Abstract
In 2010, four independent groups almost simultaneously reported the association of the novel interleukin-1 (IL-1) family member, IL-33, with inflammatory bowel disease (IBD). The findings were remarkably consistent and demonstrated that IL-33 is markedly upregulated in, and specific to, ulcerative colitis (UC). In addition, although a variety of gut-associated immune cell subsets express IL-33, the primary source appears to be the intestinal epithelium. IL-33’s receptor, ST2, a formerly orphaned IL-1 receptor-related protein, was also found to be increased in UC patients, although the cellular source of ST2 appears to be somewhat more ambiguous. In fact, emerging evidence indicates that the IL-33/ST2 axis plays a critical role in several other chronic inflammatory and immune disorders. In the gut, IL-33 has been shown to be important in the clearance of intestinal parasites, and inducing epithelial cell hyperplasia, mucus production and mucosal eosinophilic infiltration. However, despite the established trend of increased IL-33 and ST2 expression during IBD, specifically UC, the precise pathophysiologic relevance of these findings has yet to be determined. Interestingly, IL-33 has the ability to potentiate pathogenic Th2 and Th17 responses in gut-associated lymphoid tissues, while also promoting healing of damaged mucosa following inflammatory insults. Indeed, further mechanistic studies are warranted to confirm the possible dichotomous functions of IL-33 during chronic intestinal inflammation and better define its precise role in the pathogenesis of IBD. Herein, we discuss what is currently known about IL-33/ST2 in the gut and speculate as to the potential role of the IL-33/ST2 system in IBD.
Keywords: interleukin-33, ST2, inflammatory bowel disease, ulcerative colitis, crohn's disease, mucosal immunity, intestinal inflammation, alarmins, wound healing
Introduction
Since the original report in 1984 describing the sequencing and cloning of interleukin-1 (IL-1) [Auron et al. 1984], it has long been established that IL-1 and its related cytokine family members play a major role in several autoinflammatory and chronic immune diseases, including inflammatory bowel disease (IBD) (as reviewed by Dinarello [2009]). One of the earliest bodies of work in cytokine biology showed that endogenous IL-1 receptor antagonist (IL-1Ra), the natural occurring antagonist of IL-1, plays a critical role in regulating normal immune homeostasis (in this case, in the gut), and that an imbalance of IL-1 and IL-1Ra in favor of IL-1 overproduction leads to disease states, such as that observed in IBD [Casini-Raggi et al. 1995; Ferretti et al. 1994]. This concept has received recent confirmation in humans by demonstration that germ-line mutations in the IL-1Ra gene (IL-1RN) leads to a devastating chronic autoinflammatory disorder due to uncontrolled IL-1 signaling, that in children can be completely reversed by recombinant IL-1Ra administration (as reviewed by Dinarello [2009]). Other members of the IL-1 family have also been implicated in the pathogenesis of IBD, including IL-18 that was originally described as an important Th1 polarizing cytokine specifically involved in the development of Crohn’s disease (CD) [Monteleone et al. 1999; Pizarro et al. 1999]. Similar to the effects of IL-1Ra on IL-1 signaling, the IL-18 binding protein (IL-18BP) has the ability to prevent binding of IL-18 to its receptor, ultimately blocking its downstream functional effects. Neutralizing isoforms of IL-18BP were also found to be increased in the intestinal tissue of active CD patients [Corbaz et al. 2002], again underscoring the importance of maintaining homeostatic balance of IL-1 cytokine family members, this time between IL-18 and IL-18BP, to prevent chronic disease states, such as IBD.
IL-33, also known as IL-1F11, is the 11th and newest identified member of the IL-1 family (Table 1). Since 2005, when a protein highly expressed in endothelial cell nuclei, previously known as nuclear factor-high endothelial venules [Baekkevold et al. 2003], was recognized as IL-33 [Schmitz et al. 2005], a number of studies have attempted to characterize the biology and molecular interactions of IL-33, as well as postulate the potential role of this cytokine in several human disorders and mouse models of disease (reviewed in [Liew et al. 2010]). To date, most of the published studies regarding IL-33 describe its role in airway inflammation [Kurowska-Stolarska et al. 2009, 2008; Liu et al. 2009], allergy [Matsuba-Kitamura et al. 2010; Pushparaj et al. 2009; Shimizu et al. 2005], and rheumatic diseases [Palmer et al. 2009; Xu et al. 2008]. Less was known regarding the importance of this cytokine in the maintenance of intestinal homeostasis and its potential role in gut-associated diseases. However, in 2010, four independent groups reported the association of IL-33 with IBD, with elevated expression specifically in UC patients [Beltran et al. 2010; Kobori et al. 2010; Pastorelli et al. 2010; Seidelin et al. 2010]. Although emerging data is beginning to uncover the precise function of IL-33 in the gastrointestinal (GI) tract, little has been established that mechanistically addresses the role of IL-33 during normal gut homeostasis as well as during IBD. Thus, the purpose of this review is to summarize what is currently known about IL-33 as it relates to the GI system, and to provide insight into its potential role in chronic intestinal inflammation.
Table 1.
IL-1 cytokine family in inflammatory bowel disease.
| Common name | IL-1 family name | Function | Ligand-binding chain | Disease association |
|---|---|---|---|---|
| IL-1α | IL-1F1 | Agonist | IL-1R type I | CD1,2, UC1,2 |
| IL-1β | IL-1F2 | Agonist | IL-1R type I | CD1,2, UC1,2 |
| IL-1Ra | IL-1F3 | Antagonist | IL-1R type I | UC3,4,5 |
| IL-18 | IL-1F4 | Agonist | IL-18Rα | CD6,7 |
| IL-1F5 | Agonist | IL-1Rrp2 | Unknown | |
| IL-1F6 | Agonist | IL-1Rrp2 | Unknown | |
| IL-1F7 | Antagonist | IL-18Rα | Unknown | |
| IL-1F8 | Agonist | IL-1Rrp2 | Unknown | |
| IL-1F9 | Agonist | IL-1Rrp2 | Unknown | |
| IL-1F10 | Unknown | Unknown | Unknown | |
| IL-33 | IL-1F11 | Agonist | ST2 | UC8,9,10,11 |
CD, Crohn’s disease; UC, ulcerative colitis.
