Abstract
There is an urgent need for identification of a new adjuvant capable of selectively promoting an efficient immune response for use with vaccines and especially subunit vaccines. Our pervious study showed that Bursopentine (BP5) is a novel immunomodulatory peptide and has the ability to significantly stimulate an antigen-specific immune response in mice. In this study, the potential adjuvant activities of BP5 were examined in chickens by coinjection of BP5 and an inactivated avian influenza virus (AIV) (A/Duck/Jiangsu/NJ08/05 [AIV H9N2 subtype]). The results suggested that BP5 markedly elevated serum hemagglutination inhibition (HI) titers and antigen-specific antihemagglutinin (anti-HA) antibody (IgG) levels, induced both Th1 (interleukin 2 [IL-2] and gamma interferon [IFN-γ])- and Th2 (IL-4)-type cytokines, promoted the proliferation of peripheral blood lymphocytes, and increased populations of CD3+ T cells and their subsets CD4+ (CD3+ CD4+) T cells and CD8+ (CD3+ CD8+) T cells. Furthermore, a virus challenge experiment revealed that BP5 contributes to protection against homologous avian influenza virus challenge by reducing viral replication in chicken lungs. This study indicates that the combination of inactivated AIVs and BP5 gives a strong immune response at both the humoral and cellular levels and implies that BP5 is a novel immunoadjuvant suitable for vaccine design.
INTRODUCTION
The immune-promoting activity of any given vaccination strategy is set not only by the presence of the relevant antigenic components in the vaccine formulation but also by the complement of suitable adjuvants (9, 20). When incorporated into a vaccine formulation, a suitable adjuvant acts to accelerate, extend, or enhance the magnitude of a specific immune response to the vaccine antigen (6). Strategies for improving current vaccines have emphasized making currently available vaccines more efficacious by developing a better adjuvant, especially for inactivated viral and subunit vaccines.
Bursopentine (BP5; with an amino acid sequence of Cys-Lys-Asp-Val-Tyr) is a novel immunomodulatory peptide isolated from chicken bursa of Fabricius (19). As it has the ability to significantly stimulate antigen-specific immune responses at both the humoral and cellular levels in mice immunized with inactivated avian influenza viruses (AIVs) (19), its potential adjuvant activities were assessed in chickens in this study by using a model antigen of an inactivated AIV, A/Duck/Jiangsu/NJ08/05 (AIV H9N2 subtype).
In many countries, H9N2 AIVs are an enormous economic burden on the commercial poultry industry when they cause signs of mild respiratory disease and a reduction in egg production. In April 1999, two World Health Organization reference laboratories independently confirmed the isolation of avian influenza A (H9N2) viruses for the first time in humans (39). An increased risk of direct transmission of these viruses to humans is possible (21, 25, 29). Inactivated vaccines have been used to control AIV infection, but the best protection against AIV infection remains effective vaccination. Previously, it has been shown that inactivated vaccines elicit strong humoral responses, and it is commonly accepted that no adequate mucosal or cellular immunity is achieved (37). However, cellular immunity is essential for virus clearance at the end stage of many viral infections (4). Adjuvants are able to improve the quantity and quality of innate immune responses by enhancing their speed and duration and by inducing adequate adaptive immunity (31). In the current study, BP5 was used as an adjuvant for our AIV vaccination strategy to provide an effective way to prevent and control H9N2 AIV infection.
The effect of BP5 on humoral and cell-mediated immune responses induced by inactivated AIV vaccination was evaluated in 1-week-old specific-pathogen-free White Leghorn chickens. Humoral immunity was measured by detection of antigen-specific antibody titer and antihemagglutinin (anti-HA) IgG responses using the hemagglutination inhibition (HI) test and enzyme-linked immunosorbent assay (ELISA), respectively. Cell-mediated immunity was evaluated by detection of serum Th1 (interleukin 2 [IL-2] and gamma interferon [IFN-γ])- and Th2 (IL-4)-type cytokines (23) by ELISA, by measurement of chicken peripheral blood lymphocyte proliferation using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, and by measurement of chicken peripheral blood CD3+ T cells and their subsets CD4+ (CD3+ CD4+) T cells and CD8+ (CD3+ CD8+) T cells by an immunophenotyping assay. Furthermore, virus challenge experiments were assayed to evaluate the protection of activated AIV vaccine administered with BP5 against homologous avian influenza virus replication in chicken lungs.
