Abstract
Primary eosinophilic gastrointestinal disorders (EGIDs) represent a spectrum of inflammatory gastrointestinal disorders in which eosinophils infiltrate the gut in the absence of known causes for such tissue eosinophilia. EGIDs can be subgrouped as eosinophilic esophagitis (EE), eosinophilic gastroenteritis (EG), and eosinophilic colitis (EC). The least frequent manifestation of EGIDs is EC. EC is a heterogeneous entity with a bimodal age distribution, presenting with either an acute self-limited bloody diarrhea in otherwise healthy infants or as a more chronic relapsing colitis in young adults. The pathophysiology of primary EC appears related to altered hypersensitivity, principally as a food allergy in infants and T lymphocyte-mediated (i.e. non-IgE associated) in young adults. In adults, symptoms include diarrhea, abdominal pain, and weight loss. Endoscopic changes are generally modest, featuring edema and patchy granularity. Although standardized criteria are not yet established, the diagnosis of EC depends on histopathology that identifies an excess of eosinophils. Therapeutic approaches are based on case reports and small case series, as prospective randomized controlled trials are lacking. Eosinophilic colitis in infants is a rather benign, frequently food-related entity and dietary elimination of the aggressor often resolves the disorder within days. Adolescent or older patients require more aggressive medical management including: glucocorticoids, anti-histamines, leukotriene receptors antagonists as well as novel approaches employing biologics that target interleukin-5 (IL-5) and IgE. This review article summarizes the current knowledge of EC, its epidemiology, clinical manifestations, diagnosis, and treatment.
Keywords: colitis, eosinophilic colitis, eosinophilic gastrointestinal disorder, eosinophilic proctocolitis, eosinophils, gastrointestinal
The spectrum of eosinophilic gastrointestinal disorders
Primary eosinophilic gastrointestinal disorders (EGIDs) represent an unusual spectrum of inflammatory gastrointestinal disorders in which eosinophils infiltrate the gut in the absence of known causes for such tissue eosinophilia. The family of primary EGIDs has been categorized as eosinophilic esophagitis (EE), eosinophilic gastroenteritis (EG), and eosinophilic colitis (EC). Unclear is whether these disorders share a common pathophysiology or the subgroups are truly distinct [Rothenberg, 2004]. Some overlap exists, particularly between the latter two entities that reside distal to the esophagus. Gut eosinophilia likely represents an even greater spectrum, extending from those that are predominantly IgE mediated, through the primary EGIDs to those that are secondary and not IgE mediated (such as inflammatory bowel disease and celiac disease). Moreover, EGIDs frequently occur independent of blood eosinophilia, the best examples being EE and EC. This concept distinguishes the pathobiology of EGIDs from classical blood eosinophilic states such as the hypereosinophilic syndrome (HES). Classically, the hypereosinophilic syndrome exhibits markedly increased levels of blood eosinophils (≥1500/µl), while eosinophilic infiltration and mediator release damage multiple organs such as the heart, skin, and lung whose dysfunction dominants the clinical picture. In HES, the gut appears to be a rather innocent bystander with eosinophils secondarily infiltrating various sites along the gastrointestinal tract to a variable depth causing weight loss, vomiting, abdominal pain, and diarrhea. Gut eosinophilia can also develop secondary to recognized entities, such as reflux esophagitis, Helicobacter pylori gastritis, connective tissue diseases, vasculitis, inflammatory bowel disease, malignancy, and particularly helminthic infestation (Table 1).
Table 1.
Classification of eosinophilic gastrointestinal disorder (EGID).
| Primary EGIDs | Secondary Gut Eosinophilias |
|---|---|
| Primary eosinophilic esophagitis | Gastrointestinal infections: helminthes, fungi |
| Primary eosinophilic gastroenteritis | Hypereosinophilic syndrome (HES) |
| Primary eosinophilic colitis | Systemic disease (e.g. connective tissue disease, vasculitis, |
| celiac disease, inflammatory bowel disease) | |
| Allergic proctocolitis of infancy | Drugs (e.g. naproxen, clozapine, rifampicin, gold) |
Epidemiology of eosinophilic gastrointestinal disorders
Eosinophilic esophagitis
The esophagus normally lacks constituent eosinophils but can attract these inflammatory cells, the best example being in response to acid-peptic damage in gastroesophageal reflux disease. Other secondary causes of esophageal eosinophilia include helminthic and fungal infections, allergic vasculitis, scleroderma, and drug injuries.