Cominelli [1990].
Andus [1997].
Nishiyama [1994].
Ferrettiet al. [1994].
IL-33 and ST2: a complex, widely expressed cytokine system
IL-33 is widely distributed throughout various organ systems in the body and has been detected in several different cell types, mostly of nonhematopoietic origin, such as fibroblasts, adipocytes, smooth muscle cells, endothelial cells, as well as bronchial and intestinal epithelial cells [Wood et al. 2009; Moussion et al. 2008; Schmitz et al. 2005]. IL-33 has also been reported to be expressed in cells of hematopoietic origin, particularly in restricted populations of professional antigen presenting cells, such as macrophages and dendritic cells [Schmitz et al. 2005].
During the initial characterization of IL-33 in 2005, it was proposed that, similarly to other IL-1 family members, such as IL-1β and IL-18, IL-33 was synthesized as a precursor molecule (30-kDa polypeptide) that was subsequently cleaved by caspase-1 into an 18-kDa mature/bioactive form as a consequence of inflammasome activation [Schmitz et al. 2005]. However, in 2009, the ‘inflammasome paradigm’ was challenged following a series of seminal studies demonstrating that full-length (30-kDa) IL-33 is the actual bioactive form, and it became unclear as to whether an 18-kDa form exists naturally [Cayrol and Girard, 2009; Luthi et al. 2009; Talabot-Ayer et al. 2009]. Furthermore, it has also not been determined what the role is of the other processed, likely less active, forms (20–22 kDa) of IL-33 that results from caspase-3 and caspase-7 activation.
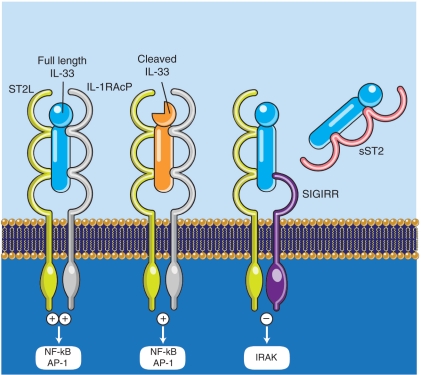
IL-33 exerts its biological effects through binding of its receptor, the formerly orphaned receptor, IL-1 receptor like 1 (IL1RL1), also known as ST2 [Schmitz et al. 2005]. Two different splice variants of ST2 have been described [Oshikawa et al. 2002], leading to the synthesis of two different proteins: ST2L, a transmembrane receptor that activates downstream signaling upon IL-33 recognition; and sST2, a soluble molecule that likely serves as a decoy receptor by binding IL-33 and blocking its biologic effects [Fagundes et al. 2007]. In order to initiate the cell signaling cascade, ST2L needs to be paired to a coreceptor, namely the IL-1 receptor accessory protein (IL1RAcP) [Palmer et al. 2008]. Both ST2 and IL1RAcP possess a Toll/IL-1 receptor (TIR) domain through which they interact with MyD88, TRAF6 and IRAK1/4, eventually leading to the activation of transcription factors, such as NF-κB and AP-1, that are able to promote the production of several pro-inflammatory mediators [Schmitz et al. 2005]. ST2 can also dimerize with a second coreceptor belonging to the IL-1R family, that is ‘single Ig IL-1R related molecule’ (SIGIRR) [Bulek et al. 2009]. SIGIRR has been shown to act as a negative regulator of the IL-33/ST2 signaling pathway, ultimately dampening IL-33’s bioactivity. As with the IL-33 isoforms and ST2 splice variants, the biologic and pathophysiologic relevance of alternative ST2/SIGIRR signaling (summarized in Figure 1) has not been thoroughly investigated.
Figure 1.
Ligand-receptor pairings and signaling pathways of the IL-33 cytokine system. Bioactive, 30-kDa IL-33 binds to cell surface ST2L and recruits its coreceptor IL-1RAcP, resulting in the initiation of a positive signaling cascade that leads to activation of several transcription factors, including NF-κB and AP-1. The 30-kDa IL-33 can also be cleaved by caspase-3 and caspase-7 into 20–22-kDa isoforms that have decreased IL-33 bioactivity and are predominantly the result of cell apoptosis. ST2L can alternatively dimerize with a second coreceptor, SIGIRR, which upon IL-33 binding, inhibits IRAK-4 and acts as a negative regulator of IL-33/ST2 signaling, ultimately dampening the bioactivity of IL-33. sST2 has the ability to bind IL-33 and primarily serves as a soluble decoy receptor that prevents IL-33/ST2L binding and positive cell signaling, further downregulating IL-33’s functional biologic activity. Illustration courtesy of Alessandro Baliani ©Copyright [2011].
