Highlights
► We studied the effects of costimulation on the immunosuppressives CsA and Aza. ► Human T cells were activated in presence of different costimulatory signals. ► CD28 but not signals via CD2, 4-1BB, ICOS or LFA-1 increased the IC50 for CsA. ► The inhibitory effects of Aza were not influenced by T cell costimulatory signals. ► This is relevant when combining immunosuppressives with costimulation blockers.
Abbreviations: Aza, azathioprine; CsA, Cyclosporine A; APC, antigen presenting cells; IC50, mean inhibitory concentration
Keywords: T cell costimulation, Immunosuppression, Cyclosporine A, azathioprine
Abstract
Immunosuppression is an important treatment modality in transplantation and human diseases that are associated with aberrant T cell activation. There are considerable differences regarding the cellular processes targeted by the immunosuppressive drugs that are in clinical use. Drugs like azathioprine (Aza) mainly act by halting proliferation of fast dividing cells, whereas others like cyclosporine A (CsA) specifically target signaling pathways in T cells. Since the outcome of T cell responses critically depends on the quality and strength of costimulatory signals, this study has addressed the interplay between costimulation and the immunosuppressive agents CsA and Aza during the in vitro activation of human T cells. We used an experimental system that allows analyzing T cells activated in the presence of selected costimulatory ligands to study T cells stimulated via CD28, CD2, LFA-1, ICOS or 4-1BB. The mean inhibitory concentrations (IC50) for Aza and CsA were determined for the proliferation of T cells receiving different costimulatory signals as well as for T cells activated in the absence of costimulation. CD28 signals but not costimulation via CD2, 4-1BB, ICOS or LFA-1 greatly increased the IC50 for CsA. By contrast, the inhibitory effects of Aza were not influenced by T cell costimulatory signals. Our results might have implications for combining standard immunosuppressive drugs with CTLA-4Ig fusion proteins, which act by blocking CD28 costimulation.
1. Introduction
T cells have important roles in allograft rejection, graft versus host diseases and autoimmune pathologies. In most cases the clinical management of these conditions requires the extensive use of immunosuppressive agents to control aberrant T cell responses. Such drugs limit and down-modulate T cell activation by targeting different cellular processes. Drugs like azathioprine (Aza) mainly act by halting proliferation of fast dividing cells, whereas others like Cyclosporine A (CsA) more specifically target T cells by interfering with signaling pathways in these cells. Since many different signals can contribute to T cell activation processes, the interplay between such signals and immunosuppressive agents might have differential effects on the outcome of T cell responses. Especially costimulatory signals generated by interaction of antigen presenting cells (APC)-expressed ligands with their T cell-expressed receptors have a crucial role in the efficient activation of T cells that recognize antigen. The interaction of CD80/CD86 with CD28 is generally regarded as the primary T cell costimulatory pathway [1]. However, there are many alternative costimulatory ligand-receptor pairs that potently enhance the proliferation, differentiation and cytokine production of T cells that recognize antigens [2–4]. Among these the CD58 – CD2, 4-1BBL – 4-1BB, ICOS-L – ICOS and CD54 – LFA-1 (CD11a/CD18) pathways are well documented to generate strong and consistent costimulatory effects in human T cells [2,5]. Costimulatory receptors belong to different molecule-families and consequently they can induce signaling events that are distinct from the pathways induced by CD28 ligation. Previous studies have shown that engagement of the CD28 costimulatory pathway greatly reduces the sensitivity of T cells to the immunosuppressive effect of CsA [6,7]. By contrast, it is not known whether triggering alternative costimulatory receptors has similar effects. Furthermore, currently there is limited knowledge how different costimulatory signals affect the immunosuppressive effects of other drugs in clinical use.
We have previously developed a cellular system termed T cell stimulator cells that allows analyzing the effect of different costimulatory signals on human T cells [5,8,9]. This system is based on cell lines engineered to express membrane-bound anti-human-CD3 antibody-fragments that trigger the TCR-complex on human T cells upon co-culture. By expressing high levels of human costimulatory ligands of interest on the T cell stimulator cells it is possible to analyze and compare human T cells that receive distinct costimulatory signals. In this study we used T cell stimulator lines expressing CD80, CD58, 4-1BBL, ICOS-L. CD54 and T cell stimulator lines expressing anti-CD3 antibody-fragments but no costimulatory molecules to activate T cells purified from healthy individuals. Using this system we determined the mean inhibitory concentrations (IC50) for CsA and Aza for the proliferation of human T cells receiving different costimulatory signals.