Primary EE is increasingly recognized as a distinct condition with esophageal symptoms, being a common cause of dysphagia, food bolus impaction, and retrosternal chest pain. The prevalence of this disorder appears to be increasing over time in geographically stable areas with consistent recording practices. Its accelerated incidence, particularly in children and young men, may represent merely heightened recognition from increased medical publications and more frequent endoscopic procedures, but more likely is a true increase in disease burden [Prasad et al. 2009]. This now well-recognized entity is found in up to 1% of the population [Furuta et al. 2007; Gonsalves, 2007; Arora and Yamazaki, 2004]. EE is not characterized by features of either EG or EC. Diagnosis is made by esophageal biopsies showing an increased number of eosinophils (>15/high-powered field) although a clear consensus is lacking [Furuta et al. 2007]. A history of atopy and other allergies (to food, drugs, and environmental allergens) may be present along with a mild peripheral eosinophilia. Its cause may have a genetic component with a family history in some 10%, evidence of food and aeroallergen hypersensitivity in many, but only a few have a history of true food anaphylaxis. It is likely not IgE-mediated.
Eosinophilic gastroenteritis
EG seems to be a misnomer as the entire gastrointestinal system can be involved: from the esophagus to the colon and even the biliary tract [Schoonbroodt et al. 1995]. EG commonly shows eosinophils selectively infiltrating the stomach (in 26–81%) and/or the small bowel (in 28–100%) [Khan and Orenstein, 2002]. There may be concurrent, although less-prominent involvement of the esophagus or colon/rectum. About half of the EG patients have atopic disorders and/or have food intolerances or allergies, especially children; here, serum IgE is often elevated. Although most patients have positive skin tests to various food antigens, such food hypersensitivities are delayed-type rather than being typical anaphylactic reactions.
Eosinophilic colitis
EC is exceptionally rare with only a few cases being reported since 1979. The absence of defined histological criteria for a specific eosinophil count in the colonic mucosa makes the diagnosis of this entity challenging. Hence, its true frequency is not clear. The clinical presentation includes abdominal pain, diarrhea (bloody or nonbloody), and/or weight loss. EC in its primary form can be associated with other atopic conditions. Colonic eosinophilia can also occur secondary to helminthic infections (e.g. pin worms, hookworms), inflammatory bowel disease, autoimmune disease (e.g. scleroderma, Churg–Strauss syndrome), celiac disease, drug reactions, and in association with the HES.
Food allergy and etiology of eosinophilic colitis
‘Atopy’ is a genetic predisposition to any excessive IgE-mediated reaction that characteristically causes eczema, allergic rhinitis (hay fever) and/or asthma. Food allergy — a part of the atopic syndrome — represents an adverse immune response toward certain food proteins [Bruijnzeel-Koomen et al. 1995]. Food hypersensitivity disorders can be classified broadly into three main categories: IgE-mediated, cell-mediated, and mixed disorders with each category having its own spectrum of clinical features. IgE-mediated food allergies typically manifest rapidly after food ingestion and can affect multiple organ systems such as the skin and respiratory system. They transpire when food-specific IgE antibodies residing on mast cells and basophils come into contact with and bind circulating food allergens. This will activate the cells to release potent mediators and cytokines. Conversely, non-IgE-mediated food allergies present as more subacute or chronic symptoms that most commonly target the gastrointestinal tract. The precise etiology of primary EC is unclear. There is undoubtedly an interaction between genetic and environmental factors as 16% of patients with EGID have family member with a similar disorder. Further, an allergic component is likely in EGIDs: 80% have a coexistent atopic disease while 62% experience specific food sensitivities [Guajardo et al. 2002]. The eosinophilic proctocolitis that develops in infants is a product of being breast fed or receiving a protein hydrolysate formula [Guajardo and Rothenberg, 2003; Hill and Milla, 1990]. Thus, the bloody diarrhea in infants with EC appears to represent an allergic colitis (dietary protein-induced proctocolitis of infancy syndrome) [Chang et al. 2002; Machida et al. 1994]. EGIDs in general have characteristics that fall between predominantly an IgE-mediated food allergy and cell-mediated hypersensitivity [Rothenberg, 2004]. In support of the role of IgE in EC, there is mast cell accumulation and degranulation in colonic tissue [Inamura et al. 2006]. Some studies, however, suggest that circulating lymphocytes sensitive to the food antigens produce the clinical symptoms in infants with this food protein hypersensitivity. Although an allergen-free diet represents one therapeutic strategy for EGIDs, only occasionally is this successful. In adults, food-related anaphylaxis is uncommon. Hence, EC in adults is more likely a non-IgE-associated disorder, acting through a CD4(+) Th2 lymphocyte-mediated mechanism [Kweon et al. 2000; Van Sickle et al. 1985].
