Abstract
The rationale for specific pharmacologic therapy in nonalcoholic steatohepatitis (NASH) is determined by the potential for disease progression and the difficulties, in many patients, of successfully implementing diet and lifestyle changes over the long term. Owing to their ability to correct insulin resistance, insulin-sensitizing agents are attractive candidates for the treatment of NASH. In this review we provide an insight into the mechanism of action, therapeutic efficacy and safety issues regarding the use of glitazones in NASH.
Keywords: nonalcoholic steatohepatitis, steatosis, fibrosis, insulin resistance, glitazones, insulin sensitizing drugs
Introduction
Nonalcoholic steatohepatitis (NASH) is becoming the leading cause of chronic liver disease and a major health issue owing to its close association with the worldwide epidemics of obesity and diabetes. A significant proportion of patients can experience disease progression with the occurrence of cirrhosis, hepatocellular carcinoma [Ascha et al. 2010; Starley et al. 2010; Paradis et al. 2009; Bugianesi et al. 2002; Ratziu et al. 2002] and end-stage liver disease [Nayak et al. 2010; Caldwell and Crespo, 2004]. This results in an increase in the overall and liver-related mortality [Soderberg et al. 2010; Gastaldelli et al. 2009b; Ong et al. 2008; dam-Larsen et al. 2004]. Patients at risk of disease progression need to be identified as not all individuals with metabolic risk factors will experience disease progression [Ratziu et al. 2010a]. Prognostic markers have mostly been derived from histological studies and found that the degree of inflammation is the strongest independent predictor for fibrosis progression [Argo et al. 2009].
Insulin resistance is an almost universal finding in primary NASH. It is the main driving force behind excessive fat accumulation in the liver but may also play a role in the initiation and perpetuation of steatohepatitis and fibrosis progression [Fabbrini et al. 2009; Fracanzani et al. 2008; Marchesini et al. 2003]. Moreover, hepatic steatosis and insulin resistance potentiate each other [Malhi and Gores, 2008; Yamaguchi et al. 2007; Bugianesi et al. 2005a]. A current model for the pathogenesis of NASH is centered on lipotoxicity [Neuschwander-Tetri, 2010a] which states that the influx of fatty acids and their derivatives through the liver induces apoptosis, oxidative stress, reticulum endoplasmic stress, activation of proinflammatroy pathways and ultimately liver cell injury. The main source of free fatty acids (FFA) reaching the liver is an uncontrolled release from insulin-resistant adipose tissue [Cusi, 2009; Petta et al. 2009]. Therefore, correcting insulin resistance, particularly at the adipose level, is a relevant aim and most therapeutic trials have focused on insulin sensitizers [Ratziu and Zelber-Sagi, 2009].
The aim of this report is to review the existing trials with glitazones in NASH with a focus on their histological, biochemical and metabolic effects.
Mechanism of action
Glitazones are agonists of PPARγ (peroxisome proliferators-activated receptor gamma) nuclear receptors, developed for the treatment of type 2 diabetes and which have been on the market for almost a decade.
The PPARγ is a member of the nuclear protein receptor superfamily which regulates the transcription of genes involved in lipid metabolism and plays a role in increasing insulin sensitivity as well as in promoting fatty acid uptake into adipocytes and adipocyte differentiation [Sharma and Staels, 2007]. PPARγ receptors are located predominantly in adipose tissue, but can be found elsewhere, to include pancreatic β cells, vascular endothelium and, to a lesser extent, in liver and skeletal muscle. Two isoforms are recognized, PPARγ1 and the less abundant PPARγ2. Obese patients have an increased expression of PPARγ2 in the adipose tissue [Sharma and Staels, 2007].
