Abstract
Infection with Borrelia burgdorferi is common in horses and ponies from the New England and mid-Atlantic regions of the United States. Here, we evaluated luciferase immunoprecipitation systems (LIPS) for profiling antibody responses against three different antigenic targets for the diagnosis of equine B. burgdorferi infection. LIPS testing of horse serum samples suspected of Lyme infection revealed that approximately 75% of the horse samples (114/159) were seropositive against the synthetic VOVO antigen, comprising repeated immunodominant C6 epitopes as well as OspC immunodominant epitopes. A comparison of VOVO and immunofluorescence assays (IFA) showed that 51% of the samples were positive in both assays (VOVO+/IFA+), 13% were VOVO−/IFA+, 21% were VOVO+/IFA−, and 15% were negative in both. To further understand humoral responses to B. burgdorferi and reconcile the diagnostic differences between IFA and VOVO, two additional B. burgdorferi LIPS tests were performed with DbpA and DbpB. Robust seropositive antibody responses against DbpA and/or DbpB were detected in 98% (79/81) of the VOVO+/IFA+ and 93% (50/54) of the discrepant samples. Additionally, some of the samples negative by both VOVO and IFA showed immunoreactivity against DbpA and/or DbpB. Overall, 94% of the suspected horse samples were seropositive by LIPS, and heat map analysis revealed that seropositive samples often were immunoreactive with at least two of the three antigens. These results suggest that LIPS tests employing multiple recombinant antigens offer a promising approach for the evaluation of antibody responses in Lyme disease.
INTRODUCTION
Lyme disease caused by the spirochete Borrelia burgdorferi represents an important problem in veterinary medicine due to the wide-spread infection of dogs, cats, and horses in areas where the organism is endemic (3, 30). While most cases of equine B. burgdorferi infection remain asymptomatic, some horses show signs of illness, including sporadic lameness, weight loss, arthritis, and encephalitis (16). Unlike humans, where the first sign of B. burgdorferi infection is erythema migrans skin lesions, the diagnosis of Lyme disease in horses is complex, and diagnostic testing is only one parameter for confirmation (16).
A variety of serological tests, including immunofluorescence assays (IFA), Western blotting, and enzyme-linked immunosorbent assays (ELISAs), have been employed to detect human antibodies to B. burgdorferi (29). Considerable progress has been made in employing defined antigenic targets, such as the C6 peptide (1, 20, 27) and other recombinant B. burgdorferi antigens, including BBK07 (18, 19) and decorin binding proteins (Dbp) DbpA and DbpB (21, 22, 24, 28, 31, 32). Although extensive testing of different antigens and assay formats has been performed for human Lyme disease (29), less is known about the optimal serological diagnosis of equine B. burgdorferi infection. Recently, the C6 SNAP test has been used for the serological diagnosis of equine Lyme disease (23, 26). Unfortunately, Chang and colleagues found that the C6 SNAP test detected only 63% of known, experimentally B. burgdorferi-infected horses, suggesting that this test is suboptimal for the diagnosis of equine infection (26). In light of the poor sensitivity of the currently available C6 SNAP test, a better understanding of humoral responses in B. burgdorferi-infected horses is needed.
Luciferase immunoprecipitation systems (LIPS) comprise a relatively new approach for the serological testing of antibodies associated with many different pathogens (5). LIPS is based on employing light-emitting Renilla luciferase-fusion proteins in a liquid-phase immunoprecipitation assay. Serologic testing by LIPS for a variety of human pathogens provides the full exploitation of antibody profiles because of its robustness, multiplex capacity, and high sensitivity and specificity (6, 11, 12, 14, 15). Recently, a LIPS test employing a synthetic VOVO antigen consisting of two variants of immunodominant epitopes of the C6 peptide and OspC achieved 98% sensitivity and 100% specificity for the diagnosis of human Lyme disease, but the findings were not statistically different from those of the C6 ELISA (10). LIPS antibody profiling of recombinant DbpA and DbpB also was highly informative (10). In this study, LIPS tests for VOVO, DbpA, and DbpB antigens were used for measuring B. burgdorferi antibodies in horses, and the results were compared to IFA results for the diagnosis of equine B. burgdorferi infection.