Clinical presentation and diagnosis of eosinophilic colitis
EC is a heterogeneous entity that affects infants and adults with the only characteristic feature being an intense eosinophilic infiltration in the colon that can be segmental or diffuse. Like EG, the presentation of EC tends to be dependent on which intestinal layer is most affected by the eosinophilic infiltration. Mucosa predominant disorder is associated with mucosal injury and presents with malabsorption, diarrhea, and protein-losing enteropathy. Transmural disease presents with colonic wall thickening and features of intestinal obstruction. Eosinophilic predominant ascites (up to 95% eosinophils) is a manifestation of serosal involvement [Ong et al. 2002; Kravis et al. 1982]. EC can present acutely with abdominal symptoms such as cecal volvulus causing intestinal obstruction [Velchuru et al. 2007], intussusception, and perforation (Table 2).
Table 2.
Possible clinical presentations of eosinophilic colitis.
| • History of allergy or atopy |
| • Abdominal pain |
| • Weight loss |
| • Diarrhea |
| Bloody |
| Nonbloody |
| • Malabsorption |
| • Peripheral eosinophilia |
| • Eosinophilic ascites |
| • Protein-losing enteropathy |
| • Intestinal obstruction |
| Volvulus |
| Intussusceptions |
| • Colonic thickening |
| • Colonic perforation |
Colon eosinophilic infiltrate can also occur secondarily to several conditions such as parasitic helminthic infections with Strongyloides stercoralis, Enterobius vermicularis, and Trichuris trichiura (whipworm) [Chandrasekhara et al. 2007; Macedo and MacCarty, 2000; Alsamman et al. 1999; Cacopardo et al. 1997] and secondary to drugs such as clozapine [Ong et al. 2002], carbamazepine [Velchuru et al. 2007], rifampicin, gold [Lange et al. 1994; Martin et al. 1981], and naproxen [Jimenez-Saenz et al. 2006; Bridges et al. 1990]. Liver transplant recipients maintained on tacrolimus as an immunosuppressive agent, are at risk of developing colonic eosinophilia; symptomatic improvement occurs when the immunosuppression is reduced [Saeed et al. 2006]. A dense eosinophilic infiltrate may initially be present in ulcerative colitis, mimicking EC and implicating a pathogenetic role for the eosinophil in inflammatory bowel disease [Uzunismail et al. 2006]. Other associations are rare and include vasculitis (e.g. Churg–Strauss syndrome). High serum IgE level (1300 U/ml) and marked submucosal infiltration with eosinophils arise in the Tolosa–Hunt syndrome, a rare neurological disorder characterized by headaches, ophthalmoplegia, and cranial nerve palsies [Kosugi et al. 2003]. Primary EC remains therefore a diagnosis of exclusion (Table 3).
Table 3.
Differential diagnosis of eosinophilic colitis.
| • Parasitic colitis |
| • Eosinophilic gastroenteritis |
| • Hypereosinophilic syndrome |
| • Inflammatory bowel disease |
| • Drug-induced colitis: |
| Nonsteroidal anti-inflammatory drugs |
| Rifampicin |
| Clozapine |
| Tacrolimus |
| Gold |
| • Allogenic bone marrow transplant |
| • Others: Tolosa–Hunt syndrome, vasculitis |
| (e.g. Churg–Strauss syndrome) |
| • Acute radiation colitis |
Allergy evaluation includes skin prick tests (SPTs) and radioallergosorbent tests (RASTs) to detect IgE antibody specific to inhaled and ingested allergens. As allergic tests in general have a high rate of false-positive results and lack sensitivity and specificity, the results of any allergy test should be interpreted cautiously. In IgE-mediated disorders (e.g. some infants with eosinophilic proctocolitis), negative SPT responses are extremely useful to confirm the absence of IgE-mediated food allergies, whereas a positive skin test suggests the presence of clinical food allergy. This makes SPTs a useful tool for infants with eosinophilic proctocolitis disease. A positive skin test however is best considered confirmatory when combined with a recent and clear-cut history of a food-induced allergic reaction to the food in question. Lastly, an eosinophilic infiltrate can be a part of acute radiation injury, developing after short-term radiotherapy for prostatic, uterine, or rectal carcinomas [Navajas León et al. 2005; Leupin et al. 2002; Dalal et al. 1992].