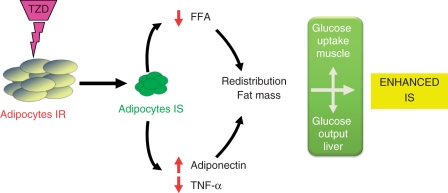
Glitazones promote the differentiation of large, insulin-resistant adipocytes into small, metabolically active, insulin-sensitive adipocytes (Figure 1). Data suggest that thiazolidinediones (TZDs) decrease FFA influx to the liver, decrease tumor necrosis factor alpha (TNFα) and resistin expression and increase adiponectin production [Gastaldelli et al. 2009a]. Increased adiponectine expression results in reduced hepatic gluconeogenesis and improved hepatic fatty acid oxidation (via increased AMP-activated protein kinase [AMPK]). Adiponectine also reduces inflammation by blocking nuclear factor κB (NFkB) and suppress hepatic stellate cell proliferation [Bugianesi et al. 2005b]. The net result is an increase in the storage of fatty acids in adipose tissue, a reduction of hepatic glucose production and a higher uptake of the glucose in the muscles. This redistribution of fat from ectopic tissue (liver, muscle) to the adipose tissue is probably the main determinant of the insulin-sensitizing action of this class of drugs.
Figure 1.
Mechanism of action of glitazones. Glitazones promote the differentiation of small, metabolically active, insulin-sensitive (IS) adipocytes from large, insulin-resistant (IR) adipocytes. This results in decreased free fatty acids (FFA) influx to the liver, decreased tumor necrosis factor alpha (TNFα) expression, increased adiponectin production and redistribution of fat mass. The consequence is an increase in the storage of fatty acids in adipose tissue, a reduction of hepatic glucose production and a higher uptake of the glucose in the muscles which results in enhanced insulin sensitivity.
Recent evidences suggest that there also are differences in lipoprotein metabolism between the two TZDs (rosiglitazone and pioglitazone), attributable to an additional PPARα activity of pioglitazone, not shared by rosiglitazone. This results in increased fatty acid oxidation and decreased hepatic de novo lipogenesis, which may explain, at least in part, the positive cardiovascular effects (improved carotid intimal medial thickness and coronary atheroma volume) with pioglitazone [Nissen et al. 2008; Betteridge, 2007; Mazzone et al. 2006].
Efficacy of glitazones in human adult NASH
Both TZDs have been studied in prospective placebo-controlled trials ranging from 6 to 24months duration. Here we discuss trials of pioglitazone and rosiglitazone, in patients with histologically proven NASH and an end-of-treatment liver biopsy. Seven trials were selected: five randomized controlled trials (RCTs) [Sanyal et al. 2010, 2004; Aithal et al. 2008; Ratziu et al. 2008; Belfort et al. 2006] and two open-label trials [Promrat et al. 2004; Neuschwander-Tetri et al. 2003] (Table 1).
Table 1.
Glitazone trials with available end-of-treatment histology.
| Trial | Drug (dose/day), n | Comparator, n | Treatment duration | End of treatment histology, n (drug/ comparator) | Percentage with diabetes | Normal ALT included | Professional diet counseling* | Run-in period |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Sanyal et al. 2004] | Pioglitazone (30 mg) + Vit E, N = 10 | Vit E, N = 10 | 6 months | 18 (9/9) | 0% | Yes | Yes | Yes (no results) |
| Belfort et al. [2006] | Pioglitazone (45 mg) N = 29 | Placebo N = 24 total 55) | 6 months | 47 (26/21) | 100% | No | Yes | Yes |
| Ratziu et al. [2008] | Rosiglitazone (8 mg), N = 32 | Placebo, N = 32 | 1 year | 63 (32/31) | 33% | No | No | No |
| Aithal et al. [2008] | Pioglitazone (30 mg), N = 37 | Placebo, N = 37 | 1 year | 61 (31/30) | 0% | Yes | No | Yes (no results) |
| Sanyal et al. [2010] | Pioglitazone (30 mg), N = 80 | Placebo, N = 84, | 2 years | 142 (70/72) | 0% | Yes | No | No |
| Open label trials | ||||||||
| Neuschwander-Tetri et al. [2003] | Rosiglitazone (8 mg), N = 25 | None | 12 months | 22 | 24% | Yes | No | No |
| Promrat et al. [2004] | Pioglitazone (30 mg), N = 18 | None | 1 year | 18 | 0% | No | No | Yes |
That is, follow-up visits with a dietician.
Vit, vitamin; ALT, alanine transaminase.
Adapted from Ratziu et al. [2010b] and Ratziu and Zelber-Sagi [2009], with permission.