MATERIALS AND METHODS
Serum samples.
Serum samples (n = 159) from Maryland horses suspected of having Lyme infection were collected from the Animal Health Laboratory, Maryland Department of Agriculture, Frederick, MD. These Maryland horse samples were previously tested by IFA and scored seropositive if immunoreactivity was detected at a serum dilution of greater than or equal to 1/128. From IFA testing of the 159 equine serum samples from Maryland, 57 were seronegative and 102 were seropositive. As potentially uninfected controls for LIPS testing, six different samples from pools of horse serum from New Zealand (Invitrogen), a region known not to be endemic for Lyme disease (4), were used. Additionally, seven human controls without B. burgdorferi infection, collected under Institutional Review Board-approved protocols at the NIH, also were evaluated.
LIPS analysis.
Extracts from three previously described antigens, VOVO, DbpA, and DbpB, that were highly informative for the diagnosis of human Lyme disease (10) were prepared and used to assess equine B. burgdorferi infection. For each antigen, serum samples were tested in duplicate using the standard liquid-phase LIPS assay (8). Briefly, 40 μl of buffer A (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100), 10 μl of diluted horse or human sera (1 μl equivalent), and 1 × 107 light units (LU) of Ruc-antigen Cos1 cell extract were added (final volume of 100 μl) to each well of a polypropylene plate and incubated for 60 min at room temperature on a rotary shaker. The 100-μl antigen-antibody reaction mixture was transferred to a 96-well filter plate containing 5 μl of a 30% suspension of protein A/G beads and further incubated at room temperature with shaking. After 60 min, a filter plate containing the retained protein A/G beads/antibody-antigen complex was washed using a BioMek robotic workstation with a vacuum manifold (8). The LU of the filter plates were measured in a Berthold LB 960 Centro microplate luminometer (Berthold Technologies, Bad Wilbad, Germany) using coelenterazine substrate mix (Promega, Madison, WI). All data represent raw antibody titers without subtracting the buffer blanks.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism software (San Diego, CA). Statistical significance was determined using Mann-Whitney U tests. Due to the wide dynamic range of the LIPS data, the results for quantitative antibody levels are reported as the geometric mean titers (GMT) ± 95% confidence intervals (CI). Although LIPS offers highly quantitative data that can be used to generate diagnostically useful serodeterminations without predetermined cutoff values (5, 7, 9), cutoff values for each of the three antigens were generated based on the uninfected New Zealand horse serum samples. A data-driven analytical approach using high-tailed intensity distribution was used to assign cutoff values for the VOVO, DbpA, and DbpB LIPS tests. For the initial evaluation of seropositivity, a standard cutoff value of 18,000 LU, which was greater than the means plus 20 standard deviations (SD) of the control New Zealand horse and known uninfected human samples, was used.
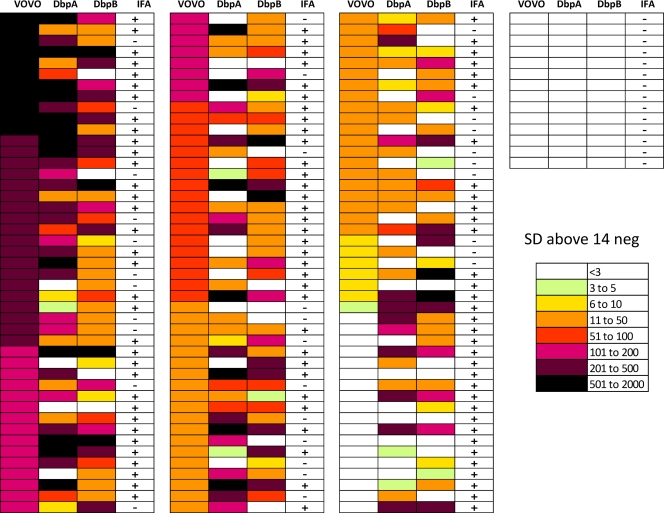
The heat map shown in Fig. 3 represents color-coded antibody titers with a palette ranging from red to green indicating high and low titers, respectively. For this heat map, the means and standard deviations from the 14 horse serum samples that were negative for both IFA and the three LIPS antigens (VOVO, DbpA, and DbpB) were used as a reference scale. Antibody titers for each antigen-antibody measurement greater than those of the negative horse sample means plus 3 SD were color coded to signify the relative numbers of SD above this value.
