Abstract
Commensal microbes in the intestine are in constant interaction with host cells and play a role in shaping the immune system. Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus salivarius are members of the chicken intestinal microbiota and have been shown to induce different cytokine profiles in mononuclear cells in vitro. The objective of the present study was to examine the effects of these bacteria individually or in combination on the induction of antibody- and cell-mediated immune responses in vivo. The birds received lactobacilli weekly via oral gavage starting on day of hatch and subsequently, at 14 and 21 days, were immunized with sheep red blood cells (SRBC), keyhole limpet hemocyanin (KLH), Newcastle disease virus vaccine, and infectious bursal disease virus vaccine. Antibody responses in serum were measured weekly for 4 weeks beginning on the day of primary immunization. The cell-mediated immune response was evaluated at 21 days postimmunization by measurement of gamma interferon (IFN-γ) production in splenocytes stimulated with inactivated vaccine antigens. L. salivarius-treated birds had significantly more serum antibody to SRBC and KLH than birds that were not treated with probiotics. L. salivarius-treated birds also had decreased cell-mediated immune responses to recall antigen stimulation. L. reuteri treatment did not significantly affect the systemic immune response, while L. acidophilus treatment increased the antibody response to KLH. These results indicate that systemic antibody- and cell-mediated immune responses can be modulated by oral treatment with lactobacilli but that these bacteria may vary in their ability to modulate the immune response.
INTRODUCTION
Probiotics are defined as live microorganisms which, when administered in adequate amounts, confer a health benefit to the host through improvements to the intestinal microbial balance (16). Lactobacilli are nonpathogenic Gram-positive inhabitants of animal intestinal microbiota that are widely used as probiotics. Although their mode of action is not completely understood, the use of these beneficial bacteria in both humans and farm animals is an area of intensive research (5, 8, 17). In the chicken, along with the ability to improve production parameters and to limit food-borne pathogens (1, 2, 19, 25, 36, 37, 49, 52, 53), treatments with various members of the Lactobacillus species have been shown to stimulate multiple aspects of the immune response. These activities include the ability to modulate chicken cytokine and chemokine gene expression (7, 11, 24), enhance the expression of Toll-like receptor (TLR) and T cell-related mRNA expression levels in the gut (44), enhance the function of T cells in newly hatched chicks (15), increase the number of intestinal epithelial lymphocytes (IELs) expressing CD3, CD4, CD8, and T cell receptor (TCR) αβ (10, 40), and improve systemic antibody response (22, 23, 26, 29, 30, 31, 51). In contrast, others have found that probiotic bacteria cannot alter the amount of serum immunoglobulin G (IgG), IgM, or IgA (3, 38, 48). The strain of Lactobacillus selected, dosage, method of preparation, and condition of animals are thought to be partially responsible for such discrepancies. These studies demonstrate that different strains of each bacterial species function differently but that many of them have immunomodulatory effects. This highlights the need to objectively examine the effect of potential probiotic bacteria for their immunomodulatory ability.
The ability of probiotic bacteria to stimulate the immune system is an additional reason for supporting their use as alternatives to antibiotics for improving animal health and protection against infectious agents. In spite of the interest in the use of probiotics in commercial poultry production, to date there is little information available on the mechanisms through which probiotic bacteria affect the chicken immune response. Given that Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus salivarius are all members of the intestinal microbiota of chickens, we conducted a series of studies to assess the influence of these bacteria on the chicken immune system. It was discovered that isolates of L. acidophilus, L. reuteri, and L. salivarius differentially altered the in vitro cytokine profiles of spleen and cecal tonsil cells (7). Specifically, we found that L. acidophilus was more effective at inducing T helper 1 (Th1) cytokines, such as gamma interferon (IFN-γ), interleukin-12 (IL-12), and IL-18, while L. salivarius induced more transforming growth factor β4 (TGF-β4) and IL-10. Further studies revealed that structural components of L. acidophilus, such as DNA, induce the in vitro expression of a number of genes in cecal tonsil mononuclear cells of chickens, including those for IFN-α, IFN-γ, and IL-18 (4). The present study was designed to further investigate the in vivo ability of three orally administered Lactobacillus bacteria, i.e., L. acidophilus, L. reuteri, and L. salivarius, to alter the antibody- and cell-mediated immune responses in chickens. We hypothesized that isolates of these three Lactobacillus species would differ in their abilities to alter the systemic immune response.
MATERIALS AND METHODS
Chickens and housing.
Newly hatched female commercial broiler chicks were obtained from Maple Leaf Hatchery (New Hamburg, ON, Canada). Birds were maintained in floor pens on clean wood shavings at the Arkell Poultry Research Station (University of Guelph, ON, Canada). Chicks were provided with free access to water and feed. The research was approved by the University of Guelph Animal Care Committee and adhered to the guidelines of the Canadian Council for Animal Care (www.ccac.ca).
Bacterial isolates, culture media, and growth conditions.
Lactobacillus acidophilus was isolated from a commercial probiotic product (Intervet, Whitby, ON, Canada), whereas L. reuteri and L. salivarius were isolated from intestinal contents of broiler chickens. Briefly, 250 mg of ileal contents was inoculated into DeMan, Rogosa, and Sharpe (MRS) broth (Becton Dickinson, Mississauga, ON, Canada), grown at 41°C under anaerobic conditions for 48 h, subcultured twice, and then diluted and plated onto MRS plates. Individual colonies were selected, and the bacteria were identified by PCR, amplification of the V3 region of the 16S rRNA gene, sequencing of the PCR products, and comparison with nonredundant nucleotides in the GenBank database using BLAST. Each Lactobacillus sp. was cultured in MRS broth (Becton Dickinson, Mississauga, ON, Canada) at 41°C without shaking. Bacteria were harvested from an overnight culture by centrifugation (5,000 × g for 15 min), and pelleted bacteria were then washed three times in phosphate-buffered saline (PBS) and diluted to 4 × 107 CFU/ml in PBS.
Experimental design.
One hundred five 1-day-old chicks were randomly allocated into seven treatment groups. Chicks received 1 × 107 CFU/chick of either L. acidophilus, L. reuteri, or L. salivarius, an equal combination (1 × 107 CFU/chick total) of all three (mix), or PBS (control group) by oral gavage weekly starting on the day of hatch and throughout the trial. There were seven groups, as follows: (i) PBS treated and nonimmunized (n = 15); (ii) L. acidophilus, L. reuteri, and L. salivarius treated (mix) and nonimmunized (n = 15); (iii) L. acidophilus treated and immunized (n = 15); (iv) L. reuteri treated and immunized (n = 15); (v) L. salivarius treated and immunized (n = 15); (vi) L. acidophilus, L. reuteri, and L. salivarius treated (mix) and immunized (n = 15); and (vii) PBS treated and immunized (n = 15).