Biochemical response
One of the most reproducible effects of glitazones is a reduction of aminotransferase levels by 30–58%. This occurs early on with therapy (starting at month 4 and almost complete by month 6) and is sustained throughout the treatment period. ALT normalization was reported in 38–100% of patients. This effect is short lived, since after drug discontinuation a return to baseline levels usually occurs within 3 months [Ratziu et al. 2008]. Longer treatment (>1 year) does not seem to have additional beneficial effects [Sanyal et al. 2010; Ratziu et al. 2010c]. However, reported trials are heterogeneous in terms of baseline ALT. Patients with normal ALT have been included in some of them [Sanyal et al. 2010, 2004; Aithal et al. 2008] (three of five RCTs) which could reduce the magnitude of the biochemical effect.
Histological response
Steatosis
Steatosis is the histologic feature most reliably improved by glitazones and was reported in all except one trial [Aithal et al. 2008]. In the one negative trial, 26% of patients had minimal steatosis at baseline (5–25%), making differences between groups harder to detect [Aithal et al. 2008]. An improvement in steatosis was reported in 47–65% of treated patients, but the magnitude of this effect was reported in only a few trials. With rosiglitazone it was 20% ranging from 30% to 60% in responders [Ratziu et al. 2008].
Necroinflammatory activity
The impact of glitazones on inflammation, ballooning and fibrosis is summarized in Table 2. Improvement of inflammation is of particular importance, since a recent study found that inflammation was the only independent predictor for fibrosis progression [Argo et al. 2009]. In RCTs, regression of inflammatory lesions was statistically significant only in two trials with pioglitazone [Sanyal et al. 2010; Belfort et al. 2006]. In one study using a lower dose of pioglitazone (30 mg/day), the improvement versus placebo was not significant after 1 year of therapy [Aithal et al. 2008]. The only RCT with rosiglitazone did not find significant changes in lobular inflammation after 1 or 2 years of therapy [Ratziu et al. 2010c, 2008]. Portal inflammation was either unchanged or worsened in one study with rosiglitazone [Neuschwander-Tetri et al. 2003].
Table 2.
Outcomes of glitazone studies for inflammation, liver cell injury and fibrosis.
| Trial | Drug | Dose/duration | LOBULAR INFLAMMATION |
BALLOONING |
FIBROSIS |
|||
|---|---|---|---|---|---|---|---|---|
| Intragroup change | Change vs. comparator | Intragroup change | Change vs. Comparator | Intragroup change | Change vs. Comparator | |||
| (trend, p) | (trend, p) | (trend, p) | (trend, p) | (trend, p) | (trend, p) | |||
| Neuschwander-Tetri et al. [2003] | Rosiglitazone | 8 mg/12 months | − | − | I, 0.003 | − | I-PSF | − |
| Promrat et al. [2004] | Pioglitazone | 45 mg/12 months | I, <0.001 | − | I, 0.001 | − | I, 0.04 | − |
| Sanyal et al. [2004] | Pioglitazone | 30 mg/6 months | − | − | I, 0.01 | NC | NC | NC |
| Belfort et al. [2006] | Pioglitazone | 45 mg/6 months | I, <0.001 | I, 0.008 | I, 0.001 | I, <0.02 | I, 0.002 | NC, 0.08 |
| Ratziu et al. [2008] | Rosiglitazone | 8 mg/12 months | NC | NC | NC | NC | NC | NC |
| Aithal et al. [2008] | Pioglitazone | 30 mg/12 months | I, 0.04 | NC | NC, 0.09 | I, 0.005 | I, 0.006 | I, 0.05 |
| Sanyal et al. [2010] | Pioglitazone | 30 mg/2 years | I, − | I, <0.001 | I,− | I, 0.01 | NC | NC |
I, improved; NC no change -, not available; PSF, Zone 3 perisinusoidal fibrosis.
Adapted from Ratziu et al. [2010b], with permission.
