu and Altman 1994; Gopalan et al. 1997). In contrast, the RNA components of archaeal and eukaryotic RNase P are not catalytically active in vitro in the absence of their respective protein subunits, despite their structural homology to the eubacterial RNAs (Altman et al. 1995; Haas et al. 1996).
Although the RNA subunit of nuclear RNase P has been characterized from a variety of eukaryotic organisms (Altman et al. 1993; Tranguch and Engelke 1993; Chamberlain et al. 1996a; Eder et al. 1996), information regarding the protein subunits of eukaryotic RNase P is limited. RNAse P isolated from human cells copurifies with an RNA (H1; 340), and at least six proteins—Rpp14, Rpp20, Rpp25, Rpp30, Rpp38, and Rpp40 (Eder et al. 1997). Genetic approaches in Saccharomyces cerevisiae have identified three essential proteins, Pop1, Pop3, and Pop4, which associate with RNase P RNA (RPR1); these proteins also associate with the RNA (NME1) of a related endoribonuclease, RNase mitochondrial RNA processing (MRP) (Schmitt and Clayton 1992; Lygerou et al. 1994; Dichtl and Tollervey 1997; Chu et al. 1997). Analysis of temperature-sensitive alleles of these proteins or depletion of these proteins in yeast cells has shown that all three have a role in tRNA processing as well as rRNA processing. It has been suggested that tRNA and rRNA processing are coordinated (Pace and Burgin 1990; Clayton 1994; Morrissey and Tollervey 1995; Lee et al. 1996).
In eukaryotes, coordination of tRNA and rRNA processing may be mediated by the activity of two related enzymes, RNase P and RNase MRP (Morrissey and Tollervey 1995; Chamberlain et al. 1996b). RNase P is essential for biosynthesis of tRNAs (Lee et al. 1991) and also appears to have a role in rRNA processing in yeast (Chamberlain et al. 1996b). RNase MRP is related to RNase P by structural similarities found in its RNA component (Forster and Altman 1990; Schmitt et al. 1993). It has been suggested that RNase P is an ancestor of RNase MRP (Morrissey and Tollervey 1995). RNase MRP was described originally as an endonuclease that cleaves RNA primers for mitochondrial DNA replication (Chang and Clayton 1987; Stohl and Clayton 1992). Recently, its role in nuclear processing of precursor rRNA (prRNA) has been established (Lygerou et al. 1996a). RNase MRP does not cleave precursor tRNAS (ptRNAs) in vitro, and depletion of NME1 RNA does not affect tRNA processing in vivo (Schmitt and Clayton 1993; Lygerou et al. 1996a). However, when proteins associated with RNase MRP are depleted or inactivated by a mutation in yeast, cleavage of ptRNAs is blocked (Lygerou et al. 1994; Chu et al. 1997; Dichtl and Tollervey 1997).
To learn more about the functions of nuclear RNase P in vivo, we report here the cloning and functional characterization of an essential protein (Rpp1, 32.2 kD) component of S. cerevisiae RNase P. Rpp1 is homologous to the human scleroderma autoimmune antigen, Rpp30, which was identified recently as a protein that copurifies with human RNase P and that is recognized by sera from patients with autoimmune disease that is referred to as Th/To antisera (Eder et al. 1997). To identify novel functions of RNase P in yeast, a strain of S. cerevisiae that conditionally expresses Rpp1 protein was constructed. Using this strain and a stain that contains an epitope-tagged RPP1 gene, we demonstrate a role for Rpp1 in processing tRNA and ribosomal RNA precursors. Depletion of Rpp1 protein revealed global defects in rRNA processing. Depletion of inactivation of proteins associated with RNase P or RNase MRP affect only a subset of the same rRNA processing events (Lygerou et al. 1994; Chu et al. 1997; Dichtl and Tollervey 1997). Based on the observed defects in rRNA processing, we suggest a possible functional interaction of RNase P with RNase MRP, and other small nucleolar ribonucleoprotein (RNP) complexes (snoRNP; for review, see Filipowicz and Kiss 1993; Fournier and Maxwell 1993; Maxwell and Fournier 1995), which is required for processing of prRNA.
Results
An essential yeast gene encodes a homolog of the human scleroderma autoimmune antigen, Rpp30
We used a combination of biochemical and genetic studies in human and S. cerevisiae cells to characterize a protein subunit of eukaryotic RNase P. We employed computational sequence search or yeast genes that have amino acid sequence similarity to biochemically identified human RNase P protein subunits (Eder et al. 1997). To determine which of the proteins associated with human RNase P might be essential components required for catalytic function (Eder et al. 1997), we searched for homologs of Rpp14, Rpp20, Rpp25, Rpp30, Rpp38, and Rpp40 in the genome of S. cerevisiae (Goffeau et al. 1996) by performing a BLAST search (blastp and tblastn algorithms; Altschul et al. 1990) of the S. cerevisiae genome database (Cherry et al. 1996). The human scleroderma autoimmune antigen, Rpp30, has the highest amino acid sequence similarity to a predicted sequence. A previously uncharacterized open reading frame (ORF), YHR062c, on the right arm of chromosome VIII has the potential to encode a protein of 32.2 kD and shares 23% amino acid sequence identity with human Rpp 30 (Fig. 1A). This yeast gene is now named RPP1 for RNase PProtein 1.
Figure 1.
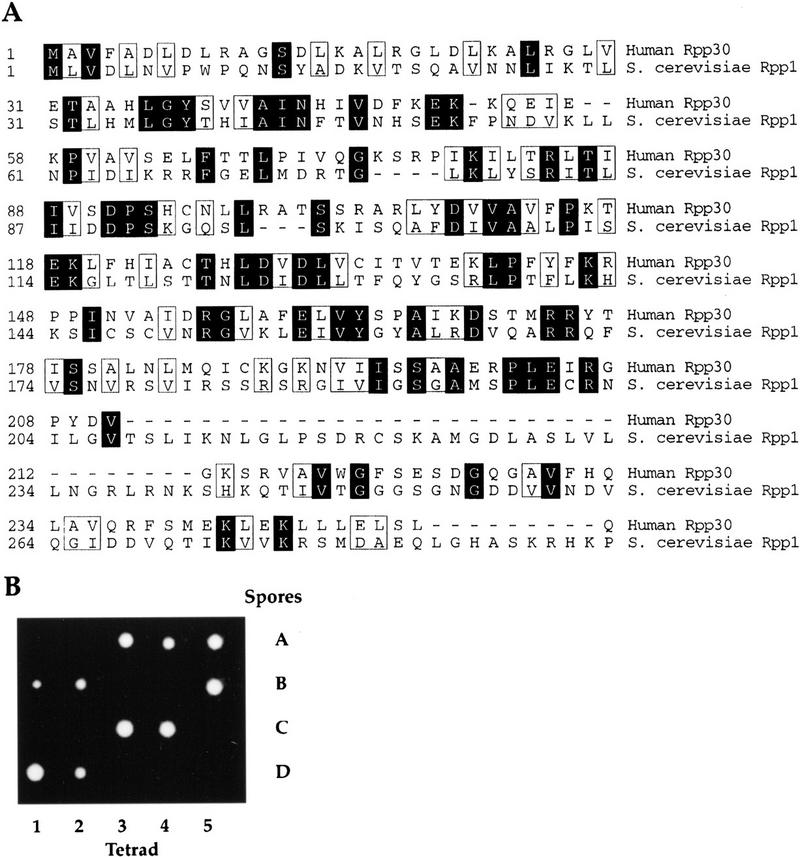
RPP1, an essential yeast gene encodes a 32.2-kD protein ortholog of the human scleroderma autoimmune antigen, Rpp30. (A) Predicted amino acid sequence of S. cerevisiae Rpp1. The protein is encoded by ORF YHR062c on chromosome VIII and its alignment with the human scleroderma autoimune antigen Rpp30 is shown. The amino acid sequences are numbered from the first methionine residue of each protein. Identical amino acids are shaded and similar amino acids are boxed. (B) The heterozygous diploid strain VS161, RPP1/rpp1::LEU2, was sporulated and dissections were performed on 20 tetrads. The four spores (A–D) derived from each of five tetrads are shown in vertical rows.
To address whether the putative protein encoded by RPP1 is an essential gene, we disrupted this gene by replacing it with the LEU2 gene (see Materials and Methods). The heterozygous RPP1/rpp1::LEU2 strain (VS161) was sporulated and subsequent tetrad analysis showed a 2:2 segregation for cell viability (Fig. 1B). All viable spores were Leu−, indicating that they had the wild-type RPP1 allele. Therefore, RPP1 is an essential gene in S. cerevisiae.
Construction of an epitope-tagged allele of RPP1
Epitope-tagged proteins are useful in the study of subunit function and interactions in large holoenzyme complexes. To determine whether or not Rpp1 associates with RPR1 RNA and RNase P activity, an epitope-tagged RPP1 strain of S. cerevisiae (VS162) was constructed (Table 1). A DNA fragment that encodes three copies of a c-myc epitope (3 × myc; TerBush and Novick 1996) was fused in-frame 3′ to the initiator ATG codon of RPP1 in a low-copy-number plasmid (pRS316), pRS316::3 × myc–RPP1. The resulting strain grew at identical rates to the wild-type cells suggesting that the 3 × myc–RPP1 allele is fully functional (Fig. 2A, lanes 2,4). Immunoblots of protein extracts from 3 × myc–RPP1 cells in anti-myc antibody (9E10) detected a polypeptide of 36 kD—the size is consistent with that predicted for the 3 × myc–Rpp1 fusion protein (Fig. 2B, lanes 2,4). Wild-type haploids (VS162A and VS162C) lacking the c-myc tag do not contain the 36-kD protein. These results show that the myc epitope-tagged Rpp1 protein migrates as a 36-kD polypeptide and is fully functional.
Table 1.