Chicks were immunized intramuscularly with 0.25 ml of 2% sheep red blood cells (SRBC) (PML Microbiologicals, Mississauga, ON, Canada) in PBS and subcutaneously with 0.25 ml of PBS containing 100 μg keyhole limpet hemocyanin (KLH) (Sigma, Oakville, ON, Canada) at 14 days posthatch, followed by a secondary immunization 1 week later with both SRBC and KLH, similarly to the protocol used previously (6). In addition, these groups were also immunized subcutaneously with Vaxxitek, a recombinant herpesvirus of turkeys (HVT) expressing the VP2 antigen of infectious bursal disease virus (IBDV) (Merial Canada Inc., Baie D'Urfré, QC, Canada) and intraocularly with Newcastle disease virus (NDV) vaccine (B1 strain) (Pfizer Canada Inc., Kirkland, QC,) at 14 days posthatch according to the manufacturer's instructions. PBS and vaccine diluent were administered as a placebo in those groups that were not immunized.
Quantification of bacteria.
Birds were euthanized at the ages of 4, 5, and 6 weeks, and ileal contents were collected. After thorough mixing of the digesta from each bird, 0.25 g was placed in a 2-ml microcentrifuge tube and DNA was extracted using the QIAamp DNA stool minikit (Qiagen Inc., Valencia, CA) according to the manufacturer's recommendations. All DNA samples of ileal digesta were analyzed for the abundance of the three species of bacteria using species-specific primers and real-time quantitative PCR as described below. The specificity of the primers used to quantify the bacteria has been previously validated (18, 46).
Serum collection.
Blood samples were collected from the wing vein on the day of immunization as well as at 7, 14, and 21 days post-primary immunization (dpi). Blood was kept at room temperature for approximately 2 h and then at 4°C overnight. Samples were centrifuged for 10 min at 580 × g, and serum was harvested and stored at −20°C.
Serological analysis.
A direct hemagglutination assay was performed to measure the antibody response to SRBC in serum according to the procedure of Haghighi and colleagues (22). Briefly, complement in the serum samples was inactivated by incubation at 56°C for 30 min. Serum samples (50 μl) were serially doubly diluted in 50 μl of PBS containing 0.05% bovine serum albumin (BSA), and 50 μl of 1% SRBC in PBS was added. Subsequently plates were shaken for 1 min and incubated for 24 h at 37°C. A positive result was recorded when at least 50% SRBC agglutination was observed. To measure anti-SRBC IgG and IgM antibodies, serum samples were treated with 0.2 M 2-mercaptoethanol (2-ME) for 30 min at 37°C. This treatment inactivates IgM, and as a result, hemagglutination observed after treatment with 2-ME is due mostly to the presence of IgG antibodies. The difference between total antibody and IgG titers determines the IgM titer.
Detection of antibodies against KLH in sera was performed using an indirect enzyme-linked immunosorbent assay (ELISA). Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat anti-chicken IgG and HRP-conjugated goat anti-chicken IgM and were purchased from MJS BioLynx Inc. (Brockville, ON, Canada). The ELISAs were performed according to the methods of Haghighi and colleagues (22). Briefly, plates were coated with 100 μl of 1-μg/ml KLH in coating buffer (pH 9.6) containing BSA (30 μg/ml) and incubated overnight at 4°C. Plates were washed three times in washing buffer (PBS with 0.05% Tween 20) and blocked (PBS with 0.05% Tween 20 and 0.25% fish gelatin) at 37°C for 1 h. Serum samples, diluted 1:200 in washing buffer, were added to the wells in duplicate, and plates were incubated for 1 h at 37°C and then washed three times. One hundred microliters of goat anti-chicken IgG or goat anti-chicken IgM conjugated to HRP was added to every well and incubated for 1 h at room temperature before the plates were washed three times. In the case of IgM ELISA, washings were done three times as described above, with the exception that the plates were incubated for 5 min in washing buffer prior to decanting the buffer. One hundred microliters of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] peroxidase substrate system (Mandel Scientific, Guelph, ON, Canada) was then added to each well, and the plates were incubated for 30 min at room temperature in the dark, after which the absorbance was measured at 405 nm. Positive-control chicken serum available in our lab and negative-control serum (fetal bovine serum) were included in each plate and to account for plate-to-plate variations. Sample/positive (Sp) ratios were determined according to the following formula: Sp = (mean of test sample − mean of negative control)/(mean of positive control − mean of negative control).
The presence of Newcastle disease virus (NDV) antibodies in serum was detected using the Synbiotics ProFLOK NDV Plus ELISA kit (Biovet Canada, Saint-Hyacinthe, QC, Canada). The titers were obtained and calculated as described by the manufacturer. The presence of serum antibodies against the VP2 antigen of IBDV was detected using the Synbiotics ProFLOK IBD Plus ELISA kit (Synbiotics, Kansas City, MO) according to the manufacturer's instructions.
Preparation of spleen mononuclear cells.
Spleens were harvested from four chickens per group at 21 and 28 days post-primary immunization. The spleens were rinsed in 1× Hanks' balanced salt solution (HBSS) and then minced with sterile scalpels. The tissue was further disrupted with the flat end of a 10-ml syringe plunger and strained through a 40-μm nylon cell strainer to obtain a single-cell suspension. The suspension was then overlaid onto a Histopaque-1077 (Sigma, Oakville, ON, Canada) density gradient and centrifuged at 400 × g for 30 min, and mononuclear cells at the interface were collected and washed twice in RPMI (Invitrogen, Burlington, ON, Canada). Cells were counted on a hemocytometer using trypan blue dye exclusion before being suspended in RPMI 1640 (Invitrogen) containing 10% fetal bovine serum, 200 U/ml penicillin, 80 μg/ml streptomycin, and 50 μg/ml gentamicin.
Mitogens and recall antigens.