Fig. 3.
Heat map analysis of antibody profiles in the horse cohort. Antibody titers to VOVO, DbpA, and DbpB are shown for each of the 159 horse serum samples. Titer values greater than the means from the 14 negative samples (14 neg) plus 3 SD were color coded from green to dark purple to signify the relative number of SD above the reference values. Each row represents one serum sample tested with the three different LIPS antigens. The samples were ranked from highest to lowest based on anti-VOVO antibody titers. The serological status by IFA is denoted for each sample tested.
RESULTS
Comparison of the VOVO LIPS test to IFA using horse serum samples.
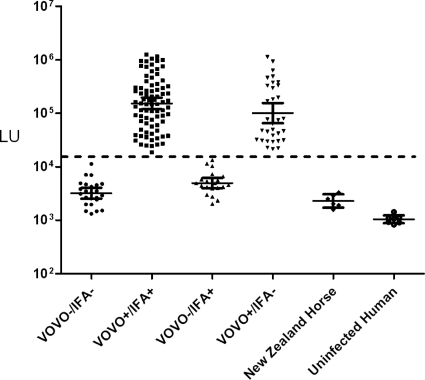
To test the effectiveness of the VOVO LIPS test and compare it to IFA for the diagnosis of equine B. burgdorferi infection, 159 horse serum samples suspected of Lyme disease were evaluated along with control serum samples. The testing of the VOVO antigen with the coded horse serum samples showed highly reproducible antibody titers ranging from 1,320 to 1,135,000 LU (Fig. 1). In contrast, the anti-VOVO antibody titer of the six horse serum samples from New Zealand, a region in which B. burgdorferi infection is not endemic, were extremely low, with a GMT of 2,295 LU (95% CI, 1,720 to 3,063 LU) (Fig. 1), which was similar to the GMT of 1,042 LU in the uninfected human controls (Fig. 1). Based on a defined high cutoff value for the VOVO antigen corresponding to 18,000 LU, 72% (114/159) of the horse samples were seropositive (Fig. 1). The GMT in the VOVO-positive samples was 135,700 LU (95% CI, 109,300 to 168,400 LU) and was markedly higher than the GMT of the VOVO-negative samples of 3,928 LU (95% CI, 3,323 to 4,642 LU). Following the decoding of sample identities, the diagnostic results from VOVO were compared to IFA results. These analyses revealed that 51% (81/159) of the suspected horse samples were positive in both assays, 13% (21/159) were VOVO−/IFA+, 21% (33/159) were VOVO+/IFA−, and 15% (24/159) were negative in both assays (Fig. 1). No statistical difference in anti-VOVO antibody titers was found between the VOVO+/IFA+ and VOVO+/IFA− samples (P > 0.54 by Mann-Whitney U test). Overall, 66% of the Maryland horse serum samples yielded the same serological status between the VOVO test and IFA, with 34% of the serum samples being discrepant. These findings highlight the marked differences in detecting anti-B. burgdorferi antibody responses between the VOVO test and IFA for the diagnosis of horse infection.
Fig. 1.
Comparison of the VOVO LIPS test to IFA for diagnosis of equine B. burgdorferi infection. Each symbol represents the antibody titer, expressed as the number of LU on the y axis (log10 scale), of a single horse serum sample determined using the VOVO LIPS test. Antibody titers of the six horse serum samples from New Zealand, a region in which B. burgdorferi infection is not endemic, and uninfected human controls also are shown. The dotted line indicates the cutoff value used to determine VOVO seropositive status. Following unblinding, the results were compared to those of the B. burgdorferi IFA, and samples were binned into four groups according to VOVO and IFA serological status. The solid horizontal lines indicate the geometric mean antibody titers in each group, and the vertical lines show the 95% confidence intervals.