In patients with EC, endoscopy may reveal edematous mucosa with a loss of the normal vascular pattern, patchy erythematous changes, and even superficial ulcerations (Figure 1). Colonic mucosal biopsies will typically show sheets of eosinophils infiltrating the lamina propria with extension through the muscularis mucosa into the submucosa, which may be edematous. Occasionally eosinophils appear within the muscularis propria. Crypt abscesses and lymphonodular hyperplasia may also be evident [Guajardo and Rothenberg, 2003; Schoonbroodt et al. 1995; Hill and Milla, 1990; Van Sickle et al. 1985] (Figure 2). In the healthy colon, eosinophil counts range from 5 up to 35 per high-powered field, with a proximal to distal gradient along the colon [Lowichik and Weinberg, 1996]. Hence, the diagnosis is contingent upon histological evidence of excessive eosinophilic infiltration of one or more segments of the gastrointestinal tract without evidence of helminthic or other underlying disease [Blackshaw and Levison, 1986; Spry, 1984; Cello, 1979; Klein et al. 1970]
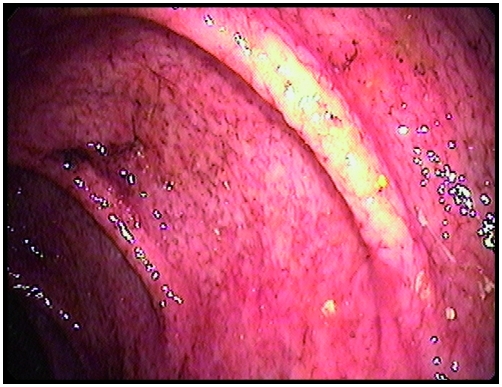
Figure 1.
Transverse colon at colonoscopy in a patient with eosinophilic colitis showing mucosal abnormalities with loss of the vascular pattern (edema), erythema, and aphthous ulceration.
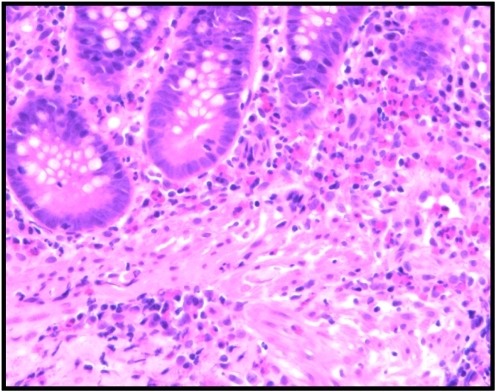
Figure 2.
Colonic biopsy of a patient with eosinophilic colitis showing sheets of eosinophils within the lamina propria, extending into the muscularis mucosa (H&E x 200).
Eosinophilic proctocolitis (allergic proctocolitis or milk-protein proctocolitis) is an entity that has been described classically in infants associated with ingestion of cow's milk and soy proteins [Lake, 2000; Raafat et al. 1990]. Thus, a bimodal age distribution of EC is apparent: an infantile form presenting at 60 days and an adolescent form later in life [Odze et al. 1995]. Infants typically have normal to soft stools with blood streaks that arises during the first 2–3 months of age. The onset of blood-tinged stools is initially erratic over several days in an otherwise healthy infant, and then wanes. In some, bloody diarrhea is pronounced and can lead to anemia. Weight gain and growth however are typically normal; the infants appear quite healthy. The abdominal examination is usually within normal limits. Fecal smear tends to show an increased polymorphonuclear cell count. Flexible sigmoidoscopy might reveal modest acute inflammation with focal erythema and friability. Mucosal biopsies are more definitive, demonstrating a dense eosinophilic infiltration of the colonic epithelium, the lamina propria, and/or even the muscularis; this can be diffuse or focal. Lymphoid nodules are frequently associated with the eosinophilic infiltration of the colonic wall, best demonstrated by cross-sectional imaging.
Treatment of eosinophilic colitis
Diet
The strong association of EGIDs with food allergies has prompted use of elimination or even elemental diets. Elemental (essential) diets contain simplified ingredients that are readily assimilated without further digestion in the form of amino acids, along with hydrolyzed fats and carbohydrates. Being devoid of whole or partial proteins, such a fundamental diet should eliminate the risk of an allergic food reaction in susceptible individuals. Elimination diets are designed to temporarily avoid certain foods for 2 weeks (the elimination phase), followed by a period of systematic reintroduction of these food groups (reintroduction phase). The purpose of this dietary protocol is to identify possible food sensitivities and the process may last for up to 9 weeks. Elimination diets include: those removing one or several whole food proteins; semi-elemental formulas; oligoantigenic diets that allow a choice of those foods (e.g. apple, corn, broccoli, sugar, salt, sweet potato, olive oil, lamb) being tested as unlikely food allergens; and lastly, the amino acid formulas in elemental form. Identification of an allergic basis is possible through a trial of an exclusively elemental diet for several weeks that yields symptomatic relief. The drawback of elemental diets is their reduced palatability making them poorly tolerated over prolonged time periods.