Role of IL-33 in enhancing mucosal defense against intestinal parasites
Original studies injecting normal mice with recombinant IL-33 showed specific effects on mucosal cell populations in the pulmonary and GI tracts; these mice developed bronchial and intestinal epithelial cell hyperplasia, as well as eosinophilic and mononuclear infiltration into the lamina propria [Schmitz et al. 2005]. The epithelial cell hyperplasia was characterized by an impressive increase in goblet cell number, leading to abundant mucus production. As such, IL-33 appears to reinforce mucosal barrier defenses towards potential invading pathogens, increasing both epithelial protection by mucus secretion and augmenting immune cell responses through the induction of Th2 cytokines, such as IL-13 and IL-5. In fact, the aforementioned functions highlight the importance of IL-33 in enhancing clearance of intestinal parasitic infestation. In an experimental mouse model of nematode infection with Trichuris muris, IL-33 was highly expressed in the cecum where T. muris was present, and upon exogenous recombinant IL-33 administration, these infected mice showed boosted parasite expulsion [Humphreys et al. 2008]. Although proliferation of natural killer (NK) cells in the mesenteric lymph nodes of treated mice was observed, T. muris clearance was abrogated in SCID mice, suggesting that IL-33-induced T- and B-cell activation is required in order to mount host defenses against this particular parasite. Additional data obtained from different animal models/organs further support the role of the IL-33/ST2 system in protecting host from parasitic and bacterial threats, such as infection with Toxoplasma gondii [Jones et al. 2010], Pseudomonas aeruginosa [Huang et al. 2007] and Leptospira [Wagenaar et al. 2009].
Interestingly, a novel immune cell population responding to IL-33 stimulation that mediates protective responses towards intestinal parasites has recently been described [Neill et al. 2010; Price et al. 2010]. These innate effector leukocytes express cell surface ST2, c-Kit, and IL-25R, and display unique phenotypic characteristics not shared by other classical immune cell populations. These cells proliferate in mouse mesenteric lymph nodes and spleens, and produce high levels of Th2 cytokines after IL-33 and IL-25 co-stimulation. These cells, uniquely coined ‘nuocytes’, represent an early source of IL-13 during intestinal helminth infestation (i.e. Nippostrongylus brasiliensis), and the lack of their activation results in severely impaired worm clearance. At the same time, Moro and colleagues characterized a similar population of innate Th2 effector cells, identified by the presence of cell surface ST2, c-Kit, Sca-1 and IL-7R. These cells reside in mesenteric adipose tissue, are organized in fat-associated lymphoid clusters, and are potently activated by IL-33, IL-25 and IL-2 [Moro et al. 2010]. Upon activation, production of IL-5 and IL-13 from these innate Th2 effector cells appear to induce histopathologic changes in the gut mucosa, including epithelial/goblet cell hyperplasia and eosinophilic infiltration, again demonstrating key features for the effective expulsion of helminth infestation.
The IL-33/ST2 axis in IBD
Given the established role of IL-1 family members, such as IL-1β and IL-18 [Cominelli and Pizarro, 1996; Reuter and Pizarro, 2004], in the development of IBD and the intestinal-specific pathologies induced by IL-33 administration in mouse models, several research groups, including ours, began to investigate the expression and possible pathogenic involvement of the IL-33/ST2 axis in IBD.
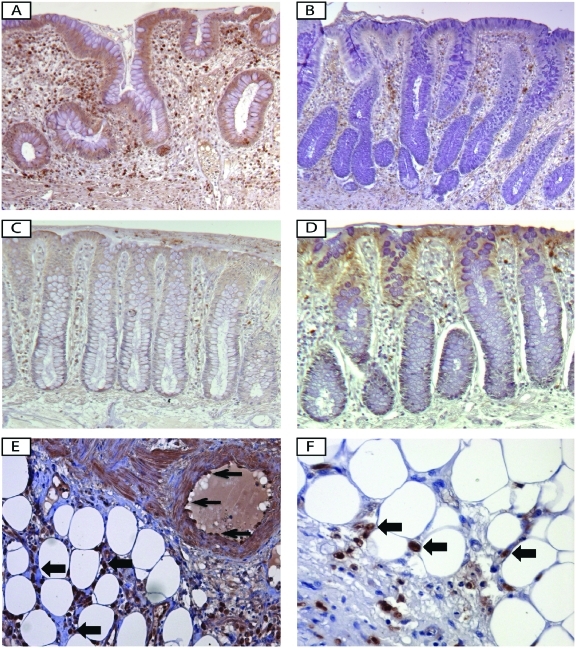
Robust data indicate that IL-33 expression is increased in the inflamed mucosa of IBD patients versus healthy controls, particularly in UC [Beltran et al. 2010; Kobori et al. 2010; Pastorelli et al. 2010; Seidelin et al. 2010]. Mucosal expression of IL-33 is mostly localized to nonhematopoietic cells, particularly intestinal epithelial cells [Beltran et al. 2010; Pastorelli et al. 2010; Seidelin et al. 2010] and myofibroblasts [Kobori et al. 2010] (Figures 2A and C). However, immunolocalization, as well as ex vivo studies on isolated intestinal mucosal cell populations, confirm that IL-33 is also expressed by a wide variety of cell types, many of which have been described in other tissue/organ systems, including fibroblasts, smooth muscle cells, endothelial cells [Miller et al. 2008; Schmitz et al. 2005], and adipocytes [Wood et al. 2009] (Figure 2E). During active UC, IL-33 expression is markedly increased in intestinal epithelial cells and infiltrating lamina propria mononuclear cells, belonging to the monocyte/macrophage and B-cell lineages [Pastorelli et al. 2010] (Figure 2A). Increased IL-33 serum concentrations were also detected in IBD patients in comparison with age-matched healthy controls [Beltran et al. 2010; Pastorelli et al. 2010]. Full-length (30-kDa) IL-33 is the form predominantly expressed in the cytoplasm and nuclei of IL-33-producing cells, whereas the only detectable form in the serum is the 20–22 kDa cleaved form of IL-33 [Pastorelli et al. 2010], which has been reported to possess reduced biologic activity [Cayrol and Girard, 2009; Luthi et al. 2009; Talabot-Ayer et al. 2009]. This important observation suggests that the presence of extracellular proteases have the ability to cleave and inactivate full-length IL-33, perhaps in an attempt to prevent possible harmful effects that may be triggered by high circulating levels of this cytokine, such as in anaphylactic shock [Pushparaj et al. 2009]. Nonetheless, increased serum levels of IL-33 in IBD patients reflect an active inflammatory state and represent a potential biomarker for disease activity.