LIPS detection of anti-DbpA and anti-DbpB antibodies in horse serum.
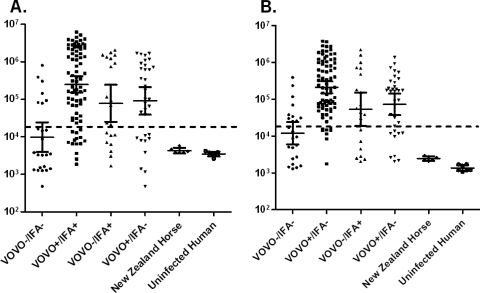
To further understand humoral responses to B. burgdorferi in these horse samples and potentially reconcile diagnostic differences between the VOVO test and IFA, two additional antigens, DbpA and DbpB, were tested by LIPS. Similarly to the anti-VOVO antibody titers, anti-DbpA and anti-DbpB antibody titers ranged from 2,000 to more than 2,000,000 LU (Fig. 2). In the control horse serum samples from New Zealand, the anti-DbpA antibodies were near the buffer baseline with a GMT of 4,249 LU (95% CI, 3,571 to 5,055 LU), which was similar to that of human uninfected control samples (Fig. 2A and data not shown). Based on a defined cutoff of 18,000 LU, the diagnostic potentials of the anti-DbpA antibody titers were further analyzed in the four VOVO/IFA subgroups (Fig. 2A). Anti-DbpA seropositivity was detected in 67/81 of the IFA+/VOVO+, 13/21 of the IFA+/VOVO−, 23/33 of the IFA−/VOVO+, and 7/24 of the IFA−/VOVO− samples (Fig. 2A and Table 1). Statistical analysis revealed that the anti-DbpA antibody titers were higher in the VOVO+/IFA+ than VOVO+/IFA− and VOVO−/IFA+ samples (P < 0.035). Similarly to the anti-DbpA antibodies, the anti-DbpB antibodies in the control New Zealand and uninfected human samples were near the buffer baseline (Fig. 2B and data not shown). However, anti-DbpB antibody seropositivity was detected in 75/81 VOVO+/IFA+, 14/21 VOVO−/IFA+, 24/33 IFA−/VOVO+, and 8/24 IFA−/VOVO− samples (Fig. 2B and Table 1). Statistical analysis revealed higher anti-DbpB antibody titers in the VOVO+/IFA+ than VOVO+/IFA− and VOVO−/IFA+ samples (P < 0.035). Of note, horse samples seropositive for DbpA and DbpB showed remarkably high levels of antibodies, with GMT of 433,700 LU (95% CI, 322,800 to 582,600 LU) and 248,600 LU (95% CI, 191,300 to 322,900 LU), respectively.
Fig. 2.
LIPS profiling of anti-DbpA and anti-DbpB antibodies. Each symbol represents the antibody titers as the numbers of LU on a log10 scale for anti-DbpA antibodies (A) and anti-DbpB antibodies (B) in the 159 Maryland horse serum samples from each of the four VOVO/IFA subgroups. Antibody titers of the six horse serum samples from New Zealand, a region in which B. burgdorferi infection is not endemic, and uninfected human controls also are shown. The dotted line indicates the standard cutoff that was used. The solid horizontal lines indicate the geometric mean antibody titers in each group, and the vertical lines show the 95% confidence intervals.
Table 1.
Serological categories and frequency of VOVO, DbpA, and DbpB antibodies
| Serological status | No. positive or negative for VOVO antibody | No. (%) positive/total no. for antibody to: |
||
|---|---|---|---|---|
| DbpA | DbpB | DbpA/B | ||
| VOVO−/IFA− | 24 Negative | 7/24 (29) | 8/24 (33) | 10/24 (42) |
| VOVO+/IFA+ | 81 Positive | 67/81 (83) | 75/81 (93) | 79/81 (98) |
| VOVO−/IFA+ | 21 Negative | 13/21 (62) | 14/21 (67) | 17/21 (81) |
| VOVO+/IFA− | 33 Positive | 23/33 (70) | 24/33 (73) | 32/33 (97) |
Overall, anti-DbpA and/or anti-DbpB antibodies were detected in 98% (79/81) of the VOVO+/IFA+ samples, 81% (17/21) of the VOVO−/IFA+ samples, 97% (32/33) of the VOVO+/IFA− samples, and 46% (10/24) of the VOVO−/IFA− samples (Table 1). In the VOVO−/IFA− samples, only 14 samples were found to be completely devoid of seropositive antibodies. These results demonstrate a high frequency of high-titer anti-Dbp antibodies in samples negative by VOVO test and IFA and suggest that many of these samples represent true B. burgdorferi-infected horse serum samples.