Strains of S. cerevisiae used in this study
| Strain
|
Genotype
|
|---|---|
| JN161 | MATa/MATα ade2-1/ade2-1 leu2-3,112/leu2-3,112 ura3-1/ura3-1 his4-260/his4-260 thr1-4/thr1-4 lys2▵NheI/lys2▵NheI |
| VS161 | MATa/MATα ade2-1/ade2-1 leu2-3,112/leu2-3,112 ura3-1/ura3-1 his4-260/his4-260 thr1-4/thr1-4 lys2▵NheI/lys2▵NheI RPP1/rpp1::LEU2/RPP1 |
| VS162 | MATa/MATα ade2-1/ade2-1 leu2-3,112/leu2-3,112 ura3-1/ura3-1 his4-260/his4-260 thr1-4/thr1-4 lys2▵NheI/lys2▵NheI RPP1/rpp1::LEU2/RPP1 + pRS316–3xmyc::RPP1 |
| VS162A | MATa ade2-1 leu2-3,112 ura3-1 his4-260 thr1-4 lys2▵NheI RPP1 + pRS316–3xmyc::RPP1 |
| VS162B | MATa ade2-1 leu2-3,112 ura3-1–his4-260–thr1-4 lys2▵NheI rpp1::LEU2 + pRS316–3xmyc::RPP1 |
| VS162C | MATα ade2-1 leu2-3,112 ura3-1 his4-260 thr1-4 lys2▵NheI RPP1 + pRS316–3xmyc::RPP1 |
| VS162D | MATα ade2-1 leu2-3,112 ura3-1–his4-260–thr1-4 lys2▵NheI rpp1::LEU2 + pRS316–3xmyc::RPP1 |
| VS162A‘ | MATa ade2-1 leu2-3,112 ura3-1 his4-260 thr1-4 lys2▵NheI RPP1 |
| VS162C‘ | MATα ade2-1 leu2-3,112 ura3-1 his4-260 thr1-4 lys2▵NheI RPP1 |
| NY1060 | MATa/MATα GAL1/GAL1 leu2-3,112/leu2-3,112 ura3-1/ura3-1 |
| VS163 | MATa/MATα GAL1/GAL1 leu2-3,112/leu2-3,112 ura3-1/ura3-1 RPP1/rpp1::LEU2 |
| VS164 | MATα GAL1 leu2-3,112 ura3-1 + pYCpGAL::rpp1 (URA3) |
| VS165 | MATα GAL1 leu2-3,112 ura3-1 + pYCpGAL (URA3) |
Figure 2.
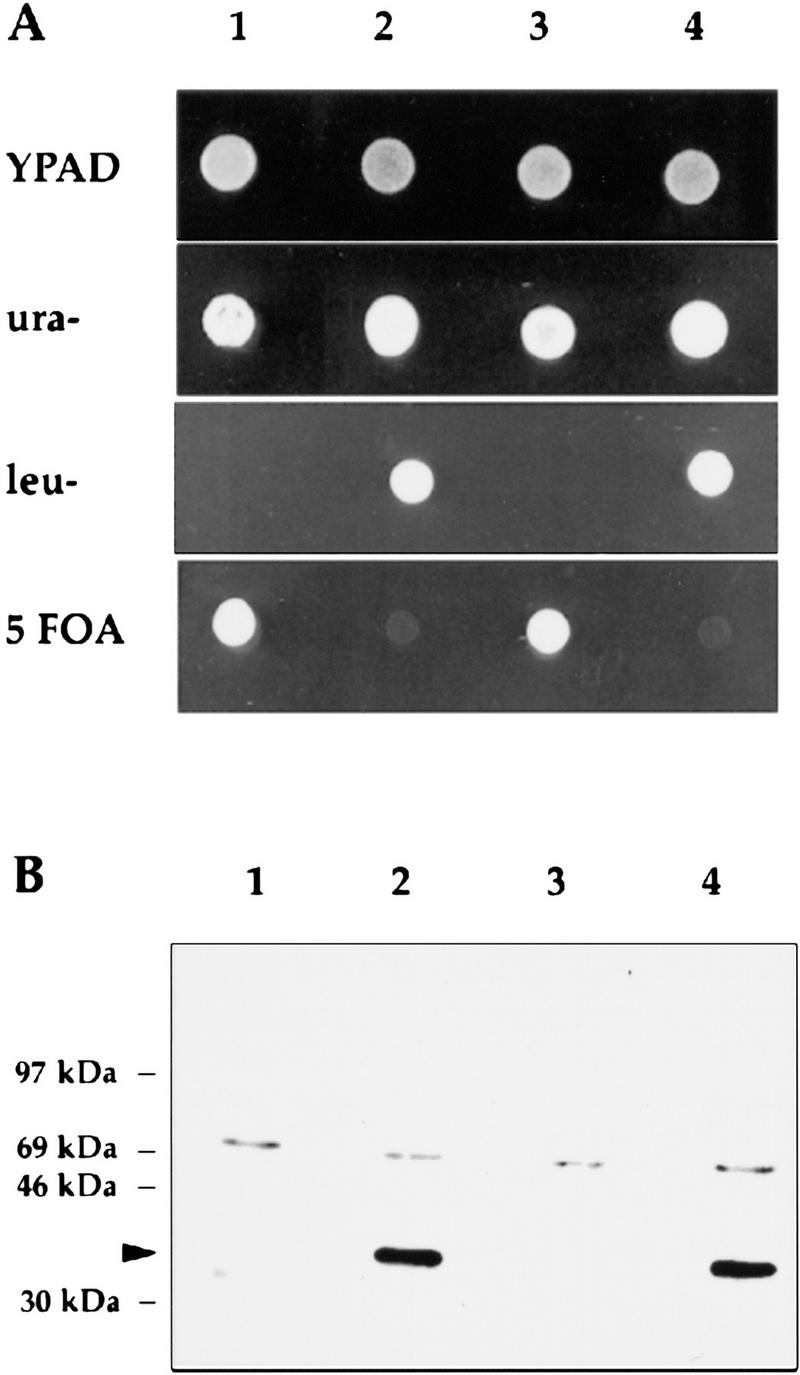
3 × myc–Rpp1 is functional and recognized by 9E10 antibody. (A) The four spores (VS162 A–D; lanes 1–4, respectively) derived from the diploid strain VS162, were dissected on rich medium plates (YPAD), and then replica-plated onto plates that contained synthetic complete medium that lacked either leucine (leu) or uracil (ura), or that contained 5-FOA. (B) Immunoblot of protein extracts prepared from the four spores VS162A′, VS162B, VS162C′, and VS162D, derived from the diploid strain VS162. All four spores are isogenic except the VS162A′ and VS162C′ spores do not have the pRS316–3 × myc–Rpp1 plasmid. (Lanes 1–4) VS162A′, VS162B, VS162C′, and VS162D, respectively. The arrowhead points to the 36-kD 3 × myc–Rpp1 fusion protein. The upper band is a nonspecifically reacting protein.
RNase P and RNase MRP RNAs coprecipitate with the 3 × myc–Rpp1 fusion protein
Whether or not Rpp1 is associated with RNase P RNA was determined by immunoprecipitation experiments (Fig. 3). Extracts from 3 × myc–RPP1 and RPP1 strains were incubated with anti-myc monoclonal antibody (9E10) and RNA was extracted from the immunoprecipitates (see Materials and Methods). The RNA was 3′ end-labeled with [32P]pCp and analyzed by denaturing gel electrophoresis (Fig. 3A,B). Immunoprecipitatd RNA was also analyzed by Northern hybridization to confirm the identity of the labeled RNAs (Fig. 3C,D). Mature RNase P RNA is the major RNA species found in the 3 × myc–Rpp1 precipitate from 3 × myc–RPP1 cells but not from the control cell lysates. Two putative precursors of RNase P RNA (Lee et al. 1991) and RNase MRP RNA are also found in immunoprecipitates from 3 × myc–RPP1 but not in control immunoprecipitates. Approximately equal levels of RNase P and RNase MRP RNAs (RPR1 and NME1, respectively) were found in end-labeled RNA derived from immunoprecipitates that were washed with buffer that contained 150 mm KCl RNAs (Fig. 3A). However, RNase P RNA is the major RNA species detected by 3′ end-labeling of RNA in 3 × myc–Rpp1 immunoprecipitates that were washed with buffer that contained 60 mm KCl (Fig. 3B). Therefore, it is possible to achieve a significant separation of the two enzymes, both physically and functionally (Lygerou et al. 1996a; and see below).
Figure 3.