Figure 2.
IL-33 and ST2 expression and localization in ulcerative colitis versus healthy controls. Immunohistochemical staining for IL-33 ((A), (C), and (E)) and ST2 ((B), (D), and (F)) in surgically resected, full-thickness colon tissues from ulcerative colitis (UC) ((A), (B), (E), and (F)) and noninflammatory control ((C) and (D)) patients show that IL-33 is localized to the intestinal epithelium and lamina propria mononuclear cells (LPMC) within the inflamed mucosa of UC patients (A), and to a lesser extent, in healthy mucosa (C). Conversely, ST2 is constitutively expressed in epithelial cells in the absence of intestinal inflammation (D), but during UC, ST2 expression in epithelial cells is lost, whereas intense ST2 staining is present within lamina propria immune cells (B). IL-33 (E) and ST2 (F) are also expressed by infiltrating immune cells within the mesenteric adipose tissue in IBD (block arrows, (E) and (F)). Clear IL-33 staining is also present in endothelial cells within the inflamed gut wall (arrows, (E)). All panels, ×20 original magnification.
The results obtained regarding IL-33 expression patterns in the gut mucosa as well as the systemic circulation of IBD patients were also confirmed in SAMP1/YitFc (SAMP) mice [Pastorelli et al. 2010], an experimental model of CD ileitis, characterized by an early Th1 immune response and a late, chronic phase of disease dominated by Th2 cytokines [Bamias et al. 2005; Rivera-Nieves et al. 2003]. In fact, IL-33 mucosal tissue levels showed a positive correlation with the severity of gut inflammation [Pastorelli et al. 2010]. The prominent involvement of IL-33 in both UC patients as well as in SAMP mice is consistent with the major role Th2 cytokines play in these particular inflammatory conditions of the gut [Xavier and Podolsky, 2007; Bamias et al. 2005; Takedatsu et al. 2004]. As such, IL-33 overexpression may represent an early event in IBD pathogenesis, driving polarization of the inflammatory response towards a Th2/UC, rather than a Th1/CD, phenotype. Moreover, as in the case of SAMP mice, IL-33 may trigger later Th2-driven immune responses in Crohn’s ileitis, exacerbating the severity of inflammation. It has recently been demonstrated that IL-33 is also specifically expressed in activated subepithelial myofibroblasts situated below ulcerative lesions in UC patients, but not in CD patients [Kobori et al. 2010], suggesting a possible function for IL-33 in wound/ulcer healing, which may be different in UC compared with CD.
Similar to IL-33, its receptor, ST2, was also found to be increased both in the intestinal mucosa and serum of IBD patients [Beltran et al. 2010; Pastorelli et al. 2010]. The intestinal tissue expression pattern of ST2 is different in healthy mucosa compared with that found in chronically inflamed IBD patients. The epithelium in macroscopically noninflamed colon displays abundant ST2, but during chronic inflammatory processes characterizing either UC or CD, epithelial-derived ST2 expression is lost/decreased and redistributed (Figure 2B and D). A marked infiltration of ST2 positive cells (both professional antigen-presenting cells and T helper cells) is evident in the lamina propria, while in the mesenteric tissue of active IBD, intense infiltration of immune cells strongly expressing ST2 are found in the perivisceral adipose tissue (Figure 2F) [Pastorelli et al. 2010]. It is likely that the latter population represents the recently described innate Th2 effector cells, that were discussed earlier [Moro et al. 2010], and whose role in idiopathic intestinal inflammation has not yet been investigated.
Of note, recruitment of ST2 positive cells into the lamina propria is a common feature shared by different intestinal inflammatory conditions, whereas epithelial changes in ST2 expression appear to be specific for IBD since nonspecific colitides (e.g. diverticulitis and infectious colitis) do not present this particular epithelial-specific pattern [Pastorelli et al. 2010]. Further analysis showed that the ST2 variant for which expression is altered in IBD epithelial cells is ST2L, IL-33’s signaling transmembrane receptor [Beltran et al. 2010; Pastorelli et al. 2010]. As such, we can extrapolate that the global increase of ST2 in both colonic biopsies and in circulating serum from IBD patients primarily represents the sST2 protein, likely produced by mucosal lamina propria mononuclear cells and to a lesser extent, intestinal epithelial cells, and potentially by circulating mononuclear cells. However, whether impaired epithelial ST2L expression is a feedback response of chronic exposure to elevated IL-33 concentrations or an intrinsic epithelial defect in IBD has yet to be determined.
Insight into the functional role of IL-33 and ST2 in the intestine during health and disease
IL-33 as a pathogenic cytokine promoting immune dysregulation and chronic gut inflammation
Despite the wealth of information regarding IL-33 and ST2 expression in IBD, functional data, needed to mechanistically understand the precise role of this novel cytokine–receptor pair in the maintenance and disruption of intestinal immune homeostasis, are still lacking. However, growing insight into the function of IL-33 in the GI system can also be derived from studies in other experimental models of chronic inflammatory disorders, such as airway inflammation and arthritis models [Liu et al. 2009; Palmer et al. 2009]. In these two settings, IL-33 demonstrates a potent pro-inflammatory effect, leading to worsening of disease states, particularly through the recruitment of activated immune cells to the site of inflammation [Komai-Koma et al. 2007] and production of several pro-inflammatory cytokines [Schmitz et al. 2005]. Along the same line, transgenic mice engineered to produce high levels of IL-33 develop spontaneous airway and pulmonary inflammation [Zhiguang et al. 2010], and treatment targeted to block the IL-33/ST2 signaling cascade has the ability to ameliorate disease in a murine model of allergic asthma [Liu et al. 2009]. This phenomenon is also observed in arthritis models where specific blockade of IL-33 by administration of anti-ST2 antibodies has the ability to dampen joint inflammation [Palmer et al. 2009]. Thus, IL-33 appears to promote chronic inflammatory conditions, enhancing both Th2 and Th1 immune responses [Smithgall et al. 2008].