Food hypersensitivities prevalence is greatest in the first few years of life, affecting some 6% of infants less than 3 years of age [Bock, 1987]. Virtually all infants who have cow's milk allergy manifest it within the first year of life. Clinical tolerance then develops in about 80% by 5 years of age [Sampson, 1999]. Sixty percent of infants with cow's milk allergy experience IgE-mediated reactions; 25% of these infants retain their sensitivity into the second decade of life [Host et al. 1995]. As certain proteins appear to trigger the development of eosinophilic proctocolitis in infants, elemental diets, at least in case reports, effect a marked clinical improvement, once the critical proteins are withdrawn [Guajardo and Rothenberg, 2003; Hill and Milla, 1990]. Therefore, it is important to eliminate any known provocative factors triggering the disorder. Eliminations diet improve steroid dependency and reverse growth failure in infants diagnosed with EG [Justinich et al. 1996]. This suggests that such elimination diets might also benefit adults with EC, although treatment of EC in older individuals usually requires medical management because IgE-associated triggers are rarely identified. Thus, as EC in infants is a rather benign entity and is food-associated, dietary elimination of the offending protein often resolves the disorder with days. Adolescent or older patients require more aggressive medical management.
Glucocorticoids and azathioprine
The beneficial effects of corticosteroids in eosinophilic disorders are largely mediated by inhibition of eosinophil growth factors: interleukin-3 (IL-3), interleukin-5 (IL-5), and granulocyte-macrophage colony-stimulating factor (GM-CSF). There are no randomized controlled trials to date on the efficacy of steroids in EGIDs or specifically in EC. Most studies employ oral prednisone dosages similar to those used in inflammatory bowel disease (i.e. 1–2 mg/kg per day) for 8 weeks, which is then tapered over 6–8 weeks, improving both clinical symptoms and pathological findings in patients with EC. Those with a more relapsing disorder may require long-term, low-dose corticosteroids for symptomatic benefit [Chen et al. 2003]. Alternatively, budesonide used at 6 mg/day PO has been reported to induce and then maintain remission for up to 2 years [Tan et al. 2001]. Secondary causes of EC (e.g. from helminthic infections or drugs) must be excluded prior to using corticosteroids to avoid exacerbating the underlying problem.
It is likely that eosinophil-active cytokines such as IL-3, IL-5 and GM-CSF play pivotal roles in eosinophilic disorders. Chemokines such as eotaxin may be involved in eosinophil recruitment. This provides the rationale for using immunomodulatory agents such as azathioprine or 6-mercaptopurine to down regulate or inhibit these mediators, resulting in less eosinophilic infiltration and symptomatic improvement. Immunomodulatory agents are worth trying in severe, refractory, or steroid-dependent EC [Rothenberg, 2004]. A combination of glucocorticosteroids and azathioprine can decrease the tissue eosinophilia and improve the diarrhea at least in EG [Copeland et al. 2004; Clouse et al. 1992].
Leukotriene receptor antagonists
Montelukast (Singulair ®) selectively blocks the action of leukotriene D4 (LTD4) on the cysteinyl leukotriene receptor Cys-LT1. This abrogates the recruitment and chemoattraction of eosinophils to many tissues including the gastrointestinal tract. In steroid-dependant patients with eosinophilic gastroenteritis, montelukast can successfully maintain them in clinical remission, yielding a safe and effective steroid-sparing therapy [Gonsalves, 2007; Friesen et al. 2004]. Montelukast at 10–40 mg for several months improves peripheral eosinophilia and some gastroduodenal symptoms in children with duodenal eosinophilia [Friesen et al. 2004]. The role of montelukast has yet to be evaluated in EC, particularly in terms of any steroid-sparing benefit.
Antihistamines and mast cell stabilizers
Ketotifen, a second-generation H1-antihistamine, blocks the calcium channel that is essential for mast cell degranulation, thus stabilizing the cell and thereby preventing the release of histamine. A course of 12 months is a safe and effective alternative to traditional systemic corticosteroids therapy in EG [Melamed et al. 1991].
Sodium cromoglycate, a mast cell stabilizer, alone or when combined with ketotifen, may have some promise as induction and maintenance therapy for EGID patients [Perez-Millan et al. 1997], but is not useful in eosinophilic esophagitis [Liacouras et al. 2005]. There are as yet no reports concerning the treatment of EC with antihistamines.
Emerging therapies with biological agents
Novel approaches to EGIDs are focusing on biologics, humanized monoclonal antibodies developed against targeted inflammatory mediators. Omalizumab, a recombinant, DNA-derived, humanized IgG1k monoclonal antibody, inhibits the binding of immunoglobulin E to the high-affinity IgE receptor, Fc epsilon R1 (FcεRI), and thus prevents an inadvertent anaphylactic reaction by limiting the degree of mediator release [Foroughi et al. 2007]. Omalizumab when given every 2 weeks for up to 8 weeks reduces absolute eosinophil count after 3–4 months of therapy and provides symptomatic improvement in EGIDs. Mepolizumab, a humanized monoclonal antibody directed against IL-5, a key cytokine involved in maturation, proliferation, and survival of eosinophils, shows promise; this antagonist at 750 mg IV every 2 weeks for 16 weeks significantly reduces tissue eosinophils in EE [Foroughi et al. 2007; Stein et al. 2006]. Further clinical studies are needed to fully validate such experimental approaches.