2. Material and methods
2.1. Antibodies, cell culture and FACS staining
293T cells and the mouse thymoma cell line Bw5147 (short designation within this work Bw) were cultured as described [9]. The ethical review board of the General Hospital and the Medical University of Vienna approved the human studies performed within this work and informed consent was obtained from the donors. PBMC were isolated from heparinised whole blood of healthy volunteer donors by standard density centrifugation with Ficoll-Paque (Amersham Bioscience, Roosendaal, Netherlands). Untouched human T cells were obtained through depletion of CD11b, CD14, CD16, CD19, CD33 and MHC-class II bearing cells with the respective mAbs by MACS (Miltenyi Biotech, Bergisch Gladbach, Germany). Untouched CD8+ and CD4+ T cells were isolated from human T cells using MACS in conjunction with antibodies to CD8 or CD4. The mAbs to CD4 (VIT4), CD8 (VIT8), CD11b (VIM12), CD14 (VIM13), CD33 (4D3), MHC-class II (1/47), CD80 (7-480), CD58 (1-456) and CD54 (5-216) were produced at our Institute. The mAb to CD14 (MEM-18) was purchased from An der Grub (Kaumberg, Austria), CD19 mAb (BU12) from Ancell (Bayport, MN), 4-1BBL from Biolegend (San Diego, CA) and ICOS-L (2D3/B7H2) from BD Pharmingen (Palo Alto, CA). FACS analysis was performed as described previously [10]. Briefly, binding of primary antibodies was detected with PE-conjugated goat-anti-mouse IgG-Fcγ specific Abs (Jackson ImmunoResearch, West Grove, PA). Expression of membrane-bound anti-CD3 antibody fragment was detected via APC-conjugated goat-anti-mouse IgG (H + L) Abs, which react with the variable regions of murine antibodies (Jackson ImmunoResearch). Fluorescence intensity is shown on a standard logarithmic scale.
2.2. T cell activation in the presence of different costimulatory molecules
Human T cells (1 × 105/well) were co-cultured for 72 h with irradiated (6000 rad) T cell stimulator cell lines (2 × 104/well) expressing high levels of membrane-bound anti-CD3 antibody-fragments and one of the following costimulatory ligands: CD80, CD58, 4-1BBL, ICOS-L, CD54 or control T cell stimulator cells expressing anti-CD3 but no human costimulatory molecules as described [5]. In some experiments 5 × 104/well T cell stimulator cells were used. Cyclosporine A (CsA; Sandimmun®, Novartis Pharma, Basel, Swiss) and azathioprine (Aza; Imurek®, GlaxoSmithKline, Greenford, GB) were added at the indicated final concentrations. To assess T cell proliferation methyl-3[H]-thymidine (final concentration: 0.025 mCi; Perkin Elmer/New England Nuclear Coorporation, Wellesley, MA) was added for the last 18 h prior harvesting of the cells. Methyl-3[H]-thymidine uptake was measured as described [8]. All T cell proliferation assays were done in triplicates, means and SD are shown.
2.3. Cytokine measurement
For cytokine measurement supernatants of T cell activation assays were collected after 48 h and pooled from triplicate wells. IFN-γ, IL-2, IL-10 and IL-13 were measured in the supernatants using the Luminex System 100 (Luminex, Texas, USA).
2.4. Statistical analyses
The half maximal inhibitory concentrations (IC50) for CsA and Aza were calculated for each experiment by blotting the percentage of proliferation (methyl-3[H]-thymidine uptake in the presence of inhibitor × 100/methyl-3[H]-thymidine uptake without inhibitor) against the log concentration of the immunosuppressive drug (mg/ml) using Graph-pad PRISM. Differences between the IC50 values for CsA and Aza of T cells stimulated in presence of different costimulatory ligands were calculated using ANOVA for repeated measures. IMB® SPSS statistics software was used for analysis and generation of Box plot graphs. Values of p ≤ 0.05 were considered statistically significant.
3. Results
3.1. T cell proliferation in the presence of different costimulatory ligands
We have previously generated a cellular system called T cell stimulator cells that allows for analysing T cells receiving distinct costimulatory signals [5]. This system is based on Bw cells, a murine thymoma line, that has been engineered to express membrane-bound anti-CD3 antibody-fragments and can thus generate “Signal 1” in human T cells by triggering their TCR-complex. By co-expressing human costimulatory ligands on these stimulator cells their contribution to T cell activation processes can readily be studied. Importantly, with this system we can analyze the net effects of individual costimulatory ligands in the absence of other accessory molecules that also regulate T cell activation on human APC.