In fact, IL-33 stimulation of unfractionated mesenteric lymph node cells from the SAMP ileitis model induces potent production of the Th2 cytokine, IL-5, as well as IL-6 and IL-17 [Pastorelli et al. 2010]. IL-5 and IL-6 are important cytokines in the SAMP model, demonstrated by neutralization experiments in which either anti-IL-5 or anti-IL-6 treatment ameliorated gut inflammation [Mitsuyama et al. 2006; Takedatsu et al. 2004]. At present, no published data is available regarding Th17 immune activation in SAMP mice; however, it is well established that IL-17 plays a pivotal role in the onset of chronic intestinal inflammation [O'Connor et al. 2009; Ahern et al. 2008; Fina et al. 2008; Zhang et al. 2006].
As such, IL-33 may represent a primary, innate cytokine capable of inducing pathogenic, adaptive immune responses in the gut. Alternatively, induction of IL-33 can occur downstream of TNF, which represents an important master cytokine in the pathogenesis of IBD [Murch et al. 1993]. TNF has the ability to increase IL-33 as well as modulate ST2 expression in intestinal epithelial cells [Pastorelli et al. 2010], as well as other cell types [Verri et al. 2010; Wood et al. 2009; Xu et al. 2008]. Administration of anti-TNF (Infliximab) for the treatment of IBD has the ability to significantly reduce circulating IL-33 protein levels in both CD, and particularly UC, patients [Pastorelli et al. 2010]; again, supporting the concept of using systemic IL-33 levels as a potential biomarker for IBD disease activity.
Although results from neutralization studies targeted at blocking the IL-33/ST2 signaling pathway in experimental colitis models have yet to be reported, a recent study in which IL-33 knockout (KO) mice were subjected to dextran sodium sulfate (DSS)-induced colitis has provided further insight into the role of IL-33 and ST2 in intestinal inflammation. Colonic inflammation induced by DSS occurs in a T-cell-independent manner and results from disruption of the epithelial barrier and consequent exposure of innate immune cells to gut luminal contents, including the bacterial microflora. IL-33 KO mice displayed less prominent inflammation, with a significant decrease in granulocyte infiltration during the acute phase of the colitis compared with wild-type littermates [Oboki et al. 2010]. Subsequently, during the recovery phase of the experiment, whereas weight recovery was markedly delayed in IL-33 KO mice, no significant difference in colonic inflammation was observed between IL-33 KO and wild-type littermates. The authors propose that in this particular model, IL-33 plays an important role in driving acute, innate immune responses, but is dispensable in the maintenance of a chronic inflammatory state. Alternatively, the possibility exists that the delayed weight recovery observed in IL-33 KO mice, but not wild-type littermates, is due to the potential role of IL-33 in restoration of gut homeostasis and mucosal healing.
It is widely accepted that intestinal epithelial cells and innate immune cells are critically involved in mucosal immune responses towards intestinal luminal pathogens and bacterial antigens. In fact, upon recognition of pathogen associated molecular patterns (PAMPs), these cells have the ability to potentiate intestinal barrier function by releasing antimicrobial peptides (i.e. defensins) [Artis, 2008; Pasparakis, 2008; Wehkamp et al. 2008], increasing mucus production [Marillier et al. 2008], and secreting pro-/anti-inflammatory mediators and cytokines [Kaser et al. 2010] in order to mount an appropriate mucosal immune response. As mentioned earlier, within the gut mucosa, IL-33 is primarily expressed in the epithelium [Beltran et al. 2010; Pastorelli et al. 2010; Seidelin et al. 2010]. Moreover, intestinal epithelial cells during active inflammation express predominantly full-length (30-kDa) IL-33, representing the most bioactive form of IL-33 with greater pro-inflammatory activity [Cayrol and Girard, 2009; Luthi et al. 2009; Talabot-Ayer et al. 2009]. It has been suggested that this full-length form of IL-33 may also function as a novel epithelial ‘alarmin’ [Cayrol and Girard, 2009; Luthi et al. 2009; Talabot-Ayer et al. 2009; Moussion et al. 2008], as increasing evidence indicates its structural and functional similarity to HMGB1 and IL-1α [Chen et al. 2007; Scaffidi et al. 2002], which represent prototypic alarmin molecules. Therefore, damaged, stressed, or necrotic cells can potentially release IL-33 as a danger signal/alarmin to alert the immune system of a local threat. In fact, loss of epithelial barrier integrity has been indicated as possible trigger for the development of pathologic gut inflammatory responses by several genetic studies in IBD patients and mouse models of colitis (reviewed by Pastorelli et al. [2008]). Therefore, it can be hypothesized that, because of either intrinsic defects (e.g. structural impairment affecting cell integrity, barrier dysfunction, etc.) or extrinsic exposure to harmful stimuli, as in the case of DSS challenge, stressed gut epithelial cells can release potent levels of IL-33 as a danger signal, initiating an inflammatory cascade that leads to intestinal inflammation.
Potential role of IL-33 as a mediator of mucosal healing and epithelial repair/restoration
An alternative role for IL-33 within the gut mucosa is slowly emerging; that is, as a trophic factor, promoting epithelial proliferation and restoration of barrier function. In fact, as previously discussed, IL-33 has the ability to potentiate epithelial defenses and enhance mucus production upon parasitic infections [Moro et al. 2010; Neill et al. 2010; Price et al. 2010; Schmitz et al. 2005]. In support of this concept, IL-33 expression has been described in subepithelial myofibroblasts, situated below ulcerated epithelium [Kobori et al. 2010], suggesting that perhaps IL-33 may also be involved in the epithelial repair process. With the aforementioned observation, together with previously reported changes in the pattern of endothelial cell IL-33 expression during angiogenesis [Kuchler et al. 2008], we can speculate that IL-33 may play a possible role in wound healing.