MATERIALS AND METHODS
Preparation of BP5.
Synthetic BP5 was purchased from Shanghai Biotech Bioscience and Technology Co., Ltd. (People's Republic of China). The sequence of the synthetic peptide was confirmed by electrospray ionization tandem mass spectrometry (ESI-MS/MS), and the purity of the synthetic peptide was >98% by reversed-phase high-performance liquid chromatography (RP-HPLC).
Viruses and chicken.
AIV A/Duck/Jiangsu/NJ08/05 (AIV H9N2 subtype) was provided by the Institute of Animal Husbandry and Veterinary Medicine, Jiangsu Academy of Agricultural Sciences (Nanjing, China). Avian influenza H9N2 virus strain JS-1 (A/Chicken/Jiangsu/JS-1/2002) was isolated and kept in our own laboratory. AIVs were cultured in the allantoic sacs of chicken embryos. The AIV hemagglutination titer of the inoculated allantoic fluid was 1:210, corresponding to 107 50% tissue culture infective doses (TCID50)/0.1 ml. The A/Duck/Jiangsu/NJ08/05 AIV was inactivated with 0.025% formaldehyde for 72 h at 4°C. Its efficacy was tested by three blind virus passages in specific-pathogen-free (SPF) eggs (19, 38), and the inactivated AIV was used as a vaccine antigen for the following experiments.
One-week-old SPF White Leghorn chickens from Qian Yuan hao Co., Ltd. (Nanjing, China), were obtained as fertilized eggs, hatched, and maintained in an isolation facility at the Poultry Research Institute (Nanjing, China). All groups of chickens were housed, handled, and immunized in accordance with the guidelines and with the approval of the local institutional animal experiment committee.
Vaccination of chickens.
SPF White Leghorn chickens were randomly divided into six experimental groups of 18 chickens each and intramuscularly immunized two times on days 0 and 14 with (i) a mixture of 400 μl AIV (A/Duck/Jiangsu/NJ08/05, 107 TCID50/0.1 ml) and 100 μl phosphate-buffered saline (PBS), (ii) a mixture of 400 μl AIV (A/Duck/Jiangsu/NJ08/05, 107 TCID50/0.1 ml) and 25, 5, or 1 mg BP5 in 100 μl PBS/kg body weight, (iii) 400 μl commercially inactivated AIV/H9N2 vaccine (an oil-formulated vaccine obtained from Qian Yuan hao Co., Ltd., Nanjing, China [107 TCID50/0.1 ml]) plus 100 μl PBS as a positive control, or (iv) 500 μl PBS as a negative control (Table 1).
Table 1.
Animal groups and the experimental designa
| Group | Vaccination on days 0 and 14b |
|---|---|
| 1 | 500 μl PBS |
| 2 | 400 μl AIVs (107 TCID50/0.1 ml) + 100 μl PBS |
| 3 | 400 μl AIVs (107 TCID50/0.1 ml) + 100 μl BP5 (25 mg/kg/0.1 ml PBS) |
| 4 | 400 μl AIVs (107 TCID50/0.1 ml) + 100 μl BP5 (5 mg/kg/0.1 ml PBS) |
| 5 | 400 μl AIVs (107 TCID50/0.1 ml) + 100 μl BP5 (1 mg/kg/0.1 ml PBS) |
| 6 | 400 μl H9N2 AIV vaccine (107 TCID50/0.1 ml) + 100 μl PBS |
All chickens were challenged on day 28.
AIVs, inactivated H9N2 avian influenza virus; H9N2 AIV vaccine, commercial H9N2 avian influenza virus vaccine prepared with oil/water as an adjuvant.
HI assay.