The mitogen concanavalin A (ConA) was purchased from Sigma. For preparation of NDV recall antigens, a viral stock of NDV strain B1, generously provided by ´Eva Nagy (Department of Pathobiology, University of Guelph), was produced by inoculating 10-day-old embryonated chicken eggs with 4 hemagglutination units (HAU) of stock virus. Infected allantoic fluid was harvested at 72 h postinfection, and virus purification was performed by continuous and discontinuous sucrose density gradient ultracentrifugation by the method described by Reynolds and Maraqa (43) at 100,000 × g for 2 h and resuspension in 2 ml of HNE buffer (5.0 mM HEPES, 0.15 M NaCl, 0.01 M EDTA, pH 7.4). IBDV VP2 antigen was prepared from the live attenuated Vaxxitek HVT + IBD vaccine (Merial Canada Inc.). One vial of the vaccine was suspended in RPMI 1640 (Invitrogen) at a concentration of 200 doses/ml. Both viruses present in the NDV and IBDV vaccines were UV inactivated in 24-well flat-bottomed plates using a maximum of 500 μl per well. Plates were UV irradiated in a UV cross-linker (FB-UVXL-1000; Fisher Biotech, CA) by 3 rounds of 1,200 mJ with a 2-min pause after each round in order to not overheat the samples. The samples were kept on ice and sonicated with maximum effect (130 W) for 30 s. The protein concentration of NDV antigen was determined with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL) according to the manufacturer's instructions. All antigen preparations were aliquoted and stored at −20°C until use.
In vitro stimulation of spleen cells.
For each of the time points, 1 ml of the spleen mononuclear cell suspension (1 × 107 cells/ml) was seeded into a 48-well flat-bottom plate. Two wells were unstimulated, two wells were stimulated with 20 μg/ml ConA (Sigma), two wells were stimulated with 1 μg/ml inactivated NDV, and two wells were stimulated with an amount of UV-inactivated Vaxxitek HVT + IBDV that correlate to 2 doses of vaccine. The optimal concentrations of all recall antigens were determined in a pilot experiment (data not shown). The cells were incubated at 41°C in a humidified 5% CO2 environment, and cells were harvested for RNA extraction at 24 h poststimulation and cell supernatant harvested for gamma interferon (IFN-γ) ELISA at 24 and 48 h poststimulation.
Chicken IFN-γ ELISA.
The concentration of IFN-γ in cell supernatants was assessed using a commercial chicken IFN-γ ELISA (Invitrogen) according to the manufacturer's instructions. The IFN-γ concentration was calculated based on comparison to the standard curve generated using known amounts of IFN-γ protein.
RNA isolation.
Total RNA was extracted from in vitro-stimulated spleen mononuclear cells cultured for 24 h with medium, inactivated NDV, or inactivated Vaxxitek HVT + IBDV using TRIzol reagent (Invitrogen) according to the manufacturer's recommendations with the addition of 10 μg glycogen (Invitrogen). Total RNA was then treated with DNase using the DNA-free kit from Ambion (Austin, TX) according to the manufacturer's instructions.
Reverse transcriptase quantitative PCR (RT-qPCR) analyses.
The cDNA synthesis was performed with 1 μg of total RNA using oligo(dT) primers and SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time quantification was performed in a LightCycler 480 instrument (Roche Diagnostics, Laval, QC, Canada) using the SYBR green dye. PCR mixtures (final volume of 20 μl) contained 10 μl of the LightCycler 480 SYBR green I master mix (Roche Diagnostics), 5 μl of a 1:20 dilution of the cDNA, and 0.25 mM each primer. The cycling conditions included an initial heat denaturation step at 95°C for 10 min, 40 cycles at 95°C for 10 s, annealing as described in Table 1 for each of the primers, and product elongation and signal acquisition (single mode) at 72°C for 10 s. Following amplification, the melting curves were determined in a three-segment cycle of 95°C for 0 s, 65°C for 15 s, and 95°C for 0 s at the continuous-acquisition mode. The temperature transition rates were set at 20°C/s except for segment three of the melting curve analysis, where it was set to 0.1°C/s. Results were analyzed with RelQuant software (Roche Diagnostics). Expression levels were normalized to β-actin expression, which was used as an internal housekeeping control.
Table 1.
Sequences and annealing temperatures for real-time quantitative PCR primers
| Amplicon | Primer sequence (5′ → 3′) |
Annealing temp (°C) | |
|---|---|---|---|
| Forward | Reverse | ||
| IL-6 | CAGGACGAGATGTGCAAGAA | TAGCACAGAGACTCGACGTT | 64 |
| IL-12p40 | TTGCCGAAGAGCACCAGCCG | CGGTGTGCTCCAGGTCTTGGG | 64 |
| IL-13 | ACTTGTCCAAGCTGAAGCTGTC | TCTTGCAGTCGGTCATGTTGTC | 60 |
| IFN-γ | ACACTGACAAGTCAAAGCCGC | AGTCGTTCATCGGGAGCTTG | 60 |
| β-Actin | CAACACAGTGCTGTCTGGTGGTA | ATCGTACTCCTGCTTGCTGATCC | 58 |
| L. acidophilus | GATCGCATGATCAGCTTATA | AGTCTCTCACTCGGCTATG | 55 |
| L. reuteri | CAGACAATCTTTGATTGTTTAG | GCTTGTTGGTTTGGGCTCTTC | 55 |
| L. salivarius | CGAAACTTTCTTACACCGAATGC | GTCCATTGTGGAAGATTCCC | 60 |
Statistical analysis.
Statistical analysis of the data was performed using analysis of variance (ANOVA) followed by Duncan's multiple-range test to compare antibody responses among groups (14). Gene expression data for treated cells were compared to those for untreated controls by the Wilcoxon matched-pairs test using GraphPad Prism version 4.00 for Windows (Graphpad Software, San Diego, CA). For all analyses, statistical significance was assessed at a P value of <0.05.
RESULTS
Anti-SRBC antibody titers.
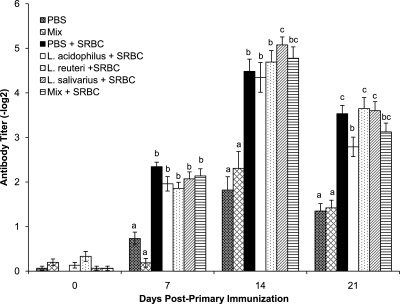
To evaluate the effects of oral treatment with Lactobacillus species alone or in combination on the systemic immune response, serum antibody responses to SRBC were compared. The antibody titers in all SRBC-immunized groups were significantly higher than those in the nonimmunized control groups (Fig. 1). At 7 days post-primary immunization (dpi), there were no differences in antibody titers among any of the treated and immunized groups. At 14 dpi, chickens in the L. salivarius treatment group had significantly higher (P < 0.05) antibody titers to SRBC than those in either the L. acidophilus, L. reuteri, or PBS treatment group. By 21 dpi the anti-SRBC titers in the L. salivarius-treated chickens were similar to those for all the other treatments with the exception of the L. acidophilus treatment group, in which titers were significantly lower (P < 0.05) than in the L reuteri, L. salivarius, and PBS treatment groups. At both 14 and 21 dpi, the chickens that were gavaged with a combination of all three bacteria had mean antibody titers that were not significantly different than the antibody titers in groups treated with the individual bacteria or with PBS.