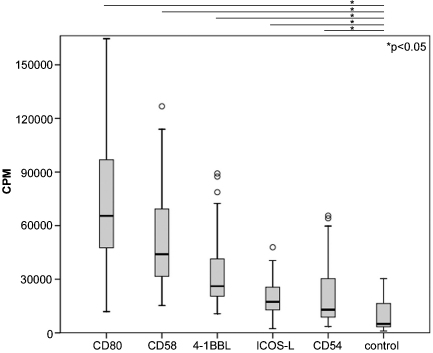
For this study we have generated and used T cell stimulator lines expressing CD80, CD58, 4-1BBL, ICOS-L, CD54 and control stimulator cells expressing anti-CD3 antibodies but no costimulatory molecules. Those molecules were chosen, because their role as costimulatory molecules is well described and moreover they represent different molecule families. Flow cytometric analysis of the resulting T cell stimulator lines demonstrate similar amounts of membrane-bound anti-CD3 and high expression levels of the respective costimulatory ligands (Fig. 1). In Fig. 2 the proliferative response of T cells stimulated with T cell stimulator lines expressing the indicated costimulatory molecules or with T cell stimulator cells expressing no costimulatory molecule is shown. The costimulatory molecules tested in these experiments differed in their potency to induce proliferation. Importantly, all T cell stimulator cells harbouring costimulatory ligands induced a stronger proliferation of T cells than the control T cell stimulator cell line expressing no human costimulatory molecules (Fig. 2).
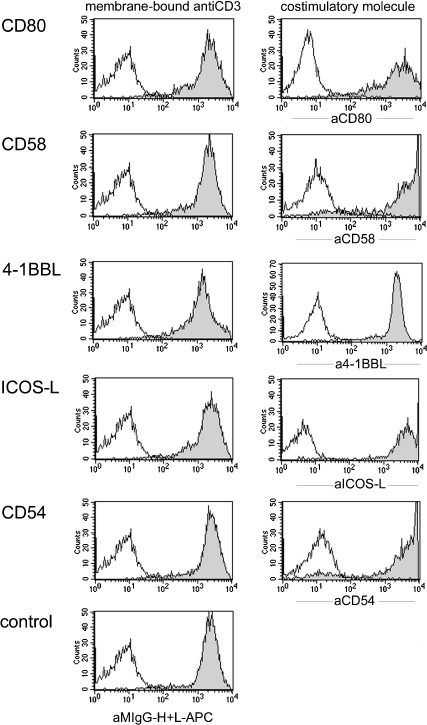
Fig. 1.
Characterisation of the T cell stimulator cells used in this study. Characterisation of T cell stimulator cells expressing anti-CD3high in conjunction with CD80, CD58, 4-1BBL, ICOS-L, CD54 or no costimulatory molecule (control) by FACS. Left panel: expression of mb-anti-CD3 antibodies on stimulator cells; open histograms: parental Bw cells. Right panel: T cell stimulator cells expressing CD80, CD58, 4-1BBL, ICOS-L or CD54 were probed with antibodies specific for these molecules (filled histograms); open histograms: reactivity of the indicated antibodies with parental Bw cells.
Fig. 2.
Proliferative response of T cells activated in presence of different costimulatory signals. Stimulator cells expressing high levels of membrane-bound anti-CD3 in conjunction with CD80, CD58, 4-1BBL, ICOS-L, CD54 or no human costimulatory molecules were co-cultured with human T cells. 3[H]-thymidine uptake was assessed following 3 days of co-culture (cpm, counts per minute). Circles indicate outliers. Box plots show the results of 10 independent experiments with T cells from different healthy donors.