On days 14 and 28 postimmunization, serum hemagglutination inhibition (HI) antibody titers of each group of chickens were evaluated with an HI test based on Hirst's principle (10). The serum was diluted 10-fold with saline before an additional 2-fold dilution with PBS was made. Standard avian influenza antigen (Harbin Veterinary Research Institute, China) with 4 hemagglutination units was then added to each diluted serum sample and mixed for approximately 15 min. An equal volume of 0.5% chicken red blood cells was added to the virus-serum mixture and incubated for 30 to 60 min before the results were read. The HI titers were defined as the highest serum dilution capable of preventing hemagglutination.
Estimation of antigen-specific antibodies (IgG).
Sera from chickens were collected on days 14 and 28 postimmunization. Specific antihemagglutinin (anti-HA) IgG of chicken sera was analyzed by ELISA. Briefly, ELISA plates were coated with a purified prokaryote-expressed recombinant JS-1 (A/Chicken/ Jiangsu/JS-1/2002, H9N2 AIV) HA protein (preserved in our laboratory, 10 μg/ml) (40). Serially diluted chicken sera were then incubated for 2 h at room temperature, followed by a 1-h incubation with horseradish peroxidase (HRP)-conjugated goat anti-chicken IgG (GenScript Co., Ltd., China). Titers at half-maximal optical densities (OD) were determined by linear interpolation between the measured points neighboring the half-maximal OD. Linear interpolation was calculated using the logarithm of the titer values. Each serum titration was repeated in triplicate.
Cytokine assays.
One day 28 postimmunization, the serum levels of Th1-type cytokines (IL-2 and IFN-γ) in chickens were determined with commercial ELISA kits (Cusabio Biotech), whereas Th2-type cytokine (IL-4) was measured by another commercial ELISA kit (R&D Systems, United Kingdom). The procedure followed the manufacturer's instructions.
Lymphocyte proliferation assay and immunophenotyping assay.
To detect changes in cellular immunity, a peripheral blood lymphocyte proliferation assay and an immunophenotyping assay were performed. Fourteen days after the second immunization (day 28), the blood samples were collected for lymphocyte separation. Peripheral blood lymphocytes were separated as described previously, with some modification (11, 28). The cell suspension from the blood was layered on Ficoll-Paque lymphocyte separation medium by density gradient centrifugation. Peripheral blood lymphocytes were obtained from the interface and washed twice with Hanks' balanced salt solution. After centrifugation, the final pellet was resuspended in RPMI 1640 medium containing 5% heat-inactivated fetal calf serum at a concentration of 2 × 106 cells per ml.
The peripheral blood lymphocyte proliferation assay was performed using a modified MTT method as described previously (13, 22). Briefly, the peripheral blood lymphocytes (2 × 106 cells/ml) were dispersed and incubated in 96-well flat-bottomed microtiter plates (80 μl/well). Another 20 μl of concanavalin A (ConA; 10 μg/ml, positive control), the recombinant JS-1 (A/Chicken/Jiangsu/JS-1/2002, H9N2 AIV) HA protein (10 μg/ml, specific antigen stimulation), or RPMI 1640 medium without antigen (negative control) was added to each well, and each sample was seeded in four wells. After 44 h of incubation at 39.5°C in a 5% CO2 incubator, 20 μl of MTT (dissolved in PBS, 5 mg/ml) (Sigma) was added to each well and the incubation was continued for another 4 h. Then 100 μl of dimethyl sulfoxide (DMSO) was added, and incubation was continued for an additional 24 h before measurement of OD at 750 nm (OD570) using an ELISA reader (Bio-Tek Instruments, VT). Cell viability is expressed as the percentage of the OD570 of cells treated with complex over the OD570 of the control samples.
Flow cytometric analysis of peripheral blood lymphocytes was carried out as previously described (30). Peripheral blood lymphocytes (2 × 106 cells/ml) were made complex with the monoclonal antibody phycoerythrin (PE)-labeled anti-chicken CD3+ and then with PE-labeled anti-chicken CD4+ and fluorescein isothiocyanate (FITC)-conjugated anti-chicken CD8+ (Southern Biotechnology) for 1 h at 4°C. PE- and FITC-conjugated isotype controls were also included. Cells were analyzed by fluorescence-activated cell sorting (BD Biosciences).
Virus challenge experiment.
Two weeks after the second vaccination, all chickens were intranasally challenged with 2