Prognosis for eosinophilic colitis
The EC that develops in infancy carries a good prognosis. It tends to spontaneously resolve, often within days. After a few years these young children can even tolerate the implicated foods. For young adults with EC, its natural history tends to become chronic with periods of activity and periods of apparent remission.
Summary
Primary EC, an emerging disorder within the primary EGIDs, although distinctly uncommon, has become better defined over the past decade. Its pathophysiology, clinical features, and natural history differ according to the age of presentation: being rather mild, self-limited, and more food-related in infants but chronic in young adults. The mode of presentation, especially in adults, depends on the colonic layer being predominantly infiltrated with eosinophils. Diagnosing primary EC is based on colonic biopsies, a situation that is especially challenging in the absence of diagnostic criteria and requires careful elimination of secondary causes. Therapeutic approaches towards EC are based on case reports and small case series but randomized controlled trials are needed to establish the best therapeutic approach.
Acknowledgment
The authors recognize the histopathology expertise and advice provided by Dr Stefan Urbanski.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- Alsamman M., Haque S., Long J.D. (1999) Strongyloidiasis colitis: a case report and review of the literature. J Clin Gastroenterol 28: 77–80 [DOI] [PubMed] [Google Scholar]
- Arora A.S., Yamazaki K. (2004) Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol 2: 523–530 [DOI] [PubMed] [Google Scholar]
- Blackshaw A.J., Levison D.A. (1986) Eosinophilic infiltrates of the gastrointestinal tract. J Clin Pathol 39: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock S.A. (1987) Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics 79: 683–688 [PubMed] [Google Scholar]
- Bridges A.J., Marshall J.B., az-Arias A.A. (1990) Acute eosinophilic colitis and hypersensitivity reaction associated with naproxen therapy. Am J Med 89: 526–527 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel-Koomen C., Ortolani C., Aas K., Bindslev-Jensen C., Bjorksten B., Moneret-Vautrin D., et al. (1995) Adverse reactions to food. European Academy of Allergology and Clinical Immunology Subcommittee. Allergy 50: 623–635 [DOI] [PubMed] [Google Scholar]
- Cacopardo B., Onorante A., Nigro L., Patamia I., Tosto S., Romano F., et al. (1997) Eosinophilic ileocolitis by Enterobius vermicularis: a description of two rare cases. Ital J Gastroenterol Hepatol 29: 51–53 [PubMed] [Google Scholar]
- Cello J.P. (1979) Eosinophilic gastroenteritis—a complex disease entity. Am J Med 67: 1097–1104 [DOI] [PubMed] [Google Scholar]
- Chandrasekhara V., Arslanlar S., Sreenarasimhaiah J. (2007) Whipworm infection resulting in eosinophilic colitis with occult intestinal bleeding. Gastrointest Endosc 65: 709–710 [DOI] [PubMed] [Google Scholar]
- Chang J.W., Wu T.C., Wang K.S., Huang I.F., Huang B., Yu I.T. (2002) Colon mucosal pathology in infants under three months of age with diarrhea disorders. J Pediatr Gastroenterol Nutr 35: 387–390 [DOI] [PubMed] [Google Scholar]
- Chen M.J., Chu C.H., Lin S.C., Shih S.C., Wang T.E. (2003) Eosinophilic gastroenteritis: clinical experience with 15 patients. World J Gastroenterol 9: 2813–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse R.E., Alpers D.H., Hockenbery D.M., Schryver-Kecskemeti K. (1992) Pericrypt eosinophilic enterocolitis and chronic diarrhea. Gastroenterology 103: 168–176 [DOI] [PubMed] [Google Scholar]
- Copeland B.H., Aramide O.O., Wehbe S.A., Fitzgerald S.M., Krishnaswamy G. (2004) Eosinophilia in a patient with cyclical vomiting: a case report. Clin Mol Allergy 2: 7–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal B.I., Das K.C., Dutta T.K., Malakar K. (1992) Local and systemic eosinophilia in patients with carcinoma of the uterine cervix undergoing radiation therapy: correlation with radiation response. Clin Oncol (R Coll Radiol) 4: 18–21 [DOI] [PubMed] [Google Scholar]