3.2. Impact of Cyclosporine A and azathioprine on human T cell proliferation acting via different costimulatory ligands
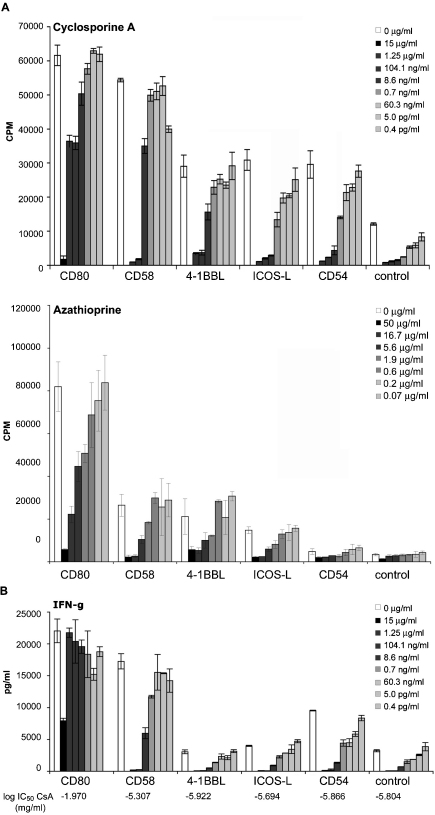
We have previously demonstrated that our system of T cell stimulator cells is an excellent tool to study the influence of immunmodulatory drugs on T cell activation [5,11].In this study we addressed whether different costimulatory signals can influence the ability of the immunosuppressive drugs CsA and Aza to inhibit human T cell proliferation. T cell stimulator cells expressing high levels of anti-CD3 in conjunction with CD80, CD58, 4-1BBL, ICOS-L, CD54 as well as T cell stimulator cells expressing no costimulatory molecules were co-cultured with human T cells in the presence of different concentrations of CsA and Aza. As shown in Fig. 3A, CsA and Aza led to a dose-dependent inhibition of T cell proliferation. Importantly, the sensitivity of CD28 costimulated T cells to the anti-proliferative effects of CsA was dramatically reduced. Furthermore, a strongly impaired immunosuppressive effect of CsA on CD28 costimulated T cells was also observed when analyzing the cytokine content of the culture supernatants: CsA concentrations of 100 ng/ml completely blocked IFN-γ production in T cells activated in the absence of CD28 signals, but had little effect on CD28 costimulated T cells (Fig. 3B). Only T cells costimulated via CD28 or CD2 produced significant amounts of IL-10, while IL-4 and IL-2 were only measured upon CD28 costimulation. CsA was also much less effective to inhibit the production of these cytokines when T cells received CD28 signals (supplementary Fig. 1, and data not shown). We also performed experiments with purified CD4 and CD8 T cells from the same donors and found that CD28 costimulation strongly reduced the anti-proliferative effect of CsA in both subsets (supplementary Fig. 2). In contrast, the T cell-inhibitory effects of Aza were not modulated by costimulatory signals (Fig. 3A).
Fig. 3.
The impact of CsA and Aza on the proliferation of human T cells receiving different costimulatory signals. Human T cells were stimulated with stimulator cells expressing high levels of anti-CD3 in conjunction with CD80, CD58, 4-1BBL, ICOS-L, CD54 or control stimulator cells. On the onset of co-culture (A) Cyclosporine A (CsA) (upper panel) or Azathioprine (Aza) (lower panel) were added at the indicated final concentrations. 3[H]-thymidine uptake was assessed following 3 days of co-culture (cpm, counts per minute). (B) The IFN-γ concentration in the culture supernatants was measured using a Luminex-based assay. Log IC50 values (mg/ml) are indicated. Data show ± SD of triplicates from one experiment. The experiment shown is representative for 10 independently performed.
3.3. IC50 values of CsA depend on the costimulatory signal
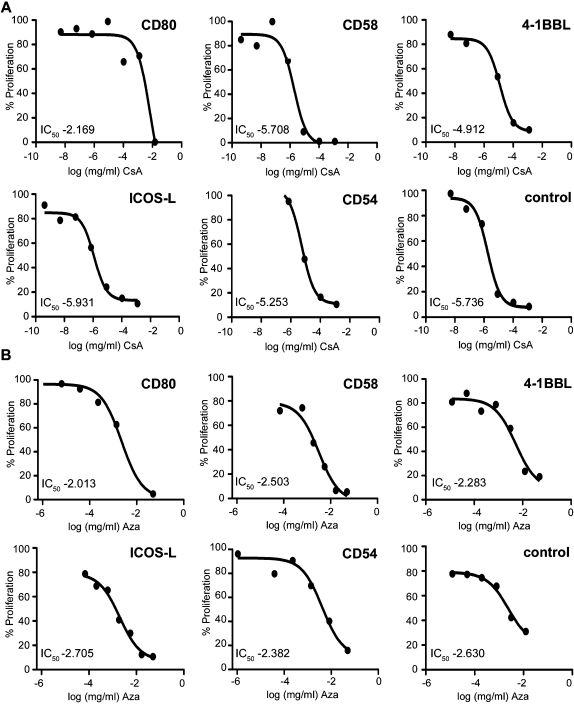
The half maximal inhibitory concentration (IC50) for CsA and Aza were calculated from dose-inhibition curves for T cells activated in the presence of T cell stimulator cells expressing the indicated costimulatory ligands. Representative dose-inhibition curves for each costimulatory molecule are shown in Fig. 4.
Fig. 4.
Dose inhibition curves for CsA (A) or Aza (B) for T cells stimulated in the presence of different costimulatory ligands and for T cells activated in absence of costimulatory signals (control). The calculated IC50 values (half maximal inhibitory concentrations) are indicated. Each curve shown is representative for ten independently performed experiments.