Whether IL-33 actually functions as a protective factor for the gut mucosa and promotes epithelial healing has yet to be proven. However, it is interesting to consider that loss and/or redistribution of ST2L expression specifically observed on the epithelium of IBD patients (Figure 2B) [Beltran et al. 2010; Pastorelli et al. 2010] may result in disruption of IL-33/ST2 signaling and consequently responsible for ineffective epithelial proliferation/repair and restoration of epithelial integrity, which would ultimately promote chronic alteration of gut immune homeostasis characterizing IBD. Similarly, it is also tempting to speculate that IL-33’s role as an epithelial alarmin may be to induce the recruitment and activation of immune cells to the site of injury that are involved in the resolution phase of inflammation and eventual wound healing.
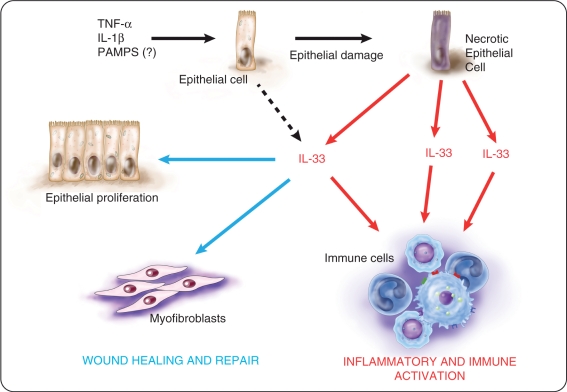
Taken together, studies indicate a possible dual, and perhaps dichotomous, role of IL-33/ST2 in the setting of chronic intestinal inflammation and IBD pathogenesis. IL-33 may have a pro-inflammatory effect on lamina propria immune cells while at the same time, promote wound healing and epithelial repair when acting on epithelial cells and myofibroblasts (summarized in Figures 3 and 4). As such, further, more mechanistic investigation is warranted to dissect the specific pathogenic, or protective, processes that may involve different cell types at different times, in order to clarify the predominant role of this complex cytokine system in gut physiology and disease.
Figure 3.
Potential dichotomous functions of IL-33 in intestinal inflammation. Pro-inflammatory stimuli, such as tumor necrosis factor (TNF), IL-1β and perhaps pathogens-associated molecular patterns (PAMPs), increase intracellular IL-33 expression in intestinal epithelial cells. Under stressful or harmful conditions that lead to damage of the epithelium, IL-33 is released by necrotic epithelial cells as a potential alarmin. Depending on the presence and abundance of ST2-bearing target cells, IL-33 may have different global effects within the gut mucosa. Encounter with activated immune cells, IL-33 may enhance immune responses that can further exacerbate the severity of inflammation. Concomitantly, IL-33 can also act on intestinal epithelial cells and myofibroblasts to induce epithelial proliferation and repair as well as promote wound healing. Illustration courtesy of Alessandro Baliani ©Copyright [2011].
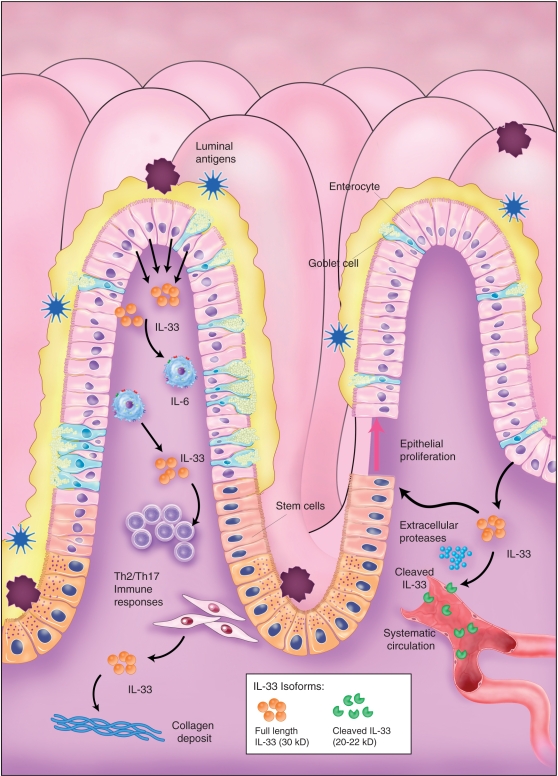
Figure 4.
Working hypothesis of IL-33’s role in inflammatory bowel disease. Bioactive IL-33 is primarily produced by intestinal epithelial cells, as well as lamina propria mononuclear cells, during active inflammation and has the ability to promote pro-inflammatory cytokine production, such as IL-6, as well as Th2 and Th17 adaptive immune responses. Intestinal myofibroblasts have also recently been reported as a source of IL-33 in inflammatory bowel disease (IBD), suggesting a speculative role in fibrosis. Bioactive IL-33 can potentially be cleaved by extracellular proteases within the gut mucosa, dampening the pro-inflammatory effects of IL-33; release of its putative 20–22-kDa products into the systemic circulation, can serve as a bioactivity disease marker for IBD. IL-33 has also been shown to promote epithelial proliferation and can alternatively be involved in epithelial repair and wound healing. Loss and/or redistribution of ST2 expression on the epithelium of IBD patients may indicate disruption of IL-33/ST2 signaling, leading to ineffective restoration of epithelial barrier integrity, facilitating translocation of luminal, bacterial products and the perpetuation of chronic intestinal inflammation. Illustration courtesy of Alessandro Baliani ©Copyright [2011].
Funding
This work was supported by the National Institutes of Health (grant number DK056762 and DK057880/PPG5 to TTP) and the Italian Ministry of University and Research PRIN (grant number 2007K4HZEJ_004 to MV).