Antibody profiles.
Based on the complexity of the antibody responses seen with IFA and LIPS testing, a heat map was used to summarize and further analyze the LIPS antibody profiles (Fig. 3). Each row represents an LIPS profile of a single Maryland horse serum sample alongside the corresponding IFA status. For this heat map, the 14 negative Maryland horse samples devoid of anti-VOVO, anti-DbpA, and anti-DbpB antibodies and also negative by IFA formed the basis for data transformation. The antibody titer values for each antigen-antibody measurement were color coded to signify the relative number of standard deviations above the means plus 3 SD as a cutoff value for the 14 negative samples. The analysis of the heat map revealed several interesting findings. An extraordinary range of antibody titers (i.e., from less than 3 to 2,000 SD above the cutoff for the 14 negative samples) was seen against these three B. burgdorferi antigens in the different horse serum samples (Fig. 3). Due to the high standard cutoff used for initial screening, several lowly positive horse samples previously negative for anti-VOVO and anti-Dbp antibodies now showed statistically elevated responses. The analysis of the LIPS antigen immunoreactive profile of each sample demonstrated that 49% (78/159) of the samples were seropositive with all three antigens, 28% (44/159) were seropositive with two antigens, and 6% (9/159) were seropositive with only one antigen. Interestingly, 33 of the horse samples that were seropositive by LIPS, all reacting with at least two LIPS antigens, failed to show seropositivity by IFA testing. Additionally, four samples seropositive by IFA testing failed to show seropositivity with any of the three LIPS antigens and may represent false positives or samples missed by the B. burgdorferi LIPS tests. These results highlight the heterogeneity and spectrum of antibody responses seen in horses against these defined B. burgdorferi antigens using these different antigens and assay formats.
DISCUSSION
This study demonstrates that LIPS shows promising results for the robust detection of equine humoral responses against B. burgdorferi antigens. In contrast to Western blot-based diagnostic testing, LIPS has the advantages of generating highly quantitative and robust antibody data against multiple, defined targets in a high-throughput capacity (6, 10). Our approach of profiling three different B. burgdorferi antigens provides novel insight into equine Lyme disease. Several different lines of evidence support the findings that 94% (145/159) of the suspected horse serum samples have humoral responses to B. burgdorferi. First, the anti-B. burgdorferi antibodies detected by LIPS assay in the suspected horse samples were substantially higher than those of the control horse serum from New Zealand. These New Zealand horse serum samples also showed baseline responses similar to those of uninfected human samples. Second, the LIPS immunoreactivity observed against multiple B. burgdorferi antigens adds confidence that a horse serum sample is truly positive. Our finding that 77% of equine samples are positive for at least two LIPS antigens supports the high likelihood that many of these horse serum samples have antibodies against B. burgdorferi due to current and/or past infection. Lastly, our results suggest that the IFA did not detect some B. burgdorferi-infected samples. While 100 of the 102 IFA-positive horse samples also were positive by LIPS, an additional 25 horse samples not positive by IFA were positive by LIPS.
A previous study using the SNAP C6 test with experimental animals showed 65% sensitivity in detecting B. burgdorferi-infected horses and ponies (26) and matched quite well with our C6-based VOVO test showing 72% sensitivity. In the SNAP C6 study, several experimentally B. burgdorferi-infected ponies were identified that were culture and PCR positive, but they did not show anti-C6 antibodies. One possible explanation for the low sensitivity of the C6 (and C6-based VOVO) test is that the C6 immunodominant peptide detects antibodies related to active infection but poorly detects past resolved infections, such as those of horses that have been treated with antibiotics (33). It is also important that B. burgdorferi infection in most horses is asymptomatic (16). For example, ponies experimentally infected with B. burgdorferi showed no clinical problems (26). Nevertheless, like the variable clinical spectrum of human Lyme disease, equine B. burgdorferi infection can cause severe illness in some horses, including Lyme neuroborreliosis (25). Future LIPS studies examining experimental B. burgdorferi infection in horses and correlating antibody titers detected with equine disease symptoms would be worthwhile.