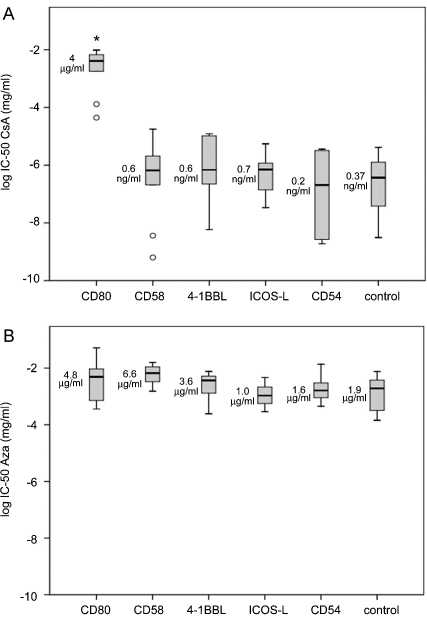
Cumulative results of the IC50 values obtained with T cells from 10 healthy donors are shown in Fig. 5. In the case of CsA the median half maximal inhibitory concentration for T cells stimulated via CD28 compared to T cells receiving no costimulatory signal was increased more than 10000-fold (median IC50 values 4 μg/ml versus 0.37 ng/ml; p ≤ 0.05, n = 10). Importantly, our results clearly demonstrate that decreased sensitivity to the immunosuppressive effects of CsA is an unique property of CD28 signals, since T cells stimulated via alternative pathways were inhibited by CsA to the same extent as T cells receiving no costimulatory signals (Fig. 5A).
Fig. 5.
IC50 values for T cell proliferation in the presence of different costimulatory signals. The cumulative results of the IC50 values for (A) CsA or (B) Aza treated human T cells activated in the presence of different costimulatory ligands. Circles indicate outliers. Box plots represent data from ten experiments independently performed. Asterisks indicate significant differences (p ≤ 0.05, n = 10). Median values are indicated.
To investigate the differential effects of CsA on CD28 signals and signals generated by alternative costimulators in more detail we used CD58 expressing stimulator cells, which induced the highest proliferation and cytokine production in human T cells besides CD80. We performed experiments where higher numbers of CD58 expressing stimulator cells were used to stimulate human T cells (50,000 instead of 20,000 cells/well). Under these conditions human T cells proliferated more compared to T cells stimulated with 20,000 CD80 expressing stimulator cells. However, the use of higher numbers of CD58-expressing stimulator cells only led to a slight increase of the IC50 value for CsA. These results clearly indicate that differences in CsA responsiveness in T cells receiving CD28 or alternative costimulatory signals are qualitative rather than quantitative (supplementary Fig. 3).
Furthermore, statistical analysis of our data clearly showed that CD28 signals are completely ineffective in reducing Aza mediated T cell suppression and the IC50 values obtained for this drug were not influenced by any of the costimulatory signals investigated in this study (Fig. 5B).
4. Discussion
CD28 is generally regarded as the primary and most potent costimulatory receptor on T cells and consequently many studies have investigated how CD28 signals contribute to T cell activation and differentiation processes. Several earlier studies have demonstrated that CD28 triggering greatly reduces the inhibitory effects of CsA on the proliferation and cytokine production of human T cells [6,7]. However, triggering of numerous alternative receptors can generate potent costimulatory signals in T cells and animal studies demonstrate that productive immune responses occur in the absence of CD28 signals [12,13]. Our study is the first to address whether alternative costimulatory signals can also interfere with CsA-mediated T cell suppression. Since activated APC express a plethora of accessory molecules, it is difficult to study the role of distinct costimulatory pathways in T cells stimulated by natural APC. We thus relied on a previously described cellular system, which allows studying human T cells that were activated in presence of individual costimulatory ligands of choice [5,9,10].
Our results clearly show that decreased sensitivity to the anti-proliferative effects of CsA is a unique feature of CD28 signals, since it was not observed with T cells activated by alternative costimulatory pathways. Although CD28 costimulation induced the strongest proliferative response we suggest that this is a qualitative effect. In support for this hypothesis we observed that IC50 values obtained for CD2 costimulated T cells, which also had very strong proliferative responses, were even slightly lower than those obtained for T cells stimulated via ICOS, which induced much less T cell proliferation and cytokine production (Figs. 2 and 5 and data not shown). Furthermore, we demonstrate that even under conditions where non-CD28 costimulation induced higher proliferative responses than CD28 costimulation, the dramatic differences in the IC50 values for CsA are not significantly affected (supplementary Fig. 3). We have recently shown that Efalizumab, a CD11a antibody, acts as a very potent inhibitor of T cell activation and that presence of CD28 costimulation is able to completely override the T cell inhibitory effects of this therapeutic antibody. Interestingly this effect was also observed with CD2 costimulation whereas ICOS, LFA-1 or 4-1BB signals were completely ineffective in reverting Efalizumab effects [11]. This indicates that T cell costimulatory signals have distinct qualities and thus can have unique abilities to interfere with immunosuppressive and immunomodulatory drugs.