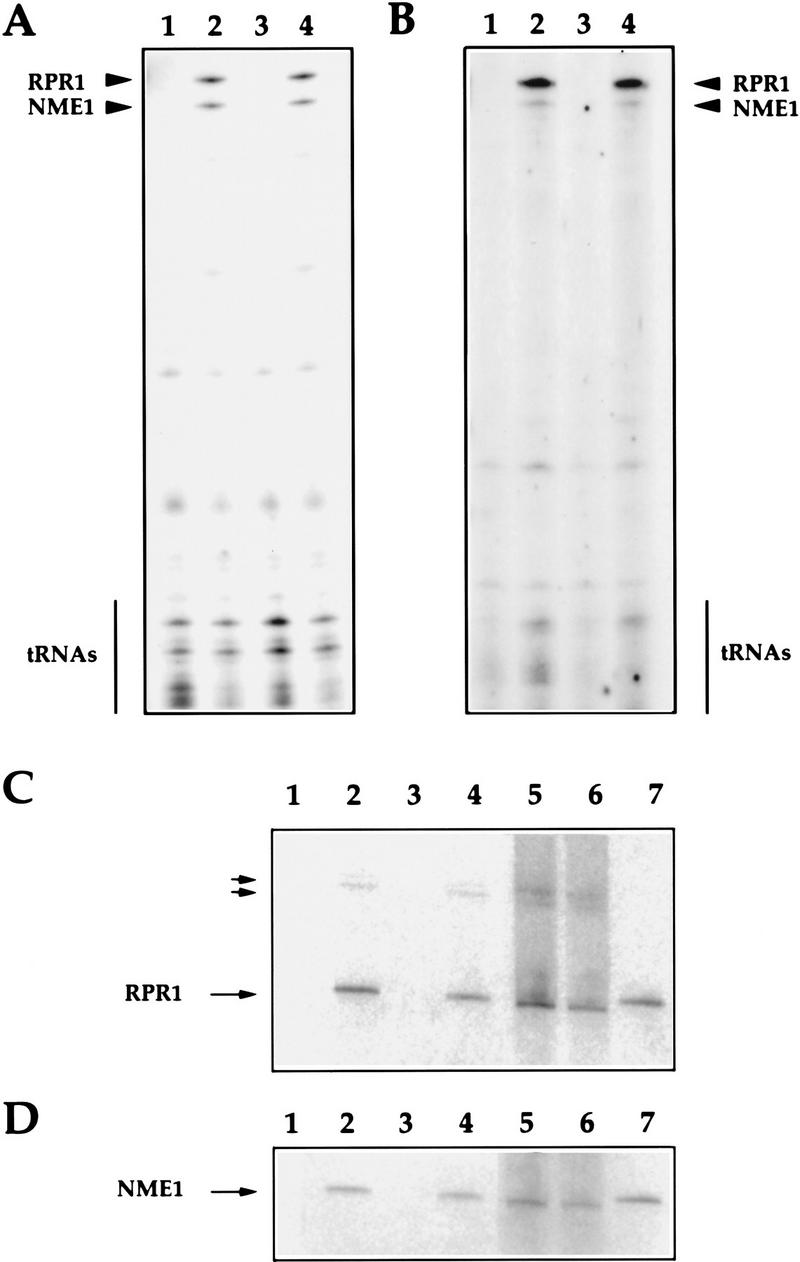
The RNA subunits of RNase P (RPR1) and RNase MRP (NME1) coprecipitate with 3 × myc–Rpp1. (A) Immunoprecipitated RNAs extracted from the 9E10 Ab–IgG–agarose beads that were incubated with protein extracts from the four spores (VS162A′, VS162B, VS162C′, and VS162D; lanes 1–4, respectively). RNA was extracted from the immunoprecipitated beads that were washed with 150 mm KCl (see Materials and Methods). The RNA was 3′ end-labeled with [5′-32P]pCp, and fractionated on a 8% polyacrylamide/7 m urea gel. (B) Immunoprecipitated RNAs derived from the same immunoprecipitated beads as in A (lanes 1–4, respectively) except that the immunoprecipitated beads were washed with 600 mm KCl prior to 3′ end-labeling of the RNA. (C) Immunoprecipitated RNAs derived from the same immunoprecipitated beads as in A were transferred to a positively charged nylon membrane (Boehringer Mannheim) by electroblotting and hybridized with a uniformly labeled DNA probe complementary to the RPR1 gene (see Materials and Methods). (Lanes 1–4) Immunoprecipitated RNA from spores VS162A′, VS162B, VS162C′, and VS162D, respectively; (lane 5) RNA from supernatant of the immunoprecipitated extract derived from spore VS162A′ after centrifugation of beads; (lane 6) RNA from supernatant of the immunoprecipitated extract derived from spore VS162B after centrifugation of beads; (lane 7) RNase P (RPR1) and RNase MRP (NME1) RNAs (0.001 pmoles each) transcribed in vitro. Two small arrows point to extended species, which may be precursors to mature RNase P RNA, RPR1 (Lee et al. 1991). The larger arrows indicate RNase P (RPR1) and RNase MRP (NME1) RNAs. (D) Same as in C, except the membrane was hybridized with a uniformly labeled DNA probe complementary to the NME1 gene (Schmitt and Clayton 1992).
The association of Rpp1 with RNaseP activity was also demonstrated by immunoprecipitation. Immunoprecipiated (and resuspended) 3 × myc–Rpp1 pellets accurately cleaved radiolabeled ptRNASer (Fig. 4) and ptRNATyr (data not shown) in vitro. We conclude that Rpp1 protein is a component of, or is tightly associated with, catalytically active RNase P holoenzyme. Indirect supporting evidence for this conclusion comes from experiments in which the human homolog of Rpp1, Rpp30, was shown not to be separable from the active holoenzyme after extensive biochemical purification (Eder et al. 1997).
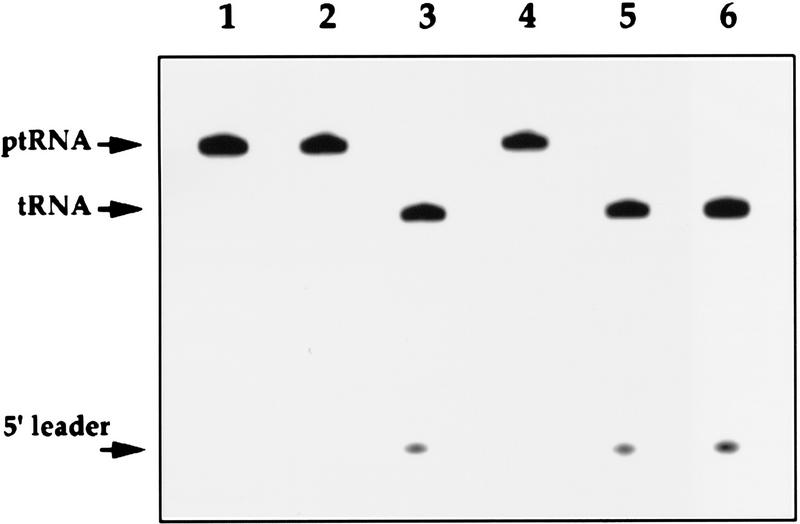
Figure 4.
RNase P activity coprecipitates with 3 × myc–Rpp1. (A) IgG–agarose pellets, to which is bound immunoprecipitated RNase P RNA, that were derived from immunoprecipitates from spores VS162A′, VS162B, VS162C′, and VS162D (as in Fig. 3B), were incubated with a uniformly labeled precursor tRNASer for 30 min at 37°C, and then fractionated on a 8% polyacrylamide/7 m urea gel (see Materials and Methods). (Lane 1) Precursor tRNASer; (lanes 2–5) immunoprecipitated RNase P from the four spores VS162A′, VS162B, VS162C′, and V6S162D, respectively; (lane 6) glycerol gradient-purified human RNase P, fraction F29 (Eder et al. 1997). Arrows indicate ptRNA, accurately processed mature tRNA, and 5′ leader sequence.
Construction of a conditional lethal allele of RPP1
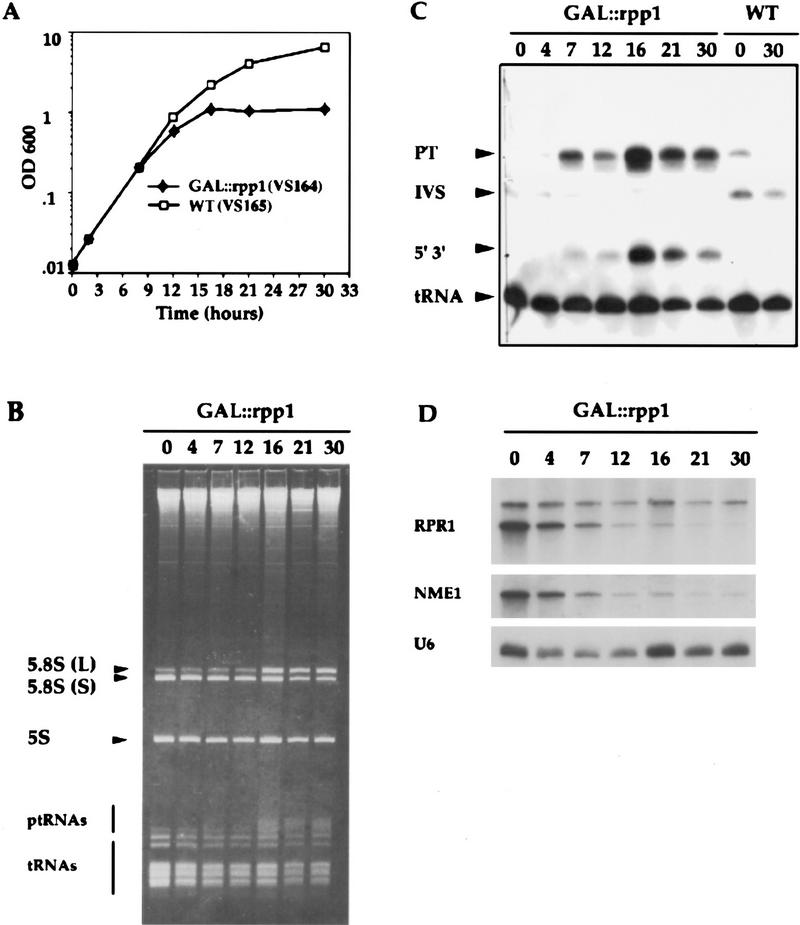
RPP1 was placed under the control of the GAL10 promoter, which allows expression of the gene in culture medium that contains galactose but suppresses expression in culture medium that contains glucose. The resulting strain, rpp1::LEU2–pGAL::rpp1 (VS164), was compared in phenotype to control strain RPP1–pGAL (VS165). In liquid culture that contained galactose, there was no difference in growth rate between GAL::rpp1 strain and the wild-type RPP1 strain. After the cultures were transferred to medium that contained glucose, cell growth continued initially with a doubling time of 2 hr. After 12–16 hr, the growth rate of the GAL::rpp1 strain declined rapidly and there was little growth after 16 hr (Fig. 5A).
Figure 5.