One of the major findings of our study was that there was marked heterogeneity in equine antibody responses to the B. burgdorferi antigens. While there was limited overlap (49.6%) between IFA and LIPS testing with three different antigens, each individual test detected a significant number of samples missed by any other single test. The analysis of test sensitivity revealed that the IFA had the lowest sensitivity of 64%, followed by DbpA with 71%, VOVO with 72%, and DbpB with 84%. In several other studies, antibodies to Dbp proteins were found to be very useful for late Lyme disease but not for early disease (17, 24). It is appropriate to point out that DbpA and DbpB, both of which are expressed by B. burgdorferi during the mammalian phase of infection, including human infection (2), share only 39% identity. Consistently with their limited homology, anti-DbpA and anti-DbpB antibody titers in the seropositive horse samples poorly correlated with each other. In contrast to our previous studies of humans (10), profiling anti-Dbp antibodies in horses appeared to markedly improve sensitivity compared to that of only examining anti-VOVO antibodies. The reason for this discrepancy may relate to horse immune differences as well as the likelihood that many of the horse serum samples represent late Lyme infection. Although not explored here, an antigen mixture of different B. burgdorferi strains by LIPS also might be useful for diagnostic screening (6, 13, 14). For example, based on the comparably low background signals of VOVO, DbpA, and DbpB, these antigens could be employed as a mixture, thereby simplifying data collection and analysis. Further large-scale epidemiological studies of humans are needed to examine in detail whether the detection of anti-Dbp antibodies improves the sensitivity of detecting human Lyme diseases beyond just evaluating anti-VOVO antibodies.
ACKNOWLEDGMENTS
This work was supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research. This research was supported in part by NIH/NIAID awards (AI080615 and AR055323) to U.P.
We are thankful to Rachel Westerlund and Laura Smith for their help with the collection and analysis of the serum samples.
P.D.B. and M.J.I. have submitted a patent application for using LIPS for detecting anti-B. burgdorferi antibodies.
Footnotes
Published ahead of print on 20 July 2011.
REFERENCES
- 1. Bacon R. M., et al. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbour A. G., et al. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect. Immun. 76:3374–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berrada Z. L., Telford S. R., III 2009. Burden of tick-borne infections on American companion animals. Top. Companion Anim. Med. 24:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Branda J. A., et al. 2010. 2-Tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin. Infect. Dis. 50:20–26 [DOI] [PubMed] [Google Scholar]
- 5. Burbelo P. D., Ching K. H., Bren K. E., Iadarola M. J. 2011. Searching for biomarkers: humoral response profiling with luciferase immunoprecipitation systems. Expert Rev. Proteomics 8:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burbelo P. D., Ching K. H., Bush E. R., Han B. L., Iadarola M. J. 2010. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev. Vaccines 9:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burbelo P. D., et al. Serological studies confirm novel astrovirus HMOAstV-C as a highly prevalent human infectious agent. PLoS One, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burbelo P. D., Ching K. H., Klimavicz C. M., Iadarola M. J. 2009. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J. Vis. Exp. 32:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burbelo P. D., et al. 2007. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem. Biophys. Res. Commun. 352:889–895 [DOI] [PubMed] [Google Scholar]