CsA acts by forming a complex with immunophilin, which inhibits the Ca2+/calmodulin-dependent serine-threonine phosphatase calcineurin. Inactive calcineurin is unable to activate the nuclear factor of activated T cells (NFAT). Previous work by Ghosh et al. has demonstrated that NFAT can be activated in a CsA-resistant pathway that is independent of calcineurin [14]. Furthermore, the authors showed that this pathway is induced via CD28, which would explain why CsA inhibition of calcineurin signaling can be overcome by CD28 costimulation. The exact mechanisms for this phenomenon have not been defined, but okadaic acid sensitive serine/threonine phosphatases have been demonstrated to mediate CsA-resistant transactivation of the IL-2 promotor presumably by targeting NFAT [15,16].
Importantly, our data demonstrate that CD28 costimulation does not result in a general insensitivity to immunosuppressive agents, since Aza reduced the proliferation of T cells stimulated via CD28 or alternative costimulatory molecules to a similar extent.
Successful clinical trials established CD28 costimulation blockade mediated by the CTLA-4Ig derivative Belatacept as emerging treatment modality to prevent acute rejection and protect renal function in kidney transplant recipients [17–19]. The results of our study suggest synergistic effects during the combined use of CTLA-4Ig and CsA, which target CD28 and alternative costimulatory pathways, respectively. In standard immunosuppressive regimen high doses of CsA might be required to overcome the low effectivity of this drug to block the activation of T cells through the CD28 pathway. Since very low concentrations of CsA are sufficient to inhibit T cells that do not receive CD28 signals, upon CD28 blockade the adverse effects of CsA could be largely avoided by administering this drug at greatly reduced doses. Current treatment regimen-in renal graft recipients aim to achieve CsA-plasma levels of 40–250 ng/ml. Based on the results of our in vitro experiments these concentrations are 100 times higher than the median IC50 values determined for human T cells activated in the absence of CD28 costimulation, which ranged from 0.6 ng/ml to 0.2 ng/ml. Moreover, these concentrations are clearly insufficient to inhibit T cells that receive strong costimulation via CD28 as their IC50 value was found to be in the μg-range (Fig. 5A). Importantly, such synergistic effects cannot be expected when CTLA-4Ig is combined with Aza and possibly other immunosuppressive agents, which halt the proliferation of T cells regardless of the costimulatory signals they receive.
In some experimental transplant models the administration of CTLA-4Ig was suggested to induce tolerance and promoted long term survival [20,21]. There are studies that found concomitant use of CsA to be detrimental to tolerance induction since it prevented activation-induced cell death (AICD) of effector T cells [22–25]. However, in these studies high concentrations of CsA were used. Moreover, other studies have not observed impairment of long-term graft survival by combined treatment with CTLA-4Ig and CsA [26] and additive effects of CTLA-4Ig and CsA were also described [27,28].
In non-human primate models CTLA-4Ig has not been shown to induce indefinite graft survival or tolerance [29,30], which might be due to an important role of CD28 independent T cell activation pathways. In human or primate T cells, the CD58-CD2 interaction functions as a second major T cell activation axis that is much more potent than other non-CD28 costimulators [2,5,31–33]. This pathway is not operative in murine T cells, since mice lack CD58. Furthermore, following chronic stimulation human but not murine CD8+ T cells loose CD28 expression and thus become dependent on alternative costimulatory pathways for activation [34]. Therefore, in humans and primates alternative costimulatory pathways are likely to have more important contributions to T cell activation processes than in rodents and consequently the efficacy of immunosuppressive drugs in blocking human T cells activated by such signals is of high interest.
5. Conclusion
The efficacy of immunosuppressive drugs in blocking human T cells, activated by different costimulatory signals, is of high interest especially under conditions where standard immunosuppressive drugs are co-administered with agents that specifically block selected costimulatory pathways. The interplay between costimulatory molecules and immunosuppressive agents should be considered when combining these drugs with costimulation blockers.
Acknowledgement
We appreciate the excellent technical assistance of Christoph Klauser, Margarete Merio, Petra Cejka and Claus Wenhart. The authors thank Dr. Michael Weber, Department of Radiology, General Hospital Medical University of Vienna, for help with statistical analysis. This study was supported by a grant from the Austrian Science Fund (FWF P21964-B20) and a grant from the Austrian National Bank (12731). Judith Leitner is supported by a Doc fForte fellowship from the Austrian Academy of Science. The authors declare no conflict of interest.