Fig. 1.
Serum anti-SRBC antibody titers determined by a direct hemagglutination assay. Error bars represent standard errors of the means, and letters that are different within a time point denote significant differences among groups (P < 0.05). Chicks received 1 × 107 CFU/chick of either L. acidophilus, L. reuteri, L. salivarius, or an equal combination (total of 1 × 107 CFU/chick) of all three bacteria (mix) or PBS (control group) by oral gavage weekly starting on the day of hatch and throughout the trial. The groups were as follows: gavaged with PBS and not immunized (PBS), gavaged with a mixture of L. acidophilus, L. reuteri, and L. salivarius and not immunized (Mix), gavaged with PBS and immunized with SRBC (PBS + SRBC), gavaged with L. acidophilus and immunized with SRBC (L. acidophilus + SRBC), gavaged with L. reuteri and immunized with SRBC (L. reuteri + SRBC), gavaged with L. salivarius and immunized with SRBC (L. salivarius + SRBC), and gavaged with a mixture of L. acidophilus, L. reuteri, and L. salivarius and immunized with SRBC (Mix + SRBC).
Anti-KLH antibodies.
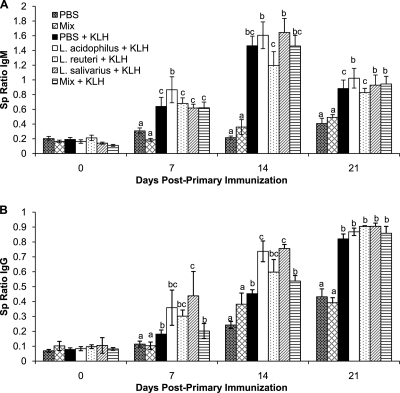
Immunization with KLH resulted in a significantly higher Sp ratio of anti-KLH IgM antibodies in the immunized chickens than in the nonimmunized control chickens (Fig. 2A). Further examination of the ability of the lactobacilli to alter the antibody-mediated immune response demonstrated that at 7 dpi, the L. acidophilus-treated chickens had significantly (P < 0.05) more anti-KLH IgM than any of the other immunized groups, including the PBS control group. At 14 dpi, none of the treatment groups were significantly different from the PBS-treated chickens; however, the L. reuteri-treated chickens had significantly (P < 0.05) less anti-KLH IgM than either the L. acidophilus- or L. salivarius-treated chickens. At 21 dpi, the Sp ratios of anti-KLH IgM antibodies in the chickens treated with L. acidophilus, L. salivarius, and mixture of all three bacteria were significantly higher than the ratios in the PBS- and L. reuteri-treated chickens.
Fig. 2.
Anti-KLH antibodies in the serum. Sample-to-positive (Sp) ratios are presented, and the error bars represent standard errors of the means. Chicks received 1 × 107 CFU/chick of either L. acidophilus, L. reuteri, L. salivarius, or an equal combination (total of 1 × 107 CFU/chick) of all three bacteria (mix) or PBS (control group) by oral gavage weekly starting on the day of hatch and throughout the trial. The groups were as follows: gavaged with PBS and not immunized (PBS), gavaged with a mixture of L. acidophilus, L. reuteri, and L. salivarius and not immunized (Mix), gavaged with PBS and immunized with KLH (PBS + KLH), gavaged with L. acidophilus and immunized with KLH (L. acidophilus + KLH), gavaged with L. reuteri and immunized with KLH (L. reuteri + KLH), gavaged with L. salivarius and immunized with KLH (L. salivarius + KLH), and gavaged with a mixture of L. acidophilus, L. reuteri, and L. salivarius and immunized with KLH (Mix + KLH). Anti-KLH IgM antibody responses (A) and anti-KLH IgG antibody responses (B) on 0, 7, 14, and 21 days post-primary immunization are shown. Letters that are different within a time point denote significant differences among groups (P < 0.05).
Similar to the observations with anti-KLH IgM antibodies, the Sp ratios of anti-KLH IgG in the KLH-immunized groups were significantly higher than those in the nonimmunized control groups (Fig. 2B). At 7 dpi, the mean anti-KLH IgG Sp ratios in the chickens treated with the individual bacteria were higher than those in the groups that were treated with PBS or the mixture of all three bacteria; however, only ratios in the L. salivarius-treated chickens reached statistical significance (P < 0.05). At 14 dpi, there was significantly more anti-KLH IgG in the chickens treated with L. acidophilus or L. salivarius than in the chickens treated with PBS or the mixture of bacteria. By 21 dpi, there was no significant difference in any of the immunized groups with regard to anti-KLH IgG.
Anti-NDV and anti-IBDV VP2 antibody titers.
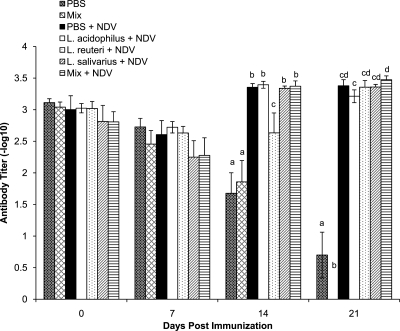
To assess the ability of lactobacilli to alter the serum antibody response to vaccination, responses to NDV and IBDV VP2 antigens were determined. After 2 weeks, the antibody titer in NDV-immunized chickens was significantly higher than that in the nonimmunized control chickens (Fig. 3). At 14 dpi, chickens treated with L. reuteri had significantly lower (P < 0.05) antibody titers to NDV than all other immunized chickens, including the chickens treated with PBS. At 21 dpi, none of the treatment groups were significantly different from the PBS-treated chickens. However, there was a significantly lower concentration of serum anti-NDV antibodies in chickens treated with L. acidophilus than in the chickens that had received a mixture of all three bacteria.
Fig. 3.
Serum anti-NDV antibody titers as determined by ELISA. Error bars represent standard errors of the means, and letters that are different within a time point denote significant differences amoung groups (P < 0.05). Chicks received 1 × 107 CFU/chick of either L. acidophilus, L. reuteri, L. salivarius, or an equal combination (total of 1 × 107 CFU/chick) of all three bacteria (mix) or PBS (control group) by oral gavage weekly starting on the day of hatch and throughout the trial. The groups were as follows: gavaged with PBS and not immunized (PBS), gavaged with a mixture of L. acidophilus, L. reuteri, and L. salivarius and not immunized (Mix), gavaged with PBS and immunized with NDV (PBS + NDV), gavaged with L. acidophilus and immunized with NDV (L. acidophilus + NDV), gavaged with L. reuteri and immunized with NDV (L. reuteri + NDV), gavaged with L. salivarius and immunized with NDV (L. salivarius + NDV), and gavaged with a mixture of L. acidophilus, L. reuteri, and L. salivarius and immunized with NDV (Mix + NDV).