- 10. Burbelo P. D., et al. 2010. Rapid, simple, quantitative, and highly sensitive antibody detection for Lyme disease. Clin. Vaccine Immunol. 17:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burbelo P. D., et al. 2009. Highly quantitative serological detection of anti-cytomegalovirus (CMV) antibodies. Virol. J. 6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burbelo P. D., et al. 2010. Distinct profiles of antibodies to Kaposi sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. J. Infect. Dis. 201:1919–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burbelo P. D., et al. 2009. Four-antigen mixture containing v-cyclin for serological screening of human herpesvirus 8 infection. Clin. Vaccine Immunol. 16:621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burbelo P. D., Leahy H. P., Iadarola M. J., Nutman T. B. 2009. A four-antigen mixture for rapid assessment of Onchocerca volvulus infection. PLoS Negl. Trop. Dis. 3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burbelo P. D., et al. 2008. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Retrovirology 5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler C. M., Houwers D. J., Jongejan F., van der Kolk J. H. 2005. Borrelia burgdorferi infections with special reference to horses. A review. Vet. Q. 27:146–156 [PubMed] [Google Scholar]
- 17. Cinco M., Ruscio M., Rapagna F. 2000. Evidence of Dbps (decorin binding proteins) among European strains of Borrelia burgdorferi sensu lato and in the immune response of LB patient sera. FEMS Microbiol. Lett. 183:111–114 [DOI] [PubMed] [Google Scholar]
- 18. Coleman A. S., Pal U. 2009. BBK07, a dominant in vivo antigen of Borrelia burgdorferi, is a potential marker for serodiagnosis of Lyme disease. Clin. Vaccine Immunol. 16:1569–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coleman A. S., et al. 2011. BBK07 immunodominant peptides as serodiagnostic markers of Lyme disease. Clin. Vaccine Immunol. 18:406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Embers M. E., Jacobs M. B., Johnson B. J., Philipp M. T. 2007. Dominant epitopes of the C6 diagnostic peptide of Borrelia burgdorferi are largely inaccessible to antibody on the parent VlsE molecule. Clin. Vaccine Immunol. 14:931–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fikrig E., et al. 1992. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J. Infect. Dis. 165:1127–1132 [DOI] [PubMed] [Google Scholar]
- 22. Gomes-Solecki M. J., et al. 2000. Recombinant chimeric Borrelia proteins for diagnosis of Lyme disease. J. Clin. Microbiol. 38:2530–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen M. G., Christoffersen M., Thuesen L. R., Petersen M. R., Bojesen A. M. 2010. Seroprevalence of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in Danish horses. Acta Vet. Scand. 52:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heikkilä T., et al. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 40:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imai D. M., et al. 2011. Lyme neuroborreliosis in 2 horses. Vet. Pathol. [Epub ahead of print.] doi:10.1177/0300985811398246 [DOI] [PubMed] [Google Scholar]
- 26. Johnson A. L., Divers T. J., Chang Y. F. 2008. Validation of an in-clinic enzyme-linked immunosorbent assay kit for diagnosis of Borrelia burgdorferi infection in horses. J. Vet. Diagn. Investig. 20:321–324 [DOI] [PubMed] [Google Scholar]
- 27. Liang F. T., et al. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J. Clin. Microbiol. 37:3990–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magnarelli L. A., Ijdo J. W., Padula S. J., Flavell R. A., Fikrig E. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marques A. R. 2010. Lyme disease: a review. Curr. Allergy Asthma Rep. 10:13–20 [DOI] [PubMed] [Google Scholar]
- 30. Morshed M. G., et al. 2006. Distribution and characterization of Borrelia burgdorferi isolates from Ixodes scapularis and presence in mammalian hosts in Ontario, Canada. J. Med. Entomol. 43:762–773 [DOI] [PubMed] [Google Scholar]
- 31. Panelius J., et al. 2003. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VlsE IR6 peptide. J. Neurol. 250:1318–1327 [DOI] [PubMed] [Google Scholar]
- 32. Panelius J., Sillanpaa H., Seppala I., Sarvas H., Lahdenne P. 2007. Antibodies to recombinant decorin-binding proteins A and B in the cerebrospinal fluid of patients with Lyme neuroborreliosis. Scand. J. Infect. Dis. 39:775–780 [DOI] [PubMed] [Google Scholar]
- 33. Philipp M. T., et al. 2005. A decline in C6 antibody titer occurs in successfully treated patients with culture-confirmed early localized or early disseminated Lyme borreliosis. Clin. Diagn. Lab. Immunol. 12:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]