K.D. and J.L. performed experiments and analyzed data. W.F.P., O.M. and G.Z. provided reagents and critically read the manuscript. J.L. and P.S. wrote the paper and designed research. All authors critically revised the manuscript and approved the final version.
Biographies

Judith Leitner received M.Sc. from the University of Vienna in 2006. Currently, doing PhD thesis about the role of alternative costimulatory pathways during the activation of human T cells, at the Institute of Immunology, Medical University of Vienna. Research interests: T cell costimulation, receptor-ligand interaction, DC, immunomodulatory drugs.

Karin Drobits joined the laboratory of Peter Steinberger to do her master degree in Biomedical Analytics. In her studies she has been focusing on the interplay between costimmulatory signals and immunosuppressive agents during the activation of human T cells.

Winfried F. Pickl is an M.D. (University of Vienna, Austria; supervisor: Walter Knapp) and board certified immunologist with 2 1/2 years of international research experience at the Department of Molecular Biology and Genetics, Harvard Medical School, Boston, MA; supervisor: Brian Seed). At present associate professor, principal investigator and head of the Division of Cellular Immunology and Immunohematology, Institute of Immunology, Medical University of Vienna. Professional skills in molecular and cellular immunology, hematology as well as blood group serology and tissue typing. His main research interest is centered around the topics lymphocyte activation and modulation, antigen presentation, immunohematology and allergy research.

Otto Majdic received his Ph.D. from the University of Vienna. Together with Johannes Stöckl he heads the Division of Immune regulation at the Institute of Immunology, Medical University of Vienna. He started his scientific career in the group of Prof. Walter Knapp where he has developed numerous widely used monoclonal antibodies against human leukocyte surface antigens. His research interest is on molecules involved in the APC-T cell interaction and in the development of antibodies with immunomodulatory capacity.

Gerhard Zlabinger, MD heads the Institute of Immunology at the Medical University of Vienna. He is board certified immunologist and full professor for Clinical and experimental Immunology. His research interests include humoral rejection of solid organ grafts, immunomodulation by bacteria and endogenous TLR-ligands.

Peter Steinberger did his Ph.D. under the supervision of Rudolf Valenta at the University of Vienna. Following postdoctoral studies with Carlos Barbas 3rd at The Scripps Research Institute in San Diego, California he joined the group of Walter Knapp at the Institute of Immunology, Medical University of Vienna. Since 2006 he is head of the Division of Immune Receptors and T cell activation at this institution. His research focuses on the role of accessory cell surface molecules during human T cell responses.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.imlet.2011.06.010.
Appendix A. Supplementary data
References
- 1.Sharpe A.H., Freeman G.J. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 2.Leitner J., Grabmeier-Pfistershammer K., Steinberger P. Receptors and ligands implicated in human T cell costimulatory processes. Immunol Lett. 2010;128:89–97. doi: 10.1016/j.imlet.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 4.Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 5.Leitner J., Kuschei W., Grabmeier-Pfistershammer K., Woitek R., Kriehuber E., Majdic O. T cell stimulator cells, an efficient and versatile cellular system to assess the role of costimulatory ligands in the activation of human T cells. J Immunol Methods. 2010;362:131–141. doi: 10.1016/j.jim.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June C.H, Ledbetter J.A., Gillespie M.M., Lindsten T., Thompson C.B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson C.B., Lindsten T., Ledbetter J.A., Kunkel S.L., Young H.A., Emerson S.G. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kober J., Leitner J., Klauser C., Woitek R., Majdic O., Stockl J. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–2688. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfistershammer K., Klauser C., Pickl W.F., Stockl J., Leitner J., Zlabinger G. No evidence for dualism in function and receptors: PD-L2/B7-DC is an inhibitory regulator of human T cell activation. Eur J Immunol. 2006;36:1104–1113. doi: 10.1002/eji.200535344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitner J., Klauser C., Pickl W.F., Stockl J., Majdic O., Bardet A.F. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuschei W., Leitner J., Majdic O., Pickl W.F., Zlabinger G., Grabmeier-Pfistershammer K. Costimulatory signals potently modulate the T cell inhibitory capacity of the therapeutic CD11a antibody Efalizumab. Clin Immunol. 