Serum antibody titers to IBDV VP2 were determined following immunization with Vaxxitek, the recombinant HVT expressing the VP2 antigen of IBDV. The antibody titers in the chickens in all groups were the highest on the day of immunization and decreased over time (data not shown). Additionally, the antibody titers in the immunized chickens were not significantly higher than those in the chickens in the nonimmunized control groups (data not shown).
IFN-γ production by splenocytes in response to recall antigen stimulation.
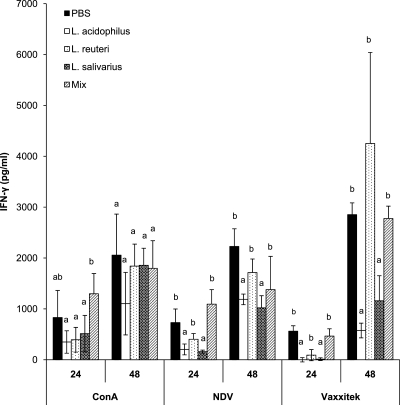
Mononuclear cells from the spleens of chickens belonging to various treatment groups were isolated at 21 and 28 days postimmunization and incubated in vitro in the presence of medium, ConA, UV-inactivated NDV, or UV-inactivated Vaxxitek vaccine. The concentration of IFN-γ in the cell culture supernatant was measured by ELISA. Cells isolated from chickens at 21 and 28 days postimmunization displayed similar profiles for IFN-γ production. However, the concentration of IFN-γ was higher at the 21-day time point; therefore, only the data for this time point were included. No IFN-γ was present in the supernatant of cultured cells treated with medium alone (data not shown). Mononuclear cells isolated from chickens treated with L. acidophilus, L. reuteri, or L. salivarius alone produced significantly less IFN-γ after stimulation with ConA at 24 h posttreatment than cells that were isolated from chickens treated with a combination of all three bacteria. At 48 h after treatment with ConA, there was no significant difference in the amount of IFN-γ produced in the supernatants of splenocytes from any of the groups (Fig. 4).
Fig. 4.
IFN-γ concentrations in the supernatants of cultured spleen mononuclear cells as determined by ELISA. Chicks received 1 × 107 CFU/chick of either L. acidophilus, L. reuteri, L. salivarius, or an equal combination (total of 1 × 107 CFU/chick) of all three bacteria (mix) or PBS (control group) by oral gavage weekly starting on the day of hatch and throughout the trial. Spleens were isolated from chickens immunized with live NDV and Vaxxitek vaccines at 2 weeks of age. Spleens were collected at 21 days postimmunization, and mononuclear cells were cultured for 24 and 48 h in the presence of ConA, UV-inactivated NDV, or UV-inactivated Vaxxitek vaccine. Error bars represent standard errors of the means, and letters that are different within a time point denote significant differences among groups (P < 0.05).
At 24 and 48 h posttreatment and in response to NDV and Vaxxitek antigens, there was significantly (P < 0.05) less IFN-γ produced by spleen mononuclear cells from L. acidophilus- and L. salivarius-treated chickens than by cells derived from chickens treated with PBS, L. reuteri, or a combination of all three bacteria (Fig. 4).
Cytokine expression by splenocytes in response to recall antigen stimulation.
RT-qPCR was performed to verify the results obtained by IFN-γ ELISA and to the further examine the cytokines expressed by the spleen mononuclear cells isolated from lactobacillus-treated chickens. Recall antigen stimulation with either NDV or Vaxxitek for 24 h significantly increased the expression (P < 0.05) of IFN-γ in all treated groups compared to unstimulated medium controls, with the exception of the spleen mononuclear cells isolated from L. acidophilus-treated chickens and stimulated with the UV-inactivated Vaxxitek vaccine (Fig. 5). The observed level of IFN-γ transcripts was consistent with the amount of IFN-γ detected in cell culture supernatant. However, comparison of the cells that were stimulated with NDV and Vaxxitek demonstrated that spleen cells isolated from chickens gavaged with PBS did not significantly (P > 0.05) differ in the abundance of IFN-γ transcripts from cells isolated from chickens treated with the various bacteria (Fig. 5).
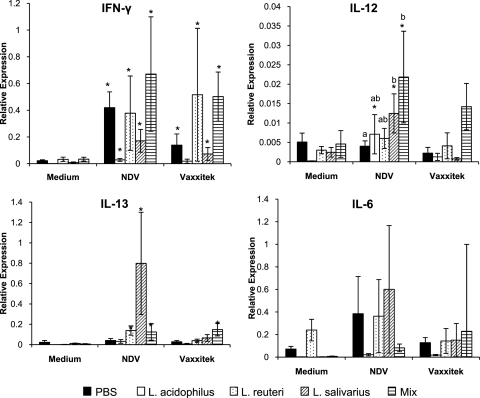
Fig. 5.
Relative expression of cytokine transcripts (IFN-γ, IL-12p40, IL-13, and IL-6) by spleen mononuclear cells after culture for 24 h with medium only, UV-inactivated NDV, or UV-inactivated Vaxxitek. Chicks received 1 × 107 CFU/chick of either L. acidophilus, L. reuteri, L. salivarius, or an equal combination (total of 1 × 107 CFU/chick) of all three bacteria (mix) or PBS (control group) by oral gavage weekly starting on the day of hatch and throughout the trial. Spleens were isolated from chickens immunized with live NDV and Vaxxitek vaccines at 2 weeks of age. Data are expressed as the relative expression of cytokine mRNA levels normalized to the expression of β-actin. *, treatment significantly different (P < 0.05) from the corresponding unstimulated medium control. Error bars represent standard errors of the means, and different letters indicate that the treatments are significantly different from the other treatments within the group.
Spleen mononuclear cells isolated from chickens treated with L. salivarius, L. acidophilus, and the mixture of all three bacteria produced significantly more IL-12p40 transcript than the medium controls after 24 h of exposure to UV-inactivated NDV (Fig. 5). Stimulation of the cells with the UV-inactivated Vaxxitek vaccine did not significantly alter the expression of IL-12p40. Comparison of the cells that were stimulated with NDV demonstrated that spleen cells isolated from chickens that were given a mixture of all three bacteria and L. salivarius expressed significantly (P < 0.05) more IL-12p40 than cells isolated from chickens gavaged with PBS (Fig. 5).