- Foroughi S., Foster B., Kim N., Bernardino L.B., Scott L.M., Hamilton R.G., et al. (2007) Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol 120: 594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen C.A., Kearns G.L., Andre L., Neustrom M., Roberts C.C., bdel-Rahman S.M. (2004) Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J Pediatr Gastroenterol Nutr 38: 343–351 [DOI] [PubMed] [Google Scholar]
- Furuta G.T., Liacouras C.A., Collins M.H., Gupta S.K., Justinich C., Putnam P.E., et al. (2007) Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133: 1342–1363 [DOI] [PubMed] [Google Scholar]
- Gonsalves N. (2007) Food allergies and eosinophilic gastrointestinal illness. Gastroenterol Clin North Am 36: 75–91, vi [DOI] [PubMed] [Google Scholar]
- Guajardo J.R., Plotnick L.M., Fende J.M., Collins M.H., Putnam P.E., Rothenberg M.E. (2002) Eosinophil-associated gastrointestinal disorders: a world-wide-web based registry. J Pediatr 141: 576–581 [DOI] [PubMed] [Google Scholar]
- Guajardo J., Rothenberg M.E. (2003) Eosinophilic esophagitis, gastroenteritis, gastroenterocolitis, and colitis. Food Allergy: Adverse Reactions to Foods and Additives Blackwell Publishing: Oxford, 217–226 [Google Scholar]
- Hill S.M., Milla P.J. (1990) Colitis caused by food allergy in infants. Arch Dis Child 65: 132–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Host A., Jacobsen H.P., Halken S., Holmenlund D. (1995) The natural history of cow's milk protein allergy/intolerance. Eur J Clin Nutr 49(Suppl. 1): S13–S18 [PubMed] [Google Scholar]
- Inamura H., Kashiwase Y., Morioka J., Suzuki K., Igarashi Y., Kurosawa M. (2006) Accumulation of mast cells in the interstitium of eosinophilic colitis. Allergol Immunopathol (Madr) 34: 228–230 [DOI] [PubMed] [Google Scholar]
- Jimenez-Saenz M., Gonzalez-Campora R., Linares-Santiago E., Herrerias-Gutierrez J.M. (2006) Bleeding colonic ulcer and eosinophilic colitis: a rare complication of nonsteroidal anti-inflammatory drugs. J Clin Gastroenterol 40: 84–85 [DOI] [PubMed] [Google Scholar]
- Justinich C., Katz A., Gurbindo C., Lepage G., Chad Z., Bouthillier L., et al. (1996) Elemental diet improves steroid-dependent eosinophilic gastroenteritis and reverses growth failure. J Pediatr Gastroenterol Nutr 23: 81–85 [DOI] [PubMed] [Google Scholar]
- Khan S., Orenstein S.R. (2002) Eosinophilic gastroenteritis: epidemiology, diagnosis and management. Paediatr Drugs 4: 563–570 [DOI] [PubMed] [Google Scholar]
- Klein N.C., Hargrove R.L., Sleisenger M.H., Jeffries G.H. (1970) Eosinophilic gastroenteritis. Medicine (Baltimore) 49: 299–319 [DOI] [PubMed] [Google Scholar]
- Kosugi S., Date K., Minagawa M., Ishikawa H., Hatakeyama K., Endo K., et al. (2003) Eosinophilic colitis accompanied by Tolosa-Hunt syndrome: report of a case. J Gastroenterol 38: 613–614 [PubMed] [Google Scholar]
- Kravis L.P., South M.A., Rosenlund M.L. (1982) Eosinophilic gastroenteritis in the pediatric patient. Clin Pediatr (Phila) 21: 713–717 [DOI] [PubMed] [Google Scholar]
- Kweon M.N., Yamamoto M., Kajiki M., Takahashi I., Kiyono H. (2000) Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest 106: 199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake A.M. (2000) Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr 30(Suppl): S58–S60 [DOI] [PubMed] [Google Scholar]
- Lange P., Oun H., Fuller S., Turney J.H. (1994) Eosinophilic colitis due to rifampicin. Lancet 344: 1296–1297 [DOI] [PubMed] [Google Scholar]
- Leupin N., Curschmann J., Kranzbühler H., Maurer C.A., Laissue J.A., Mazzucchelli L. (2002) Acute radiation colitis in patients treated with short-term preoperative radiotherapy for rectal cancer. Am J Surg Pathol 26: 498–504 [DOI] [PubMed] [Google Scholar]
- Liacouras C.A., Spergel J.M., Ruchelli E., Verma R., Mascarenhas M., Semeao E., et al. (2005) Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 3: 1198–1206 [DOI] [PubMed] [Google Scholar]