RNase P is required for processing of ptRNA and 5.8S rRNA in vivo. (A) Growth of the strain VS164 (▪), and the wild-type, isogenic strain VS165 (□), after transfer from galactose-containing to glucose-containing medium at T = 0. Cell density was measured at the times indicated and the cultures were diluted with glucose-containing medium at each time point to prevent nutritional depravation and to maintain exponetial growth. (B) RNA was extracted from VS164 following growth in glucose-containing medium at the indicated times, and was fractionated in an 8% polyacrylamide/7 m urea gel, and stained with ethidium bromide. The positions of 5.8S (L) RNA, 5.8S (S) RNA, 5S RNA, ptRNA, and mature tRNA are indicated. (C) Total RNA extracted from VS164 and VS165 was transferred to a positively charged nylon membrane (Boehringer Mannheim) by electroblotting and hybridized with a γ-32P-labeled oligonucleotide complementary to mature tRNALeu (see Materials and Methods). The position of the three processing intermediates of the ptRNA are indicated. PT is the primary transcript, which has extra sequences at both of its termini and contains an intron; IVS is the ptRNA that contains the intron but has been processed at both the 5′ and 3′ ends; 5′ 3′ is the spliced ptRNA that is unprocessed at both termini. (D) Steady-state levels of RNase P RNA (RPR1) and RNase MRP RNA (NME1) in VS164 after transfer from galactose-containing to glucose-containing medium. The upper band in the RPR1 panel is the putative precursor RPR1 RNA that has extra sequences at both termini (Lee et al. 1991). U6 snRNA levels were detected with oligo U6 (Table 2), as an internal control for RNA levels (Brow and Guthrie 1990).
Rpp1 is required for processing of ptRNA
To determine the effects of Rpp1 depletion on the biosynthesis of metabolically stable RNAs, total RNA was isolated from the GAL::rpp1 strain (VS164) and the RPP1 strain (VS165) at various times during growth in glucose-containing medium. RNA samples from VS164 were compared with those from VS165 on a denaturing gel stained with ethidium bromide and by Northern hybridization.
The effect of Rpp1 depletion on the accumulation of precursor tRNAs is shown in Figure 5B. Several RNAs began to accumulate at 7 hr after transfer to glucose-containing medium. At this time, the culture growth began to be slowed significantly. The abundance of these RNAs increased with time and their sizes were appropriate for ptRNAs. The abundance of mature tRNAs decreased accordingly. Analysis by Northern hybridization using probes complementary to tRNALeu3 (Fig. 5C), the intervening sequence (IVS) (data not shown) and 5′ leader sequence of the pre-tRNALeu3 (data not shown), showed an accumulation of tRNA that was unprocessed at both termini. After growth of the GAL::rpp1 strain in glucose for 12 hr, 5′ and 3′ unprocessed ptRNALeu3 accumulated to approximately the same level as that of mature tRNA. The level of pre-tRNALeu3 that was unspliced but processed at the 5′ end was reduced correspondingly. These results showed that the sequentially ordered removal of the 5′ leader sequence, the 3′ trailing sequence, and finally the intron of ptRNALeu3, is impaired in Rpp1-depleted cells. As this phenotype is observed in the RNase P RNA mutants, rpr1 and rpr1 (T315ΔT307) (Lee et al. 1991; Chamberlain et al. 1996b), we conclude that Rpp1 is an essential protein subunit of the catalytically active RNase P complex in vivo.
Rpp1 is required for accumulation of RNase P and RNase MRP RNAs
We investigated further the effect of Rpp1 depletion on the steady-state levels of RNase P and RNase MRP RNAs to ascertain if cells lacking Rpp1 shared phenotypic traits with previously described conditional lethal mutants of Pop1, Pop3 and Pop4, proteins that associate with both RNPs. Depletion of Rpp1 results in a decrease of the steady-state levels of RNaseP and RNase MRP RNAs (Fig. 5D). However, the RPR1 RNA precursor does not appear to decrease to the same extent as mature RPR1 RNA, even after 30 hr of Rpp1 depletion. As with Pop4 depletion (Chu et al. 1997), mature RPR1 RNA is not detectable after 21 hr of Rpp1 depletion. In contrast, depletion of Pop3 does not affect steady-state levels of these RNAs, whereas in pop1-1 steady-state levels of both mature and precursor RPR1 RNAs and NME1 RNA are reduced (Lygerou et al. 1996a; Chu et al. 1997; Dichtl and Tollervey 1997). These data suggest that the amount of Rpp1 is correlated with the maturation and stability of RPR1 and stability of NME1 in vivo. Both RNAs may be found within a large RNP complex, or alternatively, Rpp1 may be shared between the two RNP particles in vivo.
Rpp1 is required for processing of the 35S prRNA
In S. cerevisiae and other eukaryotes, rRNA is transcribed as a 35S precursor RNA that contains within it the sequences for three of the four rRNA molecules (18S, 5.8S, and 25–28S). Subsequent processing and nucleotide modifications involving endonucleolytic and exonucleolytic cleavages and methylation generate mature rRNA (for review, see Eichler and Craig 1994; Venema and Tollervey 1995; Tollervey 1996). We examined the fidelity of rRNA processing in Rpp1-depleted cells to determine if defects in this pathway were similar to those described previously for mutants that affect RNase P and RNase MRP (Shuai and Warner 1991; Lindahl et al. 1992; Schmitt and Clayton 1993; Chamberlain et al. 1996b; Lygerou et al. 1996a; Chu et al. 1997; Dichtl and Tollervey 1997). rRNAs were analyzed by gels stained with ethidium bromide and by Northern analysis with oligonucleotide probes (Table 2) to detect various prRNA species (see Figs. 5B and 6).
Table 2.
Oligonucleotides used in this study
| Oligo 1: 5‘-CAGCAGAGAGACCCGA-3‘ |
| Oligo 2: 5‘-ACTATCTTAAAAGAAGAAGC-3‘ |
| Oligo 3: 5‘-GAATTACCACGGTTATACC-3‘ |
| Oligo 4: 5‘-GCACAGAAATCTCTCACC-3‘ |
| Oligo 5: 5‘-ATGAAAACTCCACAGTG-3‘ |
| Oligo 6: 5‘-CCAGTTACGAAAATTCTTG-3‘ |
| Oligo 7: 5‘-CGCATTTCGCTGCGTTCTTCATCG-3‘ |
| Oligo 8: 5‘-ACAGAATGTTTGAGAAGGAAATG-3‘ |
| Oligo U6: 5‘-TCATCCTTATGCAGGG-3‘ |
| Oligo mtRNA: 5‘-GCATCTTACGATACCTG-3‘ |
| Oligo ltRNA: 5‘-CCAAACAACCACTTATTTGTTGA-3‘ |
| Oligo itRNA: 5‘-CACAGTTAACTGCGGTC-3‘ |
| Oligo T7MRP: 5‘-GGGAATTCGAAATTAATACGACTCACTATAGAATCCATGACCAAAGAATCGTCA-3‘ |
| Oligo 3‘ MRP: 5‘-TCCCCCGGGTGAATCCATGGACCAAGA-3‘ |
| Oligo T7ITS14: 5‘-GGGAATTCGAAATTAATACGACTCACTATAGACACTGTGGAGTTTTCATATC-3‘ |
| Oligo 3‘ ITS14: 5‘-GGGGATCCTTAAAATTTCCAGTTACGAAAATTC-3‘ |
| Oligo T7ITS16: 5‘-GGGAATTCGAAATTAATACGACTCACTATAGGCCAAACGGTGAGAGATTTCTG-3‘ |
| Oligo 3‘ ITS16: 5‘-GGGGATCCACTTTAAGAACATTGTTCGCCT-3‘ |
| Oligo MYC5: 5‘-GCGGAATGCTGGAACAGAAACTTATT-3‘ |
| Oligo T7ITS16: 5‘-GGGAATTCGAAATTAATACGACTCACTATAGGCCAAACGGTGAGAGATTTCTG-3‘ |
| Oligo 3‘ ITS16: 5‘-GGGGATCCACTTTAAGAACATTGTTCGCCT-3‘ |
| Oligo MYC3: 5‘-ACCAGCATTCCCAAATCTTCTTCAGA-3‘ |
| Oligo 5 GAL30: 5‘-GGGGATCCGCATTATAGAACCGAGAATGC-3‘ |
| Oligo 3 GAL30: 5‘-GGTCTAGAGATCCCCAAATTTTTTTGTCT-3‘ |
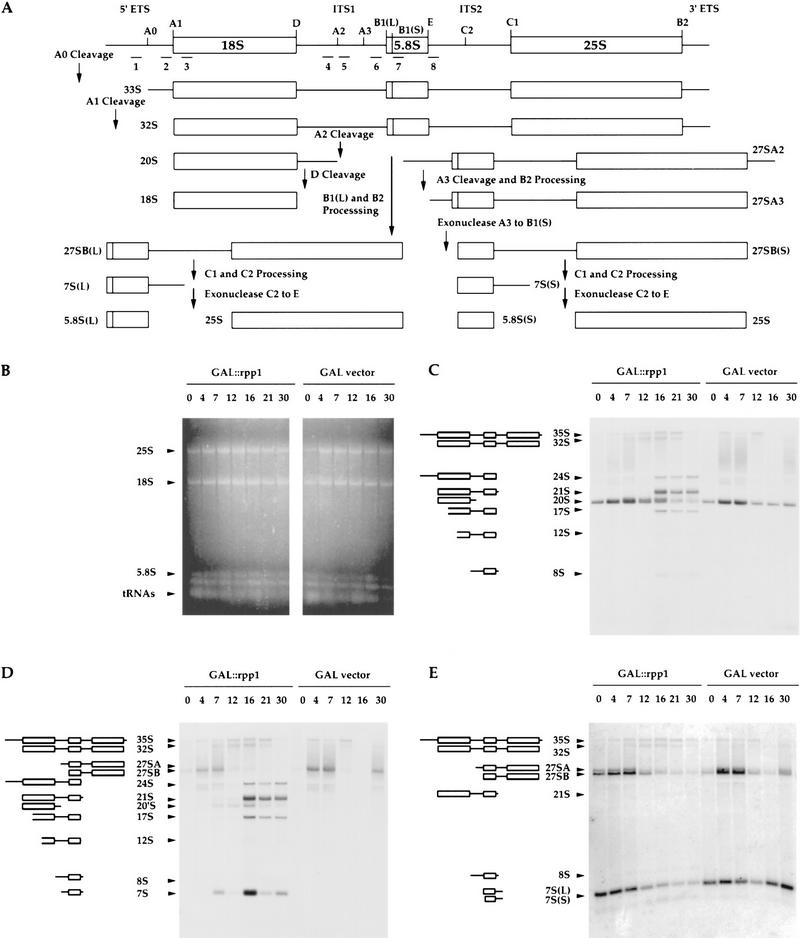
Figure 6.