Ballooning improved in 32–54% of patients which was significantly more than placebo in two RCTs with pioglitazone [Aithal et al. 2008; Belfort et al. 2006]. Rosiglitazone improved ballooning in a small uncontrolled trial [Neuschwander-Tetri et al. 2003], but no significant improvement was found in a large RCT trial after 1 or 2 years of therapy [Ratziu et al. 2010c, 2008]. Very few studies have reported on changes in the recently described NAS score. In a 6-month study [Belfort et al. 2006], the score improved in 46% of pioglitazone-treated patients by at least 2 points versus 14% in the placebo group (p = 0.02) although this could be caused by the improvement in steatosis that is a part of the score. A 1-year study with rosiglitazone failed to show significant changes in the NAS score [Ratziu et al. 2008] while a 2-year study with pioglitazone improved the NAS score significantly more often than placebo [Sanyal et al. 2010]. In this latter trial [Sanyal et al. 2010] pioglitazone failed to reach the primary endpoint, a complex composite score, at a predefined level of statistical significance of 0.025. However, the negative results of this study on the primary endpoint should be regarded with caution, because 28% of patients in the pioglitazone arm did not have ballooning on the central pathological review performed at the time of final analysis. Thus, it can be argued that some of the included patients did not have sufficient histological criteria for NASH. Further sensitivity analyses have shown that in the subset of patients with well-defined NASH, pioglitazone improved histology (except for fibrosis) including the composite endpoint more often than placebo.
Fibrosis
No study conclusively demonstrated an improvement in fibrosis. In three trials, including an uncontrolled one, improvement of fibrosis was seen in 29–61% of patients taking pioglitazone [Aithal et al. 2008; Belfort et al. 2006; Promrat et al. 2004]. Despite an end of treatment reduction, when compared with changes in placebo/control arms, improvement of fibrosis was still significant in only one study with a marginal level of statistical significance. No significant improvement of fibrosis was seen with rosiglitazone [Ratziu et al. 2010c, 2008] even after prolonged therapy, and when measured by micromorphometry, a more sensitive and quantitative technique. Thus, it becomes improbable that the lack of effect seen after 1 year could bedue to the short treatment period. Only two studies assessed changes in perisinusoidal fibrosis, one documenting no change [Ratziu et al. 2008] and the other an improvement in 35% [Neuschwander-Tetri et al. 2003].
Overall, data available so far show that the insulin-sensitizing effect of glitazones is associated with a significant improvement of aminotransferases and steatosis, most probably of inflammation but no effect on fibrosis [Ratziu et al. 2010b]. It is less likely that these results are influenced by sampling variability of liver biopsy, since in controlled trials sampling variability should affect the active treatment and placebo arms equally. Reduction in liver fat has important clinical implications, both hepatic and extrahepatic. Steatosis is the prerequisite in the sequential multistep process that leads to NASH and its progression. Steatosis is the trigger for lipid peroxidation and oxidative stress which contributes to the necroinflammatory lesions associated with steatohepatitis. It is also associated with increased hepatic expression of proinflammatory cytokines and mitochondrial dysfunction which results in increased apoptosis and decreased energy stores [Neuschwander-Tetri, 2010b]. Moreover, hepatic stellate cell activation by reactive oxygen species and lipid peroxidation products is an essential trigger for fibrogenesis [Feldstein et al. 2005]. Extrahepatic consequences of liver fat are also important since steatosis per se might aggravate hepatic insulin resistance independently of central fat. However, this beneficial effect of reduction in liver fat has been challenged by some animal studies showing that liver fat is not all bad. Blocking esterification of fatty acids into triglycerides resulted in a higher level of hepatic oxidative stress, inflammation, cell injury and fibrosis in animals fed a methionine choline-deficient (MCD) diet [Yamaguchi et al. 2007]. These observations suggest that triglycerides synthesis per se is not harmful to hepatocytes, but it rather provides a useful mechanism for protecting the liver from lipotoxicity.
Assessing the durability of the histological response to glitazone therapy is difficult since no histological follow up is available except for a series of nine pioglitazone-treated patients [Lutchman et al. 2007]. Histological criteria of steatohepatitis (steatosis, lobular inflammation, ballooning), which disappeared in all but one patient after 1 year of pioglitazone therapy, relapsed in most of the patients 1 year after treatment discontinuation. There was no worsening in fibrosis with this short follow up. Similarly, in the open-label FLIRT-2 trial, an additional 2 years of treatment with rosiglitazone did not further improve liver histology, despite a continued improvement in insulin sensitivity and aminotransferases [Ratziu et al. 2010c].