Expression of IL-13 was significantly (P < 0.05) increased by stimulation with both recall antigens in spleen mononuclear cells isolated from chickens treated with L. reuteri or all three bacteria (Fig. 5). Expression of IL-13 was significantly (P < 0.05) increased in spleen cells isolated from chickens treated with L. salivarius only after recall stimulation with NDV, and these cells expressed significantly more IL-13 than cells from chickens belonging to any of the other treatment groups, including the PBS control (P < 0.05).
The expression of IL-6 was variable (Fig. 5), and no significant differences were observed. However, spleen mononuclear cells isolated from chickens that were treated with L. salivarius and restimulated with UV-inactivated NDV tended to express more IL-6 than those from the corresponding medium control treatment (P = 0.0625).
DISCUSSION
With increasing interest in using probiotics as alternatives to antibiotic growth promoters in animal production systems, it is important to understand the role of probiotic bacteria in modulating the host immune system. Although the immunomodulatory activities of probiotic bacterial are not fully understood, it has been demonstrated that probiotic bacteria taken orally can increase cytokine levels in the serum in a bacterial species-specific manner (12, 39, 42, 45), and it is generally accepted that this is one of the ways in which probiotic bacteria alter the adaptive immune response. Others have linked changes in the systemic immune response to the ability of probiotic bacteria to enhance the ability of dendritic cells for antigen presentation (50) or changes in T cell populations and functions leading to an increase in regulatory T cells or polarization toward Th1 or Th2 responses (21, 32, 35). Given the paucity of information on the effect of treatment with probiotic bacteria on the chicken immune response, the present study was designed to examine the effects of isolates of three Lactobacillus species, L. acidophilus, L. reuteri, and L. salivarius, administered individually or in combination, on the antibody- and cell-mediated immune responses of chickens.
In the current study, gavaging chickens weekly with 1 × 107 CFU of bacteria was sufficient to significantly increase the amounts of L. acidophilus, L. reuteri, and L. salivarius bacteria present in the ileum as determined by PCR amplification with Lactobacillus species-specific primers (data not shown). The posttreatment increase in the number of lactobacilli was most significant at 4 weeks of age, and the level then returned to that for the control birds by 6 weeks of age. This observation suggested that the lactobacilli survived intestinal transit and were transiently increased in the small intestine, both of which are considered important prerequisites for modifying the host immune response. The ability of the three Lactobacillus isolates to affect the antibody-mediated immune response was analyzed by examining serum antibody response to SRBC, IgM and IgG responses to KLH, IgG response to a live NDV vaccine, and IgG response to the Vaxxitek HVT + IBDV VP2 vaccine. Immunization with Vaxxitek HVT + IBDV VP2 did not induce a significant response to the VP2 antigen of IBDV, and therefore the response to this antigen was not analyzed further. The lack of response is most likely due to the high titer of maternal antibodies present at the time of immunization. In the cases of SRBC, KLH, and NDV immunizations, the immunization protocols were effective as demonstrated by a significant increase in antibody titers in all immunized chickens compared to nonimmunized controls.
Gavage of chickens with L. acidophilus prior to immunization resulted in an increase in both IgM and IgG responses to KLH and a decrease in the agglutinating antibody response to SRBC compared to those for the control birds. Further investigation demonstrated that L. acidophilus-treated birds had less anti-SRBC IgM but more anti-SRBC IgG than the other birds (data not shown). The ability of IgM antibodies to agglutinate SRBC more efficiently than IgG (33) accounts for the agglutinating response seen in these samples. L. salivarius treatment increased the antibody response to both SRBC and KLH, while treatment with L. reuteri had no significant effect on the antibody response to SRBC or KLH. The mixture of the three bacteria had no significant effect on the antibody response to any of the antigens tested. With the exception of the chickens treated with L. reuteri at 14 dpi, immunization with the NDV vaccine generated no significant difference in antibody response among bacterial treatment groups. Although there is some evidence that administration of probiotic bacteria can induce an increase in the antibody response to NDV vaccination (41), this has not been confirmed by others (3, 48). Similarly, various results with respect to the effects of probiotics on elicitation of antibody responses to SRBC and KLH have been documented (22, 29, 47). There are a number of factors that could account for these discrepancies, including the isolates or species of bacteria, number of bacteria, route and frequency of administration, environmental and nutritional conditions, and the immune status of the host. In fact, in humans, it has been demonstrated that vaccination of the elderly with influenza vaccines results in poor seroconversion, whereas treatment with probiotics enhances the immune response to these vaccines. This observation raises the possibility that the use of probiotics may be especially warranted in cases where a suboptimal immune response to vaccination is expected (34). Zulkifli and coworkers (54) have noted that in chickens probiotics differentially affect the immune system under different environmental conditions. Those authors reported that prior to heat stress in chickens, probiotics had no significant effect on antibody production to NDV, while after heat stress, an increase in the antibody response in probiotic-treated chickens was observed (54). Another factor that requires further investigation in relation to the influence of probiotic bacteria on the immune system is the kinetics of these effects. Although the intent of the present study was not to investigate the influence of treatment with various probiotic formulations on the kinetics of immune response development, based on the data presented here, it may be concluded that these effects were rather transient, because most of these effects were most obvious by day 14 postimmunization and had subsided by 21 dpi. Finally, an important aspect that needs to be taken into consideration is the differential ability of lactobacilli to modulate immune responses to different antigens. As demonstrated in the present study, immune responses to some antigens were not significantly influenced by treatment with lactobacilli, whereas immune response to other antigens was clearly affected by treatment with bacteria. Despite the observations presented here, the underlying mechanisms of action of probiotic bacteria on the development of immune responses have yet to be clearly elucidated. These effects may be related to activation and maturation of dendritic cells and changes in the activity and number of T cells and B cells after treatment of chickens with lactobacilli.
To examine how isolates of different Lactobacillus species affect the cell-mediated immune response, cultured spleen mononuclear cells from Lactobacillus- or sham-treated chickens were stimulated with UV-inactivated recall antigens (NDV and Vaxxitek), and the production of IFN-γ and expression of the IFN-γ, IL-12, IL-6, and IL-13 genes were assessed. IFN-γ and IL-12 are typically associated with a type 1 immune responses, while IL-13 is associated with type 2 responses. IL-6 is a proinflammatory cytokine that is important for the antibody-mediated immune response due to its capability to induce the final maturation of B cells into antibody-secreting plasma cells (27). We found that after stimulation with UV-inactivated NDV or Vaxxitek vaccine, spleen mononuclear cells isolated from chickens treated with L. acidophilus or L. salivarius produced significantly less IFN-γ than splenocytes isolated from chickens gavaged with L. reuteri, the mixture of all three bacteria, or PBS. RT-qPCR analysis of IFN-γ gene expression confirmed the ELISA results. The ability of probiotic bacteria to decrease antigen-specific IFN-γ production by chicken spleen mononuclear cells has been described previously (10). This contradicts other studies that have found that oral treatment with lactobacilli increased the production of IFN-γ systemically (12, 20, 42). Our results suggest that L. acidophilus and L. salivarius may decrease the cell-mediated immune response to the antigens tested. Although the underlying mechanisms for this phenomenon are not known, it can be speculated that the decrease in IFN-γ production by spleen cells in Lactobacillus-fed chickens may reflect a selective decrease in Th1 cell activation.