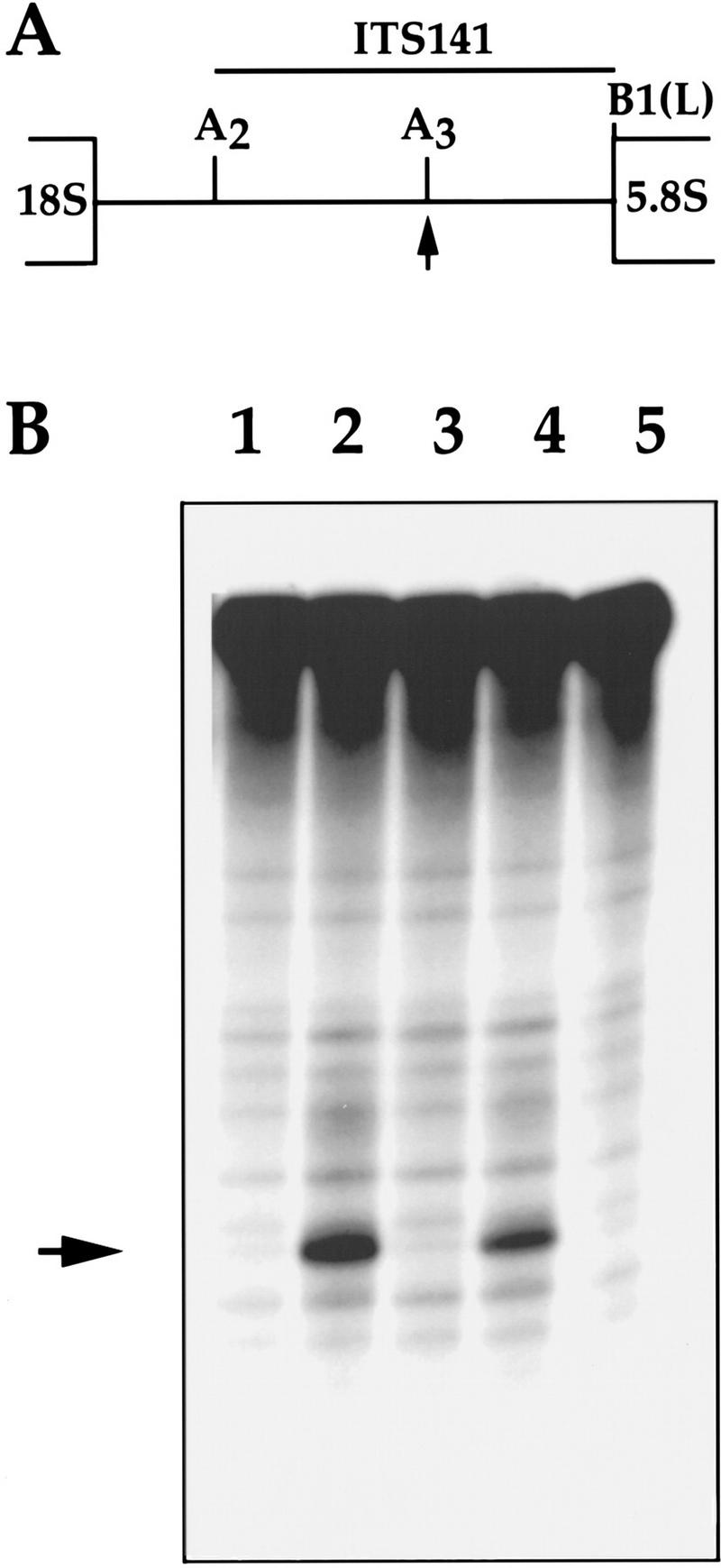
Processing of prRNA by Rpp1 immunoprecipitates. (A) Position of the ITS141 rRNA substrate relative to the 35S rRNA precursor [5′ end is at A2 site and 3′ end is at B1(L) site]. (B) Uniformly labeled rRNA transcript, ITS141 (see Materials and Methods), was incubated with 3 × myc–Rpp1 immunoprecipitates derived from the same immunoprecipitates as in Fig. 3A for 2 hr at 37°C, and then fractionated on a 8% polyacrylamide/7 m urea gel. (Lanes 1–4) ITS141 rRNA, plus IgG–agarose pellets to which are bound immunoprecipitated RNase P and RNase MRP RNAs, respectively, as in Fig. 3A. (Lane 5) ITS141 rRNA. The arrow indicates the position of two cleavage products of almost identical size from the ITS1 rRNA transcripts. The site of cleavage corresponds to the region of the A3 site in the internal transcribed sequence 1 (ITS1) (Lygerou et al. 1996).
On depletion of the Rpp1 protein, we observed multiple defects in rRNA processing at the A0 and A1 sites in the 5′ external transcribed sequence (5′ ETS), at the A2 and A3 sites within the internal transcribed sequence (ITS1), and at the E and/or C2 sites within the internal transcribed sequence 2 (ITS2) (Fig. 6). Analysis of low-molecular-weight RNA showed that synthesis of 5.8S(S) rRNA was reduced in an Rpp1-depleted strain, whereas 5.8S(L) rRNA increased (Fig. 5B). The altered ratio between 5.8S(L) and 5.8S(S) suggests a defect in cleavage at the A3 site in the ITS1 and/or other processing sites required for maturation of 5.8S rRNA in vivo (Schmitt and Clayton 1993; Henry et al. 1994). Figure 6, C and D, shows an accumulation of a 7S precursor to 5.8S rRNA after 7 hr of Rpp1 depletion. This precursor is predicted to have its 5′ end at the A2 site of ITS1 and its 3′ end at the 3′ end of 5.8S rRNA. If this prediction is proven correct, then we will be able to conclude that there is a defect in cleavage at the A3 site under the conditions we used.
In addition, two large precursors of the 18S rRNA accumulated at later times in Rpp1-depleted cells (Fig. 6C,D). The 24S rRNA precursor that contains both 5′ ETS and ITS1 sequences shows defects in cleavage at the A0, A1, A2, and A3 processing sites, and the 21S rRNA precursor shows defects in cleavage and processing at the A2 and A3 processing sites in ITS1 and at the E site in ITS2 (Fig. 6C,D). The 17S and 12S rRNA degradation intermediates represent sequences with fragmented 5′ ends within the 18S rRNA (Fig. 6C,D; Allmang et al. 1996a). Figure 6E shows depletion of the 7S(S) and 7S(L) precursors to 5.8S(S) rRNA, depletion of the 27SA and 27SB precursors to 25S rRNA, and accumulation of an 8S rRNA precursor of the 5.8S rRNA, which contains 5′ extended sequences from ITS1 and 3′ extended sequences from ITS2. These intermediates indicate defects in cleavage at the A3, and E and/or C2 processing sites of the ITS1 and ITS2, respectively. All probes also detected a moderate accumulation of the 35S rRNA primary transcript and probes 2–7 also detect the 32S precursor rRNA (Fig. 6C–E; data not shown). Despite these defects the steady-state levels of the 18S rRNA and 25S rRNA remained unchanged (Fig. 6B), suggesting delayed processing of 35S rRNA in the absence of Rpp1.
Our results show that Rpp1 is required for processing of 35S rRNA in the 5′ ETS, ITS1, and ITS2. Interestingly, some of the same processing reactions have been shown to be dependent on snoRNPs, RNase MRP, and RNase P, and the same stable rRNA degradation intermediates accumulate in cells deficient in snoRNP components (Shuai and Warner 1991; Lindahl et al. 1992; Chamberlain et al. 1996b; Venema and Tollervey 1996). However, none of the known proteins that associate with these RNP particles exhibit the same global defects in prRNA and ptRNA processing found in Rpp1-depleted cells. Interestingly, depletion of the snoRNP protein, Rrp5, results in striking similarities to Rpp1-depleted cells with respect to defects of prRNA processing (Venema and Tollervey 1996; see Discussion). Therefore, we suggest that RNase P interacts functionally with RNase MRP and perhaps other snoRNPs in the processing of rRNA in yeast.
Processing of precursor rRNA by Rpp1 immunoprecipitates
We tested whether Rpp1 is associated directly with prRNA processing activity by in vitro cleavage assay of two fragments of the 35S rRNA. We assayed cleavage in vitro by resuspended 3 × myc–Rpp1-containing immunoprecipitates of two fragments of the 35S precursor rRNA, ITS1.603 (Chamberlain et al. 1996b) and ITS1.141 (similar to Lygerou et al. 1996a). Both prRNA substrates overlap the ITS1 (see Fig. 7A, and Materials and Methods). Rpp1 immunoprecipitates, which contained both RNase P and RNase MRP RNAs, cleaved ITS1.141 (Fig. 7B) and ITS1.603 (data not shown) in the region of the A3 processing site. This result is consistent with, but does not rigorously prove, the observed defects in processing the 35S rRNA on depletion of the Rpp1 protein in vivo. However, 3 × myc–Rpp1 immunoprecipitates that were washed extensively with high ionic buffer (see Material and Methods) failed to cleave the prRNA substrates but cleaved ptRNAs in vitro (data not shown). These results suggest that in addition to RNase MRP, there is a direct role for an RNase P-containing complex in rRNA processing in vivo.