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
References
- Ahern P.P., Izcue A., Maloy K.J., Powrie F. (2008) The interleukin-23 axis in intestinal inflammation. Immunol Rev 226: 147–159 [DOI] [PubMed] [Google Scholar]
- Andus, T., Daig, R., Vogl, D., Aschenbrenner, E., Lock, G., Hollerbach, S., et al. (1997) Imbalance of the interleukin 1 system in colonic mucosa–association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut 41: 651–657. [DOI] [PMC free article] [PubMed]
- Artis D. (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8: 411–420 [DOI] [PubMed] [Google Scholar]
- Auron P.E., Webb A.C., Rosenwasser L.J., Mucci S.F., Rich A., Wolff S.M., et al. (1984) Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A 81: 7907–7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold E.S., Roussigne M., Yamanaka T., Johansen F.E., Jahnsen F.L., Amalric F., et al. (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163: 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias G., Martin C., Mishina M., Ross W.G., Rivera-Nieves J., Marini M., et al. (2005) Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology 128: 654–666 [DOI] [PubMed] [Google Scholar]
- Beltran C.J., Nunez L.E., Diaz-Jimenez D., Farfan N., Candia E., Heine C., et al. (2010) Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis 16: 1097–1107 [DOI] [PubMed] [Google Scholar]
- Bulek K., Swaidani S., Qin J., Lu Y., Gulen M.F., Herjan T., et al. (2009) The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol 182: 2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini-Raggi V., Kam L., Chong Y.J., Fiocchi C., Pizarro T.T., Cominelli F. (1995) Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol 154: 2434–2440 [PubMed] [Google Scholar]
- Cayrol C., Girard J.P. (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A 106: 9021–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. (2007) Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 13: 851–856 [DOI] [PubMed] [Google Scholar]
- Cominelli, F., Nast, C.C., Clark, B.D., Schindler, R., Lierena, R., Eysselen, V.E., et al. (1990) J Clin Invest 86: 972–980. [DOI] [PMC free article] [PubMed]
- Cominelli F., Pizarro T.T. (1996) Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther 10(Suppl. 2): 49–53; discussion 54 [DOI] [PubMed] [Google Scholar]
- Corbaz, A., ten Hove, T., Herren, S., Graber, P., Schwartsburd, B., Belzer, I., et al. (2002) IL-18 binding protein expression by endothelial cells and macrophages is up-regulated during active Crohn's disease. J Immunol 168: 3608–3616. [DOI] [PubMed]
- Dinarello C.A. (2009) Interleukin-1beta and the autoinflammatory diseases. N Engl J Med 360: 2467–2470 [DOI] [PubMed] [Google Scholar]
- Fagundes C.T., Amaral F.A., Souza A.L., Vieira A.T., Xu D., Liew F.Y., et al. (2007) ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J Leukoc Biol 81: 492–499 [DOI] [PubMed] [Google Scholar]
- Ferretti M., Casini-Raggi V., Pizarro T.T., Eisenberg S.P., Nast C.C., Cominelli F. (1994) Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest 94: 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina D., Sarra M., Fantini M.C., Rizzo A., Caruso R., Caprioli F., et al. (2008) Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 134: 1038–1048 [DOI] [PubMed] [Google Scholar]
- Huang X., Du W., Barrett R.P., Hazlett L.D. (2007) ST2 is essential for Th2 responsiveness and resistance to pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 48: 4626–4633 [DOI] [PubMed] [Google Scholar]
- Humphreys N.E., Xu D., Hepworth M.R., Liew F.Y., Grencis R.K. (2008) IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol 180: 2443–2449 [DOI] [PubMed] [Google Scholar]
- Jones L.A., Roberts F., Nickdel M.B., Brombacher F., McKenzie A.N., Henriquez F.L., et al. (2010) IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur J Immunol 40: 426–436 [DOI] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. (2010) Inflammatory bowel disease. Annu Rev Immunol 28: 573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori A., Yagi Y., Imaeda H., Ban H., Bamba S., Tsujikawa T., et al. (2010) Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol, in press in press; [DOI] [PubMed] [Google Scholar]
- Komai-Koma M., Xu D., Li Y., McKenzie A.N., McInnes I.B., Liew F.Y. (2007) IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol 37: 2779–2786 [DOI] [PubMed] [Google Scholar]
- Kuchler A.M., Pollheimer J., Balogh J., Sponheim J., Manley L., Sorensen D.R., et al. (2008) Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol 173: 1229–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M., Kewin P., Murphy G., Russo R.C., Stolarski B., Garcia C.C., et al. (2008) IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 181: 4780–4790 [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M., Stolarski B., Kewin P., Murphy G., Corrigan C.J., Ying S., et al. (2009) IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 183: 6469–6477 [DOI] [PubMed] [Google Scholar]
- Liew F.Y., Pitman N.I., McInnes I.B. (2010) Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10: 103–110 [DOI] [PubMed] [Google Scholar]
- Liu X., Li M., Wu Y., Zhou Y., Zeng L., Huang T. (2009) Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun 386: 181–185 [DOI] [PubMed] [Google Scholar]
- Luthi A.U., Cullen S.P., McNeela E.A., Duriez P.J., Afonina I.S., Sheridan C., et al. (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31: 84–98 [DOI] [PubMed] [Google Scholar]
- Marillier R.G., Michels C., Smith E.M., Fick L.C., Leeto M., Dewals B., et al. (2008) IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol 9: 11–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuba-Kitamura S., Yoshimoto T., Yasuda K., Futatsugi-Yumikura S., Taki Y., Muto T., et al. (2010) Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol 22: 479–489 [DOI] [PubMed] [Google Scholar]
- Miller A.M., Xu D., Asquith D.L., Denby L., Li Y., Sattar N., et al. (2008) IL-33 reduces the development of atherosclerosis. J Exp Med 205: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama K., Matsumoto S., Rose-John S., Suzuki A., Hara T., Tomiyasu N., et al. (2006) STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut 55: 1263–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G., Trapasso F., Parrello T., Biancone L., Stella A., Iuliano R., et al. (1999) Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol 163: 143–147 [PubMed] [Google Scholar]
- Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., et al. (2010) Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463: 540–544 [DOI] [PubMed] [Google Scholar]
- Moussion C., Ortega N., Girard J.P. (2008) The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin'? PLoS One 3: e3331–e3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch S.H., Braegger C.P., Walker-Smith J.A., MacDonald T.T. (1993) Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut 34: 1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K., et al. (2010) Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama, T., Mitsuyama, K., Toyonaga, A., Sasaki, E. and Tanikawa, K. (1994) Colonic mucosal interleukin 1 receptor antagonist in inflammatory bowel disease. Digestion 55: 368–373. [DOI] [PubMed]
- O'Connor W., Jr, Kamanaka M., Booth C.J., Town T., Nakae S., Iwakura Y., et al. (2009) A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature immunology 10: 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K., Ohno T., Kajiwara N., Arae K., Morita H., Ishii A., et al. (2010) IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A 107: 18581–18586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikawa K., Yanagisawa K., Tominaga S., Sugiyama Y. (2002) Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy 32: 1520–1526 [DOI] [PubMed] [Google Scholar]
- Palmer G., Lipsky B.P., Smithgall M.D., Meininger D., Siu S., Talabot-Ayer D., et al. (2008) The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 42: 358–364 [DOI] [PubMed] [Google Scholar]
- Palmer G., Talabot-Ayer D., Lamacchia C., Toy D., Seemayer C.A., Viatte S., et al. (2009) Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum 60: 738–749 [DOI] [PubMed] [Google Scholar]
- Pasparakis M. (2008) IKK/NF-kappaB signaling in intestinal epithelial cells controls immune homeostasis in the gut. Mucosal Immunol 1(Suppl. 1): S54–S57 [DOI] [PubMed] [Google Scholar]
- Pastorelli L., Garg R.R., Hoang S.B., Spina L., Mattioli B., Scarpa M., et al. (2010) Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A 107: 8017–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli L., Vecchi M., Cominelli F., Pizarro T.T. (2008) Genetic regulation of epithelial barrier function: Recent insights into the pathogenesis of inflammatory bowel disease. IBD Monitor 8: 124–133 [Google Scholar]
- Pizarro T.T., Michie M.H., Bentz M., Woraratanadharm J., Smith M.F., Jr, Foley E., et al. (1999) IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol 162: 6829–6835 [PubMed] [Google Scholar]
- Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J., et al. (2010) Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A 107: 11489–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj P.N., Tay H.K., H'Ng S.C., Pitman N., Xu D., McKenzie A., et al. (2009) The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A 106: 9773–9778 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reuter B.K., Pizarro T.T. (2004) Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol 34: 2347–2355 [DOI] [PubMed] [Google Scholar]
- Rivera-Nieves J., Bamias G., Vidrich A., Marini M., Pizarro T.T., McDuffie M.J., et al. (2003) Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology 124: 972–982 [DOI] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T., Bianchi M.E. (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195 [DOI] [PubMed] [Google Scholar]
- Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490 [DOI] [PubMed] [Google Scholar]
- Seidelin J.B., Bjerrum J.T., Coskun M., Widjaya B., Vainer B., Nielsen O.H. (2010) IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett 128: 80–85 [DOI] [PubMed] [Google Scholar]
- Shimizu M., Matsuda A., Yanagisawa K., Hirota T., Akahoshi M., Inomata N., et al. (2005) Functional SNPs in the distal promoter of the ST2 gene are associated with atopic dermatitis. Hum Mol Genet 14: 2919–2927 [DOI] [PubMed] [Google Scholar]
- Smithgall M.D., Comeau M.R., Yoon B.R., Kaufman D., Armitage R., Smith D.E. (2008) IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 20: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Takedatsu H., Mitsuyama K., Matsumoto S., Handa K., Suzuki A., Funabashi H., et al. (2004) Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol 34: 1561–1569 [DOI] [PubMed] [Google Scholar]
- Talabot-Ayer D., Lamacchia C., Gabay C., Palmer G. (2009) Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem 284: 19420–19426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri W.A., Jr, Souto F.O., Vieira S.M., Almeida S.C., Fukada S.Y., Xu D., et al. (2010) IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis 69: 1697–1703 [DOI] [PubMed] [Google Scholar]
- Wagenaar J.F., Gasem M.H., Goris M.G., Leeflang M., Hartskeerl R.A., van der Poll T., et al. (2009) Soluble ST2 levels are associated with bleeding in patients with severe Leptospirosis. PLoS Negl Trop Dis 3: e453–e453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J., Koslowski M., Wang G., Stange E.F. (2008) Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol 1(Suppl. 1): S67–S74 [DOI] [PubMed] [Google Scholar]
- Wood I.S., Wang B., Trayhurn P. (2009) IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun 384: 105–109 [DOI] [PubMed] [Google Scholar]
- Xavier R.J., Podolsky D.K. (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434 [DOI] [PubMed] [Google Scholar]
- Xu D., Jiang H.R., Kewin P., Li Y., Mu R., Fraser A.R., et al. (2008) IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A 105: 10913–10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zheng M., Bindas J., Schwarzenberger P., Kolls J.K. (2006) Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis 12: 382–388 [DOI] [PubMed] [Google Scholar]
- Zhiguang X., Wei C., Steven R., Wei D., Wei Z., Rong M., et al. (2010) Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett 131: 159–165 [DOI] [PubMed] [Google Scholar]