Metabolic response
Changes in insulin resistance were assessed by clamp studies or surrogate markers such as hyperinsulinemia or the HOMA index in both diabetic [Ratziu et al. 2008; Belfort et al. 2006] and nondiabetic patients [Sanyal et al. 2010, 2004; Promrat et al. 2004].
In diabetic patients, both TZDs induced a decrease in insulin levels of 30–34%, as well as a significant reduction in serum glucose and HOMA [Ratziu et al. 2008; Belfort et al. 2006]. Moreover a 1.9- to 2.8-fold increase in adiponectin was documented with both glitazones. Changes in serum adiponectin levels correlated negatively with a reduction in steatosis [Ratziu et al. 2008; Lutchman et al. 2006]. It has been shown that the increase in adiponectin levels and the improvement in insulin resistance with glitazones are causally related through hepatic effects [Gastaldelli et al. 2009a], namely a decrease in hepatic glucose production and an increase in hepatic adenosine monophosphate-activated protein kinase. Importantly, improvement in insulin sensitivity was well correlated with a reduction in liver fat and aminotransferase values but not in necroinflammatory lesions. After drug discontinuation, aminotransferases serum insulin and HOMA were still maintained at 3 months [Ratziu et al. 2008] but rose above baseline 1 year after, while adiponectin declined [Lutchman et al. 2007].
Interestingly, the decline in HOMA index was significantly higher in patients with steatosis reduction (93%) although 59% of patients with unchanged steatosis also experienced a reduction in HOMA [Ratziu et al. 2008]. These findings suggest that correcting insulin resistance is necessary but not sufficient for treating NASH and that, at least in some patients, different pathways (such as inflammatory or fibrotic cascades) should be targeted.
Adverse effects of glitazones
The main drawback of using glitazones is their safety profile. Therefore, potential hepatic benefit of this class of drugs needs to be weighed against long-term safety issues, of particular concern being cardiovascular toxicity, osteoporosis and weight gain.
Glitazones and cardiovascular toxicity
Significant concerns have been raised about thepotential of both glitazones to increase cardiovascular morbidity and related mortality. Cardiovascular risk appears to be drug related rather than a class effect, and seemed to be higher with rosiglitazone than pioglitazone.
Concerns about adverse cardiovascular effects were first raised in 2007, when a meta-analysis of data from 42 clinical trials found a significant increase in the relative risk of myocardial infarction (odds ratio [OR] = 1.43, 95% confidence interval [CI], 1.03–1.98; p = 0.03), and of death from cardiovascular causes (OR = 1.64 95% CI, 0.98–2.74; p = 0.06), among type 2 diabetics treated with rosiglitazone [Nissen and Wolski, 2007].
Following this meta-analysis an unplanned interim analysis of RECORD trial, the largest, randomized, long-term trial of the cardiovascular safety of rosiglitazone compared with other drug interventions for type 2 diabetes, was performed [Home et al. 2007]. This analysis was inconclusive, with data being insufficient to determine whether the drug was associated with an increase in the risk of myocardial infarction or death from cardiovascular causes. The finals results of RECORD trials published in 2009 are inconclusive about effects on myocardial infarction, and conclude that rosiglitazone increase the risk of heart failure but does not increase the risk of overall cardiovascular morbidity or mortality compared with standard glucose-lowering drugs [Home et al. 2009]. The RECORD trial has certain built-in limitations, particularly its open-label design, its relatively small size (for a cardiovascular trial) and the choice of the primary endpoint. Two longer-term, double-blind RCTs of rosiglitazone (DREAM and ADOPT) were completed around the time of the original meta-analysis. These trials did not show an increased mortality, but had numerically (not statistically significant) higher rates of myocardial infarction in the rosiglitazone arms [Gerstein et al. 2006; Kahn et al. 2006].