Figure 7.
Effects of Rpp1 depletion on the processing of 35S prRNA. (A) Schematic plan of the rRNA processing pathway (Venema and Tollervey 1995). Steady-state levels of rRNA, prRNA intermediates, and aberrant rRNA species were detected by ethidium bromide staining and by Northern hybridization with oligonucleotide probes 1–7. (B) Total RNA extracted from VS164 and VS165 was obtained after transfer from galactose-containing to glucose-containing medium at the times indicated, and separated in a l.2% agarose gel stained with ethidium bromide. Arrows indicate 25S rRNA, 18S rRNA, 5.8S rRNA, and tRNAs. (C–E) The gel shown in B was transferred to a positively charged nylon membrane (Boehringer Mannheim) by capillary diffusion and hybridized with γ-32-labeled oligonucleotide probes 4–6 (see Materials and Methods). Oligonucleotide 4 (C) is complementary to the ITS1, between 3′ end of 18S rRNA and the A2 site (position 157–180), oligonucleotide 5 (D) is complementary to the ITS1, between A2 and A3 sites (position 119–236), and oligonucleotide 8 (E) is complementary to the ITS2, between the 3′ end of 5.8S rRNA and C2 sites (position −3 to +21). The 24S precursor rRNA is predicted to extend from the 5′ end of the ETS to the 3′ end of 5.8S rRNA, and represents a product of the 35S rRNA precursor that is cleaved in ITS2 in the absence of cleavage at the A0, A1, A2, and A3 sites. The 21S precursor rRNA appears to be comprised of rRNA species that are predicted to extend from the 5′ end of 18S rRNA to the 3′ end of 5.8S rRNA, and also have 3′ ends that extend 3′ to the 3′ end of 5.8S rRNA, and may be heterogeneous. The 20′S rRNA species is predicted to extend from the 5′ end of 18S rRNA to the region of the A3 site in the ITS1. The 17S′ and 12S′ aberrant rRNA species represent stable degradation intermediates, which may have fragmented 5′ ends that correspond to sequences within 18S rRNA and 3′ ends close to the 3′ end of 5.8S rRNA. The 8S rRNA species is predicted to extend from the 5′ end of ITS1 to the 3′ extended ends of 5.8S rRNA. The 7S RNA is the precursor of the 5.8S rRNA, which is predicted to extend from the region of the A2 site in the ITS1 to the 3′ end of 5.8S rRNA.
Discussion
We have cloned a gene encoding a protein subunit of nuclear RNase P from S. cerevisiae based on its homology to human Rpp30. Yeast Rpp1 is homologous to the human scleroderma autoimmune antigen, Rpp30, which was described previously as an autoantigen that copurifies with at least six other Rpp protein subunits of the human RNase P—Rpp14, Rpp20, Rpp25, Rpp30, Rpp38, and Rpp40 (Eder et al. 1997). Using computer database searches (blastp and tblastn algorithms), we compared the predicted amino acid sequences of human Rpp proteins with the S. cerevisiae genome database. This analysis revealed that Rpp1 is one of three yeast proteins with amino acid sequence similarity to the human Rpp proteins. A second such yeast protein is Pop4, a subunit of yeast RNase P and RNase MRP (Chu et al. 1997), which is related in amino acid sequence to a previously uncharacterized human Rpp protein, Rpp29 (P. Eder, N. Jarrous, and S. Altman, unpubl.). The third yeast, protein, Rpp2, shares amino acid similarity with Rpp20 (V. Stolc and S. Altman, unpubl.)
Rpp1 is a small basic protein with a predicted molecular mass of 32.2 kD and pI 9.76. It does not have any previously identified RNA-binding domains. In vitro, Rpp1 remains associated with the mature RNase P RNA and RNase P activity, even in buffers of high ionic strength. Rpp1 also associates with RNase MRP RNA and an rRNA processing activity (cleavage at the A3 site), ascribed previously to RNase MRP (Lygerou et al. 1996a). However, in contrast to RNase P, RNase MRP RNA and the rRNA processing activity can be separated from Rpp1 significantly after high-salt washes. Furthermore, the human Rpp30 does not associate with RNase MRP (N. Jarrous and S. Altman, unpubl.) Whether yeast RNase P cleaves the prRNA substrates directly in vivo is unknown. Proposals for the secondary structure of ITS1 that are based on phylogenetic analysis and chemical mapping of ITS1 (Van Nues et al. 1994; Allmang et al. 1996b) and computational folding of the ITS1.141 prRNA substrate, do not show a common structural feature of an RNase P substrate (a helical segment at the junction of a single-stranded region; Altman et al. 1995). Moreover, prRNA substrates used in this study are not cleaved at the A3 site in vitro by reconstituted E. coli RNase P (V. Stolc and S. Altman, unpubl.), which has a broader substrate specificity than the eukaryotic RNase P (Yuan and Altman 1994). Therefore, in vivo, yeast Rpp1 may be a shared subunit of a large RNP complex containing both RNase P and RNase MRP, and perhaps other snoRNPs.
On depletion of Rpp1 in vivo, we found a defect in ptRNA processing, an indication that Rpp1 is associated with RNase P activity. Surprisingly, we also found an rRNA processing defect characterized by the absence of cleavage at the A0 and A1 sites in the 5′ ETS, and at the A2 and A3 sites in ITS1, and at the E and/or C2 sites in ITS2 of the primary 35S rRNA transcript. To our knowledge, Rpp1 is the only RNase P or RNase MRP protein, that on depletion simultaneously inhibits cleavages at all of these major processing sites of 35S rRNA and is required for ptRNA processing. Moreover, processing of prRNA at sites A0, A1, A2, A3, E, and/or C2 may be coordinated by RNase P, as depletion of RNase MRP RNA alone results only in defects at the A3 site (Schmitt and Clayton 1993).
Apart from RNase MRP, other essential snoRNPs may be involved directly in the mechanism of rRNA processing (for recent reviews, see Eichler and Craig 1994; Venema and Tollervey 1995; Tollervey 1996). In contrast to the Rpp1 phenotype, depletion- or temperature-sensitive alleles of these snoRNAs or their associated proteins show no defect in ptRNA processing and, with the exception of snoRNP protein Rrp5, no single component is defective for processing at all of the processing sites in the 5′ ETS, ITS1, and ITS2. For example, Gar1p, Nop1p, Sof1p, as well as snoRNA U3, U14, and snR30-depleted cells, and strains lacking snR10 are defective at A0, A1, and A2 sites without detectable inhibition of the A3 site (Tollervey 1987; Li et al. 1990; Hughes and Ares 1991; Tollervey 1991; Girard et al. 1992; Jansen et al. 1993; Morrissey and Tollervey 1993). The prRNA processing phenotype of Rrp5-depleted strains differs from that of Rpp1 in that it is defective in maintaining accumulation of 25S rRNA, 18S rRNA, and relative levels of rRNA intermediates (Venema and Tollervey 1996). Interestingly, as in Rpp1-depleted cells, cleavage at the A3 site is also defective in Pop1, Pop3, Pop4, and RNase MRP (NME1) conditional mutants (Shaui and Warner 1981; Lindahl et al. 1992; Schmitt and Clayton 1993; Lygerou et al. 1996a; Chu et al. 1997; Dichtl and Tollervey 1997). However, in contrast to Rpp1, a direct role of Pop1 in catalysis of ptRNAs is unknown, as the human Pop1 homolog (Lygerou et al. 1995b) is not found in highly purified human RNase P (Eder et al. 1997; N. Jarrous and S. Altman, unpubl.). Therefore, although Rpp1 shares rRNA processing defects with known RNase P, RNase MRP, and snoRNPs, it is the only described protein that has both ptRNA processing defect as well as rRNA processing defects at the A0, A1, A2, A3, and E, and/or C2 processing sites.
Materials and methods
Strains, media, and general procedures
S. cerevisiae strains used in this work are listed in Table 1. The composition of the media with appropriate nutrients for plasmid maintenance and S. cerevisiae growth and handling techniques were used as described (Guthrie and Fink 1991). Unless stated otherwise, all techniques for manipulating DNA, RNA, and oligonucleotides were performed according to standard procedures (Sambrook et al. 1989). The identities of all constructs were verified by sequence analysis. Oligonucleotides used in this study are listed in Table 2.
Gene disruption
A genomic clone spanning the RPP1 locus was identified using the S. cerevisiae Genome Database (SGD) and obtained from the American Tissue Culture Collection (ATCC) as cosmid 8025. Clone 8025 was sequenced previously (Johnston et al. 1994). A 1.4-kb BamHI–XbaI fragment encoding the RPP1 gene was subcloned from cosmid 8025 into pBluescript (SK) vector (Stratagene) cut with BamHI–XbaI to generate pRPP1SK. The RPP1 gene was disrupted by replacing a 0.6-kb NcoI–NdeI fragment with the LEU2 gene, thereby deleting 65% of the coding sequence. The rpp1::LEU2 construct was integrated at the RPP1 genomic locus in diploid strain JN161 (VS161) and correct replacement of one allele was verified by PCR digest analysis: Oligonucleotides 5GAL30 and 3GAL30 were used to amplify genomic DNA isolated from VS161, resulting PCR-amplified fragment (2.7 kb) encoding LEU2 and flanking sequences of Rpp1 was mapped with restriction enzymes. The heterozygous RPP1/rpp1::LEU2 strain (VS161) was sporulated and tetrad analysis showed a 2:2 segregation for cell viability. Dissections were performed on 20 tetrads. The same disruption and analysis was performed for strain VS163, which was disrupted with the rpp1::LEU2 construct.