2011;139:199–207. doi: 10.1016/j.clim.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Shahinian A., Pfeffer K., Lee K.P., Kundig T.M., Kishihara K., Wakeham A. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 13.Cassell D.J. Validity of the two-signal model for activation of CD28-deficient T lymphocytes: quantitative characterization of an alternative costimulatory function of dendritic cells. Scand J Immunol. 2001;53:346–356. doi: 10.1046/j.1365-3083.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh P., Sica A., Cippitelli M., Subleski J., Lahesmaa R., Young H.A. Activation of nuclear factor of activated T cells in a cyclosporin A-resistant pathway. J Biol Chem. 1996;271:7700–7704. doi: 10.1074/jbc.271.13.7700. [DOI] [PubMed] [Google Scholar]
- 15.Nebl G., Meuer S.C., Samstag Y. Cyclosporin A-resistant transactivation of the IL-2 promoter requires activity of okadaic acid-sensitive serine/threonine phosphatases. J Immunol. 1998;161:1803–1810. [PubMed] [Google Scholar]
- 16.Murphy L.L., Hughes C.C. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. J Immunol. 2002;169:3717–3725. doi: 10.4049/jimmunol.169.7.3717. [DOI] [PubMed] [Google Scholar]
- 17.Larsen C.P., Grinyo J., Medina-Pestana J., Vanrenterghem Y., Vincenti F., Breshahan B. Belatacept-Based Regimens Versus a Cyclosporine A-Based Regimen in Kidney Transplant Recipients: 2-Year Results From the BENEFIT and BENEFIT-EXT Studies. Transplantation. 2010;90:1528–1535. doi: 10.1097/TP.0b013e3181ff87cd. [DOI] [PubMed] [Google Scholar]
- 18.Rostaing L., Massari P., Garcia V.D., Mancilla-Urrea E., Nainan G., Rial M.D. Switching from Calcineurin Inhibitor-based Regimens to a Belatacept-based Regimen in Renal Transplant Recipients: A Randomized Phase II Study. Clin J Am Soc Nephrol. 2011;6:430–439. doi: 10.2215/CJN.05840710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincenti F., Blancho G., Durrbach A., Friend P., Grinyo J., Halloran P.F. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21:1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenschow D.J., Zeng Y., Thistlethwaite J.R., Montag A., Brady W., Gibson M.G. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 21.Wekerle T., Sayegh M.H., Ito H., Hill J., Chandraker A., Pearson D.A. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 22.Larsen C.P., Elwood E.T., Alexander D.Z., Ritchie S.C., Hendrix R., Tucker-Burden C. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Zheng X.X., Li X.C., Zand M.S., Strom T.B. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation. 1998;66:1387–1388. doi: 10.1097/00007890-199811270-00021. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Li X.C., Zheng X.X., Wells A.D., Turka L.A., Strom T.B. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 25.Gao W., Lu Y., El Essawy B., Oukka M., Kuchroo V.K., Strom T.B. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sho M., Sandner S.E., Najafian N., Salama A.D., Dong V., Yamada A. New insights into the interactions between T-cell costimulatory blockade and conventional immunosuppressive drugs. Ann Surg. 2002;236:667–675. doi: 10.1097/00000658-200211000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hale D.A., Gottschalk R., Maki T., Monaco A.P. Use of CTLA4-Ig in combination with conventional immunosuppressive agents to prolong allograft survival. Transplantation. 1997;64:897–900. doi: 10.1097/00007890-199709270-00018. [DOI] [PubMed] [Google Scholar]
- 28.Bolling S.F., Lin H., Wei R.Q., Linsley P., Turka L.A. The effect of combination cyclosporine and CTLA4-Ig therapy on cardiac allograft survival. J Surg Res. 1994;57:60–64. doi: 10.1006/jsre.1994.1110. [DOI] [PubMed] [Google Scholar]
- 29.Levisetti M.G., Padrid P.A., Szot G.L., Mittal N., Meehan S.M., Wardrip C.L. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159:5187–5191. [PubMed] [Google Scholar]
- 30.Kirk A.D., Harlan D.M., Armstrong N.N., Davis T.A., Dong Y., Gray G.S. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meuer S.C., Hussey R.E., Fabbi M., Fox D., Acuto O., Fitzgerald K.A. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984;36:897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- 32.Moingeon P., Chang H.C., Sayre P.H., Clayton L.K., Alcover A., Gardner P. The structural biology of CD2. Immunol Rev. 1989;111:111–144. doi: 10.1111/j.1600-065x.1989.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 33.Liversidge J., Dawson R., Hoey S., McKay D., Grabowski P., Forrester J.V. CD59 and CD48 expressed by rat retinal pigment epithelial cells are major ligands for the CD2-mediated alternative pathway of T cell activation. J Immunol. 1996;156:3696–3703. [PubMed] [Google Scholar]
- 34.DeBenedette M.A., Shahinian A., Mak T.W., Watts T.H. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.