- Lowichik A., Weinberg A.G. (1996) A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol 9: 110–114 [PubMed] [Google Scholar]
- Macedo T., MacCarty R.L. (2000) Eosinophilic ileocolitis secondary to Enterobius vermicularis: case report. Abdom Imaging 25: 530–532 [DOI] [PubMed] [Google Scholar]
- Machida H.M., Catto Smith A.G., Gall D.G., Trevenen C., Scott R.B. (1994) Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr 19: 22–26 [DOI] [PubMed] [Google Scholar]
- Martin D.M., Goldman J.A., Gilliam J., Nasrallah S.M. (1981) Gold-induced eosinophilic enterocolitis: response to oral cromolyn sodium. Gastroenterology 80: 1567–1570 [PubMed] [Google Scholar]
- Melamed I., Feanny S.J., Sherman P.M., Roifman C.M. (1991) Benefit of ketotifen in patients with eosinophilic gastroenteritis. Am J Med 90: 310–314 [PubMed] [Google Scholar]
- Navajas León F.J., Lucendo Villarín A.J., Erdozain Sosa J.C., Carrión Alonso G., Martín Chávarri S., Gómez Senent S., et al. (2005) Eosinophilia and actinic enteritis due to radiotherapy for prostatic adenocarcinoma. Rev Esp Enferm Dig 97: 759–761 [DOI] [PubMed] [Google Scholar]
- Odze R.D., Wershil B.K., Leichtner A.M., Antonioli D.A. (1995) Allergic colitis in infants. J Pediatr 126: 163–170 [DOI] [PubMed] [Google Scholar]
- Ong G.Y., Hsu C.C., Changchien C.S., Lu S.N., Huang S.C. (2002) Eosinophilic gastroenteritis involving the distal small intestine and proximal colon. Chang Gung Med J 25: 56–61 [PubMed] [Google Scholar]
- Perez-Millan A., Martin-Lorente J.L., Lopez-Morante A., Yuguero L., Saez-Royuela F. (1997) Subserosal eosinophilic gastroenteritis treated efficaciously with sodium cromoglycate. Dig Dis Sci 42: 342–344 [DOI] [PubMed] [Google Scholar]
- Prasad G.A., Alexander J.A., Schleck C.D., Zinsmeister A.R., Smyrk T.C., Elias R.M., et al. (2009) Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 7: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raafat F., Castro R., Booth I.W. (1990) Eosinophilic proctitis with giant cells: a manifestation of cow's milk protein intolerance. J Pediatr Gastroenterol Nutr 11: 128–132 [DOI] [PubMed] [Google Scholar]
- Rothenberg M.E. (2004) Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 113: 11–28 [DOI] [PubMed] [Google Scholar]
- Saeed S.A., Integlia M.J., Pleskow R.G., Calenda K.A., Rohrer R.J., Dayal Y., et al. (2006) Tacrolimus-associated eosinophilic gastroenterocolitis in pediatric liver transplant recipients: role of potential food allergies in pathogenesis. Pediatr Transplant 10: 730–735 [DOI] [PubMed] [Google Scholar]
- Sampson H.A. (1999) Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol 103: 717–728 [DOI] [PubMed] [Google Scholar]
- Schoonbroodt D., Horsmans Y., Laka A., Geubel A.P., Hoang P. (1995) Eosinophilic gastroenteritis presenting with colitis and cholangitis. Dig Dis Sci 40: 308–314 [DOI] [PubMed] [Google Scholar]
- Spry, C.J.F. (1984) Eosinophilic gastroenteritis. In: Bouchier, I.A.D., Allan, R.N., Hodgson, H.J.F. et al. (eds), Textbook of Gastroenterology. Balliere Tindall: London, pp. 596–598.
- Stein M.L., Collins M.H., Villanueva J.M., Kushner J.P., Putnam P.E., Buckmeier B.K., et al. (2006) Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol 118: 1312–1319 [DOI] [PubMed] [Google Scholar]
- Tan A.C., Kruimel J.W., Naber T.H. (2001) Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur J Gastroenterol Hepatol 13: 425–427 [DOI] [PubMed] [Google Scholar]
- Uzunismail H., Hatemi I., Dogusoy G., Akin O. (2006) Dense eosinophilic infiltration of the mucosa preceding ulcerative colitis and mimicking eosinophilic colitis: report of two cases. Turk J Gastroenterol 17: 53–57 [PubMed] [Google Scholar]
- Van Sickle G.J., Powell G.K., McDonald P.J., Goldblum R.M. (1985) Milk- and soy protein-induced enterocolitis: evidence for lymphocyte sensitization to specific food proteins. Gastroenterology 88: 1915–1921 [DOI] [PubMed] [Google Scholar]
- Velchuru V.R., Khan M.A., Hellquist H.B., Studley J.G. (2007) Eosinophilic colitis. J Gastrointest Surg 11: 1373–1375 [DOI] [PubMed] [Google Scholar]