Construction of expression plasmids
To generate myc-epitope-tagged RPP1, three myc epitope domains (3 × myc) were PCR-amplified from the 3 × mycSEC8 construct (TerBush and Novick 1996) using oligonucleoitdes MYC5 and MYC3, and then cut with BsmI. The PCR fragment was subcloned into the BsmI site of pRPP1SK plasmid to generate p3 × myc–RPP1SK. A 1.5-kb BamHI–XbaI fragment from p3 × myc–RPP1SK was subcloned into pRS316 plasmid to generate pRS316–3 × myc::RPP1. The coding sequence of RPP1 was PCR-amplified from pRPP1SK using oligonucleotides 5GAL30 and 3GAL30, cut with BamHI and XhoI, and subcloned into modified pYCp33 vector that contained the GAL1–10 promoter to generate pYCp–GAL::rpp1. To generate rRNA ITS1 substrate, ITS1.141 (similar to Lygerou et al. 1996a), oligonucleotides T7ITS14 and 3′ ITS14 were used to PCR-amplify yeast genomic DNA and then the 141-bp fragment was cloned into EcoRI and BamHI sites in pUC19 (New England Biolabs). To generate rRNA ITS1 substrate, ITS1.603 (Chamberlain et al. 1996b), oligonucleotides T7ITS16 and 3′ ITS16 were used to PCR amplify yeast genomic DNA and the amplified fragment was subsequently subcloned into pUC19 the same way as for the ITS1.141 fragment. The NME-coding sequence was amplified by PCR from yeast genomic DNA using T7MRP and 3′ MRP oligonucleotides, and subcloned into EcoRI and SmaI sites of pUC19 to generate pUCT7MRP.
Strain construction
The diploid strain heterozygous for the RPP1 deletion (VS161) was transformed with pRS316::3 × myc–Rpp1 plasmid and sporulated. After sporulation and dissection, spores disrupted for RPP1, but harboring the plasmid encoded 3 × myc–Rpp1 fusion protein, were viable. Only two haploid cells (VS162A′ and VS162C′), derived from sporulation of a single tetrad, were viable after the loss of the pRS316–3 × myc–RPP1 plasmid during selection on plates that contain 5-fluoro-orotic acid (5-FOA): These haploids were Leu−, showing that they had lost the pRS316–3 × –RPP1 plasmid and had the wild-type RPP1 allele. The other two haploids (VS162B and VS162D) were not viable without the pRS316–3 × myc–RPP1 plasmid during selection on 5-FOA plates and were Leu+, showing that they had the 3 × myc–tagged RPP1 allele. The same results were obtained after dissection of five additional tetrads. The growth rate of two haploid rpp1:LEU2 cells (VS162B and VS162D), which depend on a low-copy-number episomal plasmid encoding the 3 × myc–RPP1 (pRS316–3 × myc–RPP1) for viability, was identical to that of the wild-type haploid cells (VS162A and VS162C).
VS163 was transformed with pYCp–GAL::rpp1 plasmid (VS164) or a control plasmid, pYCp–GAL (VS165), sporulated and germinated on medium that contained galactose. Four viable spores were obtained from several independent tetrads and the presence of the plasmid-encoded GAL::rpp1 fusion gene was verified on ura− and leu− plates containing galactose.
Immunoprecipitation
Extracts were prepared by lysis of yeast cells (25 ml, OD600 = 0.5) with glass beads (Guthrie and Fink 1991). Extracts were clarified by centrifugation three times at 15,000g for 10 min. The final supernatants were used in immunoprecipitations by adding 4 μg of 9E10 antibody to the protein extract derived from spores VS162A′, VS162B, VS162C′, and VS162D, and incubated for 2.5 hr, followed by addition of 50 μl of 100 mg/ml of anti-mouse IgG (whole molecule) agarose (Sigma) in IP150 buffer (150 mm KCl, 10 mm Tris-Cl (pH 7.9), 0.1% NP-40, and 0.1% NaN3). Pellets were washed five times with IP150 or IP600 (same composition as IP150, except 600 mm KCl). RNA was extracted from the immunoprecipitated beads by adding 100 μl of IPR buffer (100 mm Tris-Cl at pH 7.5, 100 mm EDTA at pH 8.0, 150 mm NaCl, 1% SDS), followed by two extractions with phenol and one extraction with phenol/chloroform/isoamylalcohol solution (25:24:1). RNA was precipitated with ethanol.
Assays for RNase P activity and rRNA processing activity
IgG–agarose pellets, to which are bound immunoprecipitated RNase P and RNase MRP RNAs, were washed with IP150 and incubated with labeled ptRNASer (Tollervey et al. 1991) for 30 min at 37°C in 1× BB (10 mm HEPES at pH 8.0, 400 mm NH4OAc, 10 mm Mg2OAc, 5% glycerol). rRNA transcripts ITS1.141 and ITS603 (Chamberlain et al. 1996b) were incubated with IgG-pellets to which are bound immunoprecipitated RNase P and RNase MRP RNAs (washed with IP150) for 2 hr at 37°C in 1× PMB (20 mm Tris-HCl at pH 8.0, 10 mm MgCl2, 1 mm EDTA, 50 mm KCl, 2 units of RNasin, and 50 mg/ml of BSA). IgG pellets, to which is bound RNase P RNA, were washed with IP600 and incubated with either ptRNASer, ITs141, or ITS603 as above. The tRNA substrate, ITS141, and ITS603 rRNA substrates were labeled uniformly with [32P]GTP (3000 mCi/mmole, Amersham) and purified on an 8% polyacrylamide/7 m urea gel. The assays were performed with 0.5 nm ptRNA, 3.2 nm ITS141 RNA, or 0.5 nm ITS603 RNA. The RNA products were fractionated on an 8% polyacrylamide/7 m urea gel.
RNA analysis
Total RNA was isolated by disruption of cell pellets (25 ml, OD600 = 0.5), resuspended in AE buffer [50 mm NaAc at pH 5.3, 10 mm EDTA, 10 mnm RNase inhibitor, vanadyl ribonucleoside complex (GIBCO BRL)] and equal volume of phenol and chloroform, with glass beads at 65°C for 15 min. Phenol/chloroform extractions were repeated four times at 65°C for 15 min, followed by an extraction with chloroform at 25°C and ethanol precipitation. Northern hybridization was performed as described previously (Guerrier-Takada et al. 1995). All oligonucleotides were end-labeled with T4 polynucleotide kinase (New England Biolabs) and [γ-32P]ATP (Amersham). NME1 EcoRI–SmaI DNA fragment from pUCT7MRP and EcoRI–SmaI DNA fragment from pScRNAP (Lee et al. 1991) were labeled uniformly with [32P]dCTP (Amersham).
Acknowledgments
We thank Y. Barral, J. Novak, S. Reck-Peterson, B. Rockmill, S. Roeder, and A. Smith for yeast strains and helpful advice, D.R. TerBush and P. Novick for yeast strains and 9E10 antibody, K. Ross for the GAL plasmid, and P.S. Eder, N. Jarrous, V. Gopalan, and C. Guerrier-Takada for discussion and useful comments. We are grateful to M. Snyder and several other colleagues for a critical reading of the manuscript. V.S. was supported by a predoctoral training grant in cell biology from the U.S. Public Health Service (U.S. PHS) to Yale University. Research in the laboratory of S.A. was funded by grant GM-19422 from the U.S. PHS.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL sidney.altman@qm.yale.edu; FAX (203) 432-5713.