Splenocytes isolated from the chickens treated with L. salivarius produced less IFN-γ transcript and protein and had a corresponding increase in IL-13 and IL-6 gene expression. This along with the higher antibody response to some of the antigens examined in the present study indicates that giving oral L. salivarius may favor the development of a type 2-biased immune response. It is well established that the gastrointestinal microbiota has the capacity to modulate the balance of the different Th cells (Th1, Th2, Th3, and Treg) and their associated cytokines (9). The ability of L. salivarius to induce a type 2 phenotype in vitro has been previously demonstrated (13). Drago and colleagues (13) observed that two of the four L. salivarius strains tested on a human macrophage cell line induced the production of IL-4 and IL-5, two cytokines associated with a type 2 immune response. The significance of a type 2 phenotype is the development of B cells and the immunoglobulin isotype switching required for the production of antibodies (28).
In the case of the antibody- and cell-mediated immune responses, feeding a combination of all three bacteria moderated the phenotype seen with individual species. This highlights the need to examine the ability of the individual bacteria to alter the immune response when using multispecies probiotics to ensure that the desired effect is not being negated.
Regardless of the mechanisms, this work highlights the fact that different species of Lactobacillus vary in their ability to enhance systemic antibody responses. L. salivarius and L. acidophilus both demonstrated weak immunity-enhancing effects. Further studies are needed to determine the mechanisms by which these bacteria modulate the immune response and also to determine if production parameters and pathogen control can be similarly influenced by these bacteria.
ACKNOWLEDGMENTS
We thank ´Eva Nagy, University of Guelph, for her generous donation of the NDV used for recall stimulation and Lloyd Weber, Lloyd Weber Poultry Health Consulting Services, for his generous donation of the NDV vaccine.
Jennifer Brisbin is a recipient of a Postgraduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC). This study was supported by the Canadian Poultry Research Council, Poultry Industry Council, NSERC, Saskatchewan Chicken Industry Development Fund, and Agriculture and Agri-Food Canada.
Footnotes
Published ahead of print on 6 July 2011.
REFERENCES
- 1. Angel R., Dalloul R. A., Doerr J. 2005. Performance of broiler chickens fed diets supplemented with a direct-fed microbial. Poult. Sci. 84:1222–1231 [DOI] [PubMed] [Google Scholar]
- 2. Awad W. A., Ghareeb K., Abdel-Raheem S., Böhm J. 2009. Effects of dietary inclusion of probiotic and symbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88:49–55 [DOI] [PubMed] [Google Scholar]
- 3. Balevi T., Uçan U. S., Coşkun B., Kurtoglu V., Cetingül I. S. 2001. Effect of dietary probiotic on performance and humoral immune response in layer hens. Br. Poult. Sci. 42:456–461 [DOI] [PubMed] [Google Scholar]
- 4. Brisbin J. T., et al. 2008. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev. Comp. Immunol. 32:563–574 [DOI] [PubMed] [Google Scholar]
- 5. Brisbin J. T., Gong J., Sharif S. 2008. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 9:101–110 [DOI] [PubMed] [Google Scholar]
- 6. Brisbin J. T., et al. 2008. Influence of in-feed virginiamycin on the systemic and mucosal antibody response of chickens. Poult. Sci. 87:1995–1999 [DOI] [PubMed] [Google Scholar]
- 7. Brisbin J. T., Gong J., Parvizi P., Sharif S. 2010. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 17:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callaway T. R., et al. 2008. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim. Health Res. Rev. 9:217–225 [DOI] [PubMed] [Google Scholar]
- 9. Corthésy B., Gaskins H. R., Mercenier A. 2007. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 137:781S–790S [DOI] [PubMed] [Google Scholar]
- 10. Dalloul R., Lillehoj H., Shellem T., Doerr J. 2003. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult. Sci. 82:62–66 [DOI] [PubMed] [Google Scholar]
- 11. Dalloul R. A., Lillehoj H. S., Tamim N. M., Shellem T. A., Doerr J. A. 2005. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp. Immunol. Microbiol. Infect. Dis. 28:351–361 [DOI] [PubMed] [Google Scholar]
- 12. De Simone C., Vesely R., Bianchi-Salvadori B., Jirillo E. 1993. The role of probiotics in modulation of the immune system in man and animals. Int. J. Immunother. 9:23–28 [Google Scholar]
- 13. Drago L., Nicola L., Iemoli E., Banfi G., De Vecchi E. 2010. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res. Notes 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duncan D. B. 1955. Multiple range and multiple F test. Biometrics 11:1–42 [Google Scholar]
- 15. Dunham H. J., Williams C., Edens F. W., Casas I. A., Dobrogosz W. J. 1993. Lactobacillus reuteri immunomodulation of stressor-associated diseases in newly hatched chickens and turkeys. Poult. Sci. 72:103 [Google Scholar]
- 16. FAO/WHO 2002. Working group report on drafting guidelines for the evaluation of probiotics in food, 30 April to 1 May, London, United Kingdom, and Ontario, Canada [Google Scholar]
- 17. Forsythe P., Bienenstock J. 2010. Immunomodulation by commensal and probiotic bacteria. Immunol. Invest. 39:429–448 [DOI] [PubMed] [Google Scholar]
- 18. Furet J. P., Quénée P., Tailliez P. 2004. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int. J. Food Microbiol. 97:197–207 [DOI] [PubMed] [Google Scholar]
- 19. Gérard P., Brézillon C., Quéré F., Salmon A., Rabot S. 2008. Characterization of cecal microbiota and response to an orally administered Lactobacillus probiotic strain in the broiler chicken. J. Mol. Microbiol. Biotechnol. 14:115–122 [DOI] [PubMed] [Google Scholar]
- 20. Gill H. S., Rutherfurd K. J., Prasad J., Gopal P. K. 2000. Enhancement of natural and aquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br. J. Nutr. 83:167–176 [DOI] [PubMed] [Google Scholar]
- 21. Gill H. S., Rutherfurd K. J., Cross M. L. 2001. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J. Clin. Immunol. 21:264–271 [DOI] [PubMed] [Google Scholar]
- 22. Haghighi H. R., et al. 2005. Modulation of antibody-mediated immune response by probiotics in chickens. Clin. Diagn. Lab. Immunol. 12:1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haghighi H. R., et al. 2006. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 13:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haghighi H. R., Abdul-Careem M. F., Dara R. A., Chambers J. R., Sharif S. 2008. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 126:225–233 [DOI] [PubMed] [Google Scholar]
- 25. Higgins J. P., et al. 2010. Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella enteritidis in neonatal broilers. Poult. Sci. 89:243–247 [DOI] [PubMed] [Google Scholar]
- 26. Huang M. K., et al. 2004. Effects of lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult. Sci. 83:788–795 [DOI] [PubMed] [Google Scholar]
- 27. Jones S. A. 2005. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175:3463–3468 [DOI] [PubMed] [Google Scholar]
- 28. Kabir S. M. L. 2009. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 10:3531–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kabir S. M. L., Rahman M. M., Rahman M. B., Rahman M. M., Ahmed S. U. 2004. The dynamics of probiotics on growth performance and immune response in broilers. Int. J. Poult. Sci. 3:361–364 [Google Scholar]
- 30. Karimi Torshizi M. A., Moghaddam A. R., Rahimi S., Mojgani N. 2010. Assessing the effect of administering probiotics in water or as feed supplement on broiler performance and immune response. Br. Poult. Sci. 51:178–184 [DOI] [PubMed] [Google Scholar]
- 31. Koenen M. E., van der Hulst R., Leering M., Jeurissen S. H., Boersma W. J. 2004. Development and validation of a new in vitro assay for selection of probiotic bacteria that express immune-stimulating properties in chickens in vivo. FEMS Immunol. Med. Microbiol. 40:119–127 [DOI] [PubMed] [Google Scholar]
- 32. Lavasani S., et al. 2010. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 5:e9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leitner G., Melamed D., Drabkin N., Heller E. D. 1990. An enzyme-link immunosorbent assay for detection of antibodies against Escherichia coli: association between indirect hemagglutination test and survival. Avian Dis. 34:58–62 [PubMed] [Google Scholar]
- 34. MacDonald T. T., Bell I. 2010. Probiotics and the immune response to vaccines. Proc. Nutr. Soc. 69:442–446 [DOI] [PubMed] [Google Scholar]
- 35. Mazmanian S. K., Liu C. H., Tzianabos A. O., Kasper D. L. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118 [DOI] [PubMed] [Google Scholar]
- 36. Mountzouris K. C., Balaskas C., Xanthokos I., Tzivinikou A., Fegeros K. 2009. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 50:467–468 [DOI] [PubMed] [Google Scholar]
- 37. Mountzouris K. C., et al. 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86:309–317 [DOI] [PubMed] [Google Scholar]
- 38. Mountzouris K. C., et al. 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobins, and cecal microflora composition. Poult. Sci. 89:58–67 [DOI] [PubMed] [Google Scholar]
- 39. Niers L. E., et al. 2005. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin. Exp. Allergy 35:1481–1489 [DOI] [PubMed] [Google Scholar]
- 40. Noujaim J. C., et al. 2008. Detection of T lymphocytes in intestine of broiler chicks treated with Lactobacillus spp. and challenged with Salmonella enterica serovar Enteritidis. Poult. Sci. 87:927–933 [DOI] [PubMed] [Google Scholar]
- 41. Ogawa T., Asai Y., Sakamoto H., Yasuda K. 2006. Oral immunoadjuvant activity of Lactobacillus casei subsp. casei in dextran-fed layer chickens. Br. J. Nutr. 95:430–434 [DOI] [PubMed] [Google Scholar]
- 42. Paturi G., Phillips M., Jones M., Kailasapathy K. 2007. Immune enhancing effects of Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 in mice. Int. J. Food Microbiol. 115:115–118 [DOI] [PubMed] [Google Scholar]
- 43. Reynolds D. L., Maraqa A. D. 2000. Protective immunity against Newcastle disease: the role of cell-mediated immunity. Avian Dis. 44:145–154 [PubMed] [Google Scholar]
- 44. Sato K., et al. 2009. Immunomodulation on gut-associated lymphoid tissue of neonatal chicks by immunobiotic diets. Poult. Sci. 88:2532–2538 [DOI] [PubMed] [Google Scholar]
- 45. Schultz M., et al. 2003. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J. Dairy Res. 70:165–173 [DOI] [PubMed] [Google Scholar]
- 46. Song Y., et al. 2000. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 187:167–173 [DOI] [PubMed] [Google Scholar]
- 47. Taheri H. R., Moravej H., Tabandeh F., Zaghari M., Shivazad M. 2010. Efficacy of combined or single use of Lactobacillus crispatus LT116 and L. johnsonii LT171 on broiler performance. Br. Poult. Sci. 51:580–585 [DOI] [PubMed] [Google Scholar]
- 48. Talebi A., Amirzadeh B., Mokhtari B., Gahri H. 2008. Effects of a multi-strain probiotic (PrimaLac) on performance and antibody response to Newcastle disease virus and infectious bursal disease vaccination in broiler chickens. Avian Pathol. 37:509–512 [DOI] [PubMed] [Google Scholar]
- 49. Timmerman H. M., Veldman A., van den Elsen E., Rombouts F. M., Beynen A. C. 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 85:1383–1388 [DOI] [PubMed] [Google Scholar]
- 50. Tsai Y. T., Cheng P. C., Fan C. K., Pan T. M. 2008. Time-dependent persistence of enhanced immune response by a potential probiotic strain Lactobacillus paracasei subsp. Paracasei NTU 101. Int. J. Food Microbiol. 128:219–225 [DOI] [PubMed] [Google Scholar]
- 51. Yurong Y., Ruiping S., Shimin Z., Yibao J. 2005. Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens. Arch. Anim. Nutr. 59:237–246 [DOI] [PubMed] [Google Scholar]
- 52. Zhang G., Ma L., Doyle M. P. 2007. Potential competitive exclusion bacteria from poultry inhibitory to Campylobacter jejuni and Salmonella. J. Food Prot. 70:867–873 [DOI] [PubMed] [Google Scholar]
- 53. Zhang G., Ma L., Doyle M. P. 2007. Salmonellae reduction on poultry by competitive exclusion bacteria Lactobacillus salivarius and Streptococcus cristatus. J. Food Prot. 70:874–878 [DOI] [PubMed] [Google Scholar]
- 54. Zulkifli I., Abdullah N., Azrin N. M., Ho Y. W. 2000. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br. Poult. Sci. 41:593–597 [DOI] [PubMed] [Google Scholar]