An update of the 2007 US Food and Drug Administration (FDA) meta-analysis, including 52 trials, was performed in 2010 and supported the original concern that rosiglitazone increases the risk of heart attacks, and thereby might increase the risk of cardiovascular death and all-cause death, when compared with placebo or non-TZD diabetes drugs. In addition, the vast majority of these events in the meta-analysis come from trials of 12 months duration or less. Therefore, the hypothesis raised by this meta-analysis is that the risk of myocardial infarction, and potentially other serious cardiovascular events, occurs promptly after exposure to rosiglitazone, during the first year of therapy.
Recently the FDA decided to further restrict the use of rosiglitazone in the US, and to continue the ongoing cardiovascular safety trial, called TIDE (Thiazolidinedione Intervention with Vitamin D Evaluation), to compare rosiglitazone to other diabetes treatments such as pioglitazone. GSK was asked to perform a re-adjudication of the RECORD study. In Europe the European Medicines Agency (EMEA) decided to suspend the marketing authorization of rosiglitazone following a review by the Committee for Medicinal Products for Human Use (CHMP), initiated on 9 July 2010.
Unlike rosiglitazone, pioglitazone has not been associated with increased risks of cardiovascular events or mortality. The prospective, placebo-controlled PROactive trial of 5238 subjects with diabetes and known cardiovascular disease demonstrated no increase in risk of all-cause mortality, nonfatal myocardial infarction and stroke in patients with type 2 diabetes who have a high risk of macrovascular events [Dormandy et al. 2005]. This was confirmed by a subsequent meta-analysis [Lincoff et al. 2007]. The risk of congestive heart failure (CHF) in PROactive trial, 38% over the entire treatment period, was significantly higher in the pioglitazone group but the mortality rate from heart failure was not different in the placebo and the pioglitazone arms [Dormandy et al. 2005]. The only predictive factors for severe heart failure were older age >65 years and obesity [Lincoff et al. 2007]. The current recommendations are to avoid the use of pioglitazone in patients with severe heart failure.
Weight gain
Weight gain is a well-recognized side effect of glitazones mainly because of expansion of peripheral and subcutaneous adipose tissue. The weight gain with glitazones is associated with an increase in peripheral adipose tissue and a concomitant decrease in visceral fat content. This fat redistribution is explained by PPARγ agonist-induced remodeling of abdominal fat tissue, characterized by differentiation of preadipocytes into small fat cells in subcutaneous fat depots and apoptosis of differentiated large adipocytes (hypertrophic adipocytes) in visceral and/or subcutaneous fat depots. Fat is thus cleared from muscle and liver and redirected into inert storage sites, which could explain why glitazones improve adipose tissue insulin resistance and glucose metabolism despite weight gain [Miyazaki et al. 2002]. This increase in body weight is not associated with an increased cardiometabolic risk.
Weight gain most frequently develops within the first few months of treatment and appears to plateau thereafter, although there can be additional weight gain over time. In the largest pioglitazone trial, a significant weight gain was noted by week24 and progressed over the course of the study, with a mean of 4.7 kg at week 96. In rosiglitazone trials, a weight gain of more than 3 kg was noted in 30% and 36% of the patients after one and two additional years of treatment, respectively [Ratziu et al. 2010c, 2008].
Bone loss and fractures
Recently, clinical studies have confirmed that TZDs can damage the bones although the confounding adverse effect of diabetes per se cannot be ruled out. Clinical studies demonstrated a decreased cortical bone mass in hands and feet in patients with type 1 diabetes mellitus. Studies in rodent models and humans indicate that glitazones impairs osteoblastic function, resulting in reduced bone formation and bone mass but do not affect bone resorption in vivo [Grey, 2008].
A post hoc analysis of ADOPT trial [Kahn et al. 2006] reported a significantly higher incidence of fractures in the appendicular skeleton in women but not in men. A recent meta-analysis confirmed that overall, use of glitazones significantly increase the risk of fracture in women but not in men and is also associated with significant changes in bone mineral density at the lumbar spine and hip [Loke et al. 2009].
Conclusions
By correcting insulin resistance, glitazones are logical drug candidates for the treatment of NASH. Although imperfect, the existing glitazones studies highlight the methodological challenges for future clinical trials. Whether the partial efficacy of glitazones on both biochemical and histological outcomes can be improved by better selection of patients and better identification of predictors of response needs to be further determined. Given the complexity of mechanisms involved in the progression of the disease, simply correcting insulin resistance will not be enough for a majority of patients. Combining insulin-sensitizing agents with hepatoprotective or anti-inflammatory/antifibrotic drugs in nonresponders or partial responders is a very attractive option for future therapeutic strategies.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
VR has worked as an occasional consultant regarding NAFLD projects for Axcan Pharma, Astellas, Genentech, Gilead, Pfizer, Roche, Sanofi-Aventis.
The FLIRT and FLIRT2 trials were conducted with partial financial support from GSK.
References
- Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I., et al. (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135: 1176–1184 [DOI] [PubMed] [Google Scholar]
- Argo C.K., Northup P.G., Al-Osaimi A.M., Caldwell S.H. (2009) Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 51: 371–379 [DOI] [PubMed] [Google Scholar]
- Ascha M.S., Hanouneh I.A., Lopez R., Tamimi T.A., Feldstein A.F., Zein N.N. (2010) The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 51: 1972–1978 [DOI] [PubMed] [Google Scholar]
- Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J., et al. (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307 [DOI] [PubMed] [Google Scholar]
- Betteridge D.J. (2007) Effects of pioglitazone on lipid and lipoprotein metabolism. Diabetes Obes Metab 9: 640–647 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Leone N., Vanni E., Marchesini G., Brunello F., Carucci P., et al. (2002) Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 123: 134–140 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., McCullough A.J., Marchesini G. (2005a) Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 42: 987–1000 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Pagotto U., Manini R., Vanni E., Gastaldelli A., de Iasio R., et al. (2005b) Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab 90: 3498–3504 [DOI] [PubMed] [Google Scholar]
- Caldwell S.H., Crespo D.M. (2004) The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol 40: 578–584 [DOI] [PubMed] [Google Scholar]
- Cusi K. (2009) Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin Liver Dis 13: 545–563 [DOI] [PubMed] [Google Scholar]
- Dam-Larsen S., Franzmann M., Andersen I.B., Christoffersen P., Jensen L.B., Sorensen T.I., et al. (2004) Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut 53: 750–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy J.A., Charbonnel B., Eckland D.J., Erdmann E., Massi-Benedetti M., Moules I.K., et al. (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366: 1279–1289 [DOI] [PubMed] [Google Scholar]
- Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W., et al. (2009) Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 106: 15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein A.E., Papouchado B.G., Angulo P., Sanderson S., Adams L., Gores G.J. (2005) Hepatic stellate cells and fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 3: 384–389 [DOI] [PubMed] [Google Scholar]
- Fracanzani A.L., Valenti L., Bugianesi E., Andreoletti M., Colli A., Vanni E., et al. (2008) Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48: 792–798 [DOI] [PubMed] [Google Scholar]
- Gastaldelli A., Harrison S.A., Belfort-Aguilar R., Hardies L.J., Balas B., Schenker S., et al. (2009a) Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 50: 1087–1093 [DOI] [PubMed] [Google Scholar]
- Gastaldelli A., Kozakova M., Hojlund K., Flyvbjerg A., Favuzzi A., Mitrakou A., et al. (2009b) Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 49: 1537–1544 [DOI] [PubMed] [Google Scholar]
- Gerstein H.C., Yusuf S., Bosch J., Pogue J., Sheridan P., Dinccag N., et al. (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Grey A. (2008) Skeletal consequences of thiazolidinedione therapy. Osteoporos Int 19: 129–137 [DOI] [PubMed] [Google Scholar]
- Home P.D., Pocock S.J., Beck-Nielsen H., Curtis P.S., Gomis R., Hanefeld M., et al. (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373: 2125–2135 [DOI] [PubMed] [Google Scholar]
- Home P.D., Pocock S.J., Beck-Nielsen H., Gomis R., Hanefeld M., Jones N.P., et al. (2007) Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med 357: 28–38 [DOI] [PubMed] [Google Scholar]
- Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P., et al. (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443 [DOI] [PubMed] [Google Scholar]
- Lincoff A.M., Wolski K., Nicholls S.J., Nissen S.E. (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298: 1180–1188 [DOI] [PubMed] [Google Scholar]
- Loke Y.K., Singh S., Furberg C.D. (2009) Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 180: 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutchman G., Modi A., Kleiner D.E., Promrat K., Heller T., Ghany M., et al. (2007) The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology 46: 424–429 [DOI] [PubMed] [Google Scholar]
- Lutchman G., Promrat K., Kleiner D.E., Heller T., Ghany M.G., Yanovski J.A., et al. (2006) Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol 4: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Malhi H., Gores G.J. (2008) Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 28: 360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., et al. (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–923 [DOI] [PubMed] [Google Scholar]
- Mazzone T., Meyer P.M., Feinstein S.B., Davidson M.H., Kondos G.T., D'Agostino R.B., Sr, et al. (2006) Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296: 2572–2581 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Mahankali A., Matsuda M., Mahankali S., Hardies J., Cusi K., et al. (2002) Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87: 2784–2791 [DOI] [PubMed] [Google Scholar]
- Nayak N.C., Vasdev N., Saigal S., Soin A.S. (2010) End-stage nonalcoholic fatty liver disease: evaluation of pathomorphologic features and relationship to cryptogenic cirrhosis from study of explant livers in a living donor liver transplant program. Hum Pathol 41: 425–430 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A. (2010a) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52: 774–788 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A. (2010b) Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr Gastroenterol Rep 12: 49–56 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Oliver D., Bacon B.R. (2003) Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38: 1008–1017 [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Nicholls S.J., Wolski K., Nesto R., Kupfer S., Perez A., et al. (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299: 1561–1573 [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Wolski K. (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471 [DOI] [PubMed] [Google Scholar]
- Ong J., Pitts A., Younossi Z. (2008) Increased overall mortality and liver-related mortality in nonalcoholic fatty liver disease. J Hepatol 49: 608–612 [DOI] [PubMed] [Google Scholar]
- Paradis V., Zalinski S., Chelbi E., Guedj N., Degos F., Vilgrain V., et al. (2009) Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 49: 851–859 [DOI] [PubMed] [Google Scholar]
- Petta S., Muratore C., Craxi A. (2009) Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis 41: 615–625 [DOI] [PubMed] [Google Scholar]
- Promrat K., Lutchman G., Uwaifo G.I., Freedman R.J., Soza A., Heller T., et al. (2004) A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 39: 188–196 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Bellentani S., Cortez-Pinto H., Day C., Marchesini G. (2010a) A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 53: 372–384 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Bonyhay L., Di Martino V., Charlotte F., Cavallaro L., Sayegh-Tainturier M.H., et al. (2002) Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology 35: 1485–1493 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Caldwell S., Neuschwander-Tetri B.A. (2010b) Therapeutic trials in nonalcoholic steatohepatitis: Insulin sensitizers and related methodological issues. Hepatology 52: 2206–2215 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Charlotte F., Bernhardt C., Giral P., Halbron M., Lenaour G., et al. (2010c) Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: Results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51: 445–453 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L., et al. (2008) Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135: 100–110 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Zelber-Sagi S. (2009) Pharmacologic therapy of non-alcoholic steatohepatitis. Clin Liver Dis 13: 667–688 [DOI] [PubMed] [Google Scholar]
- Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., et al. (2010) Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N Engl J Med 362: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A.J., Mofrad P.S., Contos M.J., Sargeant C., Luketic V.A., Sterling R.K., et al. (2004) A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2: 1107–1115 [DOI] [PubMed] [Google Scholar]
- Sharma A.M., Staels B. (2007) Review: Peroxisome proliferator-activated receptor gamma and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 92: 386–395 [DOI] [PubMed] [Google Scholar]
- Soderberg C., Stal P., Askling J., Glaumann H., Lindberg G., Marmur J., et al. (2010) Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 51: 595–602 [DOI] [PubMed] [Google Scholar]
- Starley B.Q., Calcagno C.J., Harrison S.A. (2010) Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51: 1820–1832 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yang L., McCall S., Huang J., Yu X.X., Pandey S.K., et al. (2007) Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45: 1366–1374 [DOI] [PubMed] [Google Scholar]