References
- Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes & Dev. 1994;8:3021–3031. doi: 10.1101/gad.8.24.3021. [DOI] [PubMed] [Google Scholar]
- Allmang C, Henry Y, Morrissey JP, Wood H, Petfalski E, Tollervey D. Processing of the yeast pre-rRNA at sites A(2) and A(3) is linked. RNA. 1996a;2:63–73. [PMC free article] [PubMed] [Google Scholar]
- Allmang C, Henry Y, Wood H, Morrissey JP, Petfalski E, Tollervey D. Recognition of cleavage site A(2) in the yeast pre-rRNA. RNA. 1996b;2:51–62. [PMC free article] [PubMed] [Google Scholar]
- Altman S, Kirseborm L, Talbot S. Recent studies of ribonuclease P. FASEB J. 1993;7:7–14. doi: 10.1096/fasebj.7.1.7916700. [DOI] [PubMed] [Google Scholar]
- ————— . Recent studies of RNase P. In: Soll D, RajBhandary UL, editors. tRNA: Structure biosynthesis and function. Washington, DC.: American Society for Microbiology; 1995. pp. 67–78. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bothwell ALM, Stark BC, Altman S. Ribonuclease P substrate specificity: Cleavage of a bacteriophage φ/80-induced RNA. Proc Natl Acad Sci. 1974;73:1912–1916. doi: 10.1073/pnas.73.6.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell ALM, Garber RL, Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5S RNA. J Biol Chem. 1976;251:7709–7716. [PubMed] [Google Scholar]
- Brow DA, Guthrie C. Transcription of a yeast U6 snRNA gene requires a polymerase III promoter element in a novel position. Genes & Dev. 1990;4:1345–1356. doi: 10.1101/gad.4.8.1345. [DOI] [PubMed] [Google Scholar]
- Chamberlain JR, Tranguch AJ, Pagan-Ramos E, Engelke DR. Eukaryotic nuclear RNase P: Structures and function. Prog Nucleic Acid Res Mol Biol. 1996a;55:87–119. doi: 10.1016/s0079-6603(08)60190-7. [DOI] [PubMed] [Google Scholar]
- Chamberlain JR, Pagan-Ramos E, Kindelberger DW, Engelke DR. An RNase P RNA subunit mutation affects ribosomal RNA processing. Nucleic Acids Res. 1996b;24:3158–3166. doi: 10.1093/nar/24.16.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DD, Clayton DA. Mouse RNase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substate. Cell. 1987;56:131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Cherry, J.M., C. Adler, C. Ball, S. Dwight, S. Chervitz, Y. Jia, G. Juvik, S. Weng, and D. Botstein. 1996. Saccharomyces Genome Database. http://genome-www.stanford.edu/Saccharomyces/1996. [DOI] [PMC free article] [PubMed]
- Chu S, Zengel JM, Lindahl L. A novel protein shared by RNase MRP and RNase P. RNA. 1997;3:382–391. [PMC free article] [PubMed] [Google Scholar]
- Clayon D. A nuclear function for RNase MRP. Proc Natl Acad Sci. 1994;91:4615–4617. doi: 10.1073/pnas.91.11.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr SC, Brown JW, Pace NR. The varieties of ribonuclease P. Trends Biochem Sci. 1992;17:178–182. doi: 10.1016/0968-0004(92)90262-8. [DOI] [PubMed] [Google Scholar]
- Dichtl, B. and D. Tollervey. 199. POP3p is essential for the activity of RNase MRP and RNase P ribonucleopoteins in vivo. EMBO J. 16: 417–429. [DOI] [PMC free article] [PubMed]
- Eder PS, Srinivasan A, Fishman MC, Altman S. The RNA subunit of ribonuclease P from the zebrafish, Danio rerio. J Biol Chem. 1996;271:21031–21036. doi: 10.1074/jbc.271.35.21031. [DOI] [PubMed] [Google Scholar]
- Eder PS, Kekuda R, Stolc V, Altman S. Characterization of two scleroderma autoimmune antigens that copurify with human ribonuclease P. Proc Natl Acad Sci. 1997;94:1101–1106. doi: 10.1073/pnas.94.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler DC, Craig N. Processing of eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1994;49:197–239. doi: 10.1016/s0079-6603(08)60051-3. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Kiss T. Structure and function of nucleolar snRNPs. Mol Biol Reports. 1993;18:149–156. doi: 10.1007/BF00986770. [DOI] [PubMed] [Google Scholar]
- Forster AC, Altman S. Similar cage-shaped structure for the RNA components of all ribouclease P and ribonuclease MRP emzymes. Cell. 1990;62:407–409. doi: 10.1016/0092-8674(90)90003-w. [DOI] [PubMed] [Google Scholar]
- Founier MJ, Maxwell ES. The nucleolar snRNAs: Catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993;18:131–135. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- Girard JP, Lehtonen H, Caizergues-Ferrer M, Amalric F, Tollervey D, Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992;11:673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG. Life with 6000 genes. Science. 1996;274:563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- Gopalan V, Baxevanis AD, Landsman D, Altman S. Analysis of the functional role of conserved residues in the protein subunit of ribonuclease P from Escherichia coli. J Mol Biol. 1997;267:818–829. doi: 10.1006/jmbi.1997.0906. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C, Li Y, Altman S. Artificial regulation of gene expression in Escherichia coli by RNase P. Proc Natl Acad Sci. 1995;92:11115–11119. doi: 10.1073/pnas.92.24.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:1–539. [PubMed] [Google Scholar]
- Hartmann RK, Heinrich J, Schlegl J, Schuster H. Precursor of C4 antisense RNA of bacteriophages P1 and P7 in a substrate for RNase P of Escherichia coli. Proc Natl Acad Sci. 1995;92:5822–5826. doi: 10.1073/pnas.92.13.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas ES, Armbruster DW, Vucson BM, Daniels CJ, Brown JW. Comparative analysis of ribonuclease P RNA structure in Archaea. Nucleic Acids Res. 1996;24:1252–1259. doi: 10.1093/nar/24.7.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Y, Wood H, Morrissey JP, Petfalski E, Kearsey S, Tollervey D. The 5′ end of yeast 5.8S rRNA is generated by exonucleases from an upstream cleavage site. EMBO J. 1994;13:2452–2463. doi: 10.1002/j.1460-2075.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5′ external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1992;10:4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Tollervey D, Hurt EC. A U3 snoRNP protein with homology to splicing factor PRP4 and G betadomains is required for ribosomal RNA processing. EMBO J. 1993;12:2549–2558. doi: 10.1002/j.1460-2075.1993.tb05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Andrews S, Brinkman R, Cooper J, Ding H, Dover J, Du Z, Favello A, Fulton L, Gattung S, et al. Complete nucleotide sequence of Saccharomyces cerevisiae chromosome VIII. Science. 1994;265:2077–2082. doi: 10.1126/science.8091229. [DOI] [PubMed] [Google Scholar]
- Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Matera AG, Ward DC, Craft J. Association of RNase mitochondrial RNA processing enzyme with ribonuclease P in higher ordered structures in the nucleolus: A possible coordinate role in ribosome biogenesis. Proc Natl Acad Sci. 1996;93:11471–11476. doi: 10.1073/pnas.93.21.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Rohlman CE, Molony LA, Engelke DR. Characterization of RPR1, an essential gene encoding the RNA component of Saccharomyces cerevisiae nuclear RNase P. Mol Cell Biol. 1991;11:721–730. doi: 10.1128/mcb.11.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Zagorski J, Fournier MJ. Depletion of U14 small nuclear RNA (snR128) disrupts production of 18S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:1145–1152. doi: 10.1128/mcb.10.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L, Archer RH, Zengel JM. A new rRNA processing mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 1992;20:295–301. doi: 10.1093/nar/20.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Altman S. Differential evolution of substrates for an RNA enzyme in the presence and absence of its protein cofactor. Cell. 1994;77:1093–1100. doi: 10.1016/0092-8674(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Lygerou Z, Mitchell P, Petfalski E, Seraphin B, Tollervey D. The POP1 gene encodes protein component common to the RNase MRP and RNase P ribonucleoproteins. Genes & Dev. 1994;8:1423–1433. doi: 10.1101/gad.8.12.1423. [DOI] [PubMed] [Google Scholar]
- Lygerou Z, Allmang C, Seraphin B. Accurate processing of a eukaryotic precursor ribosomal RNA by ribonuclease MRP in vitro. Science. 1996a;272:268–270. doi: 10.1126/science.272.5259.268. [DOI] [PubMed] [Google Scholar]
- Lygerou Z, Pluk H, van Venrooij WJ, Seraphin B. hPop1: An autoantigenic protein subunit shared by the human RNase P and RNase MRP ribonucleoproteins. EMBO J. 1996b;15:5936–5948. [PMC free article] [PubMed] [Google Scholar]
- Maxwell ES, Fournier MJ. The small nucleolar RNAs. Annu Rev Biochem. 1995;64:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- Morrissey JP, Tollervey D. Yeast snR30 is a small nucleolar RNA required for 18S rRNA synthesis. Mol Cell Biol. 1993;13:2469–2477. doi: 10.1128/mcb.13.4.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Birth of the snoRNPs: The evolution of RNase MRP and the eukaryotic pre-rRNA-processing system. Trends Biochem Sci. 1995;20:78–82. doi: 10.1016/s0968-0004(00)88962-8. [DOI] [PubMed] [Google Scholar]
- Pace NR, Burgin AB. In: The ribosome. Hill WE, editor. Washington, DC.: American Society for Microbiology; 1990. p. 418. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schmnitt ME, Clayton DA. Yeast site-specific ribonucleoprotein endoribonuclese MRP contains an RNA component homologus to mammalian RNAse MRP RNA and essential for cell viability. Genes & Dev. 1992;6:1975–1985. doi: 10.1101/gad.6.10.1975. [DOI] [PubMed] [Google Scholar]
- ————— Nuclear RNase MRP is required for correct processing of pre-5.8S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:7935–7941. doi: 10.1128/mcb.13.12.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt ME, Bennett JL, Dairaghi DJ, Clayton DA. Secondary structure of RNase MRP RNA as predicted by phylogenetic comparison. FASEB J. 1993;7:208–213. doi: 10.1096/fasebj.7.1.7678563. [DOI] [PubMed] [Google Scholar]
- Shuai K, Warner JR. A temperature sensitive mutant of Saccharomyces cerevisiae defective in pre-rRNA processing. Nucleic Acid Res. 1991;19:5059–5064. doi: 10.1093/nar/19.18.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl LL, Clayton DA. Saccharomyces cerevisiae contains an RNase MRP that cleaves at a conserved mitochondrial RNA sequence implicated in replication priming. Mol Cell Biol. 1992;12:2561–2569. doi: 10.1128/mcb.12.6.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;23:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO J. 1987;6:4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Small nucleolar RNAs guide ribosomal RNA methylation. Science. 1996;273:1056–1057. doi: 10.1126/science.273.5278.1056. [DOI] [PubMed] [Google Scholar]
- Tollervey D, Lehtonen H, Carmo-Fonseca M, Hurt EC. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991;10:573–583. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch AJ, Engelke DR. Comparative structural analysis of nuclear RNase P RNAs from yeast. J Biol Chem. 1993;268:14045–14055. [PubMed] [Google Scholar]
- Van Nues RW, Rientjes JMJ, Van der Sande CAFM, Zerp SF, Sluiter C, Venema J, Planta RJ, Raue HA. Separate structural elements within internal transcribed spacer 1 of Saccharomyces cerevisiae precursor ribosomal RNA direct the formation of 17S and 26S rRNA. Nucleic Acids Res. 1994;22:912–929. doi: 10.1093/nar/22.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J, Tollervey D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae. Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]
- ————— RRP5 is required for formation of both 18S and 5.85 rRNA in yeast. EMBO J. 1996;15:5701–5714. [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Altman S. Substrate recognition by human RNase P: Identification of small, model substrates for the enzyme. EMBO J. 1995;14:159–168. doi: 10.1002/j.1460-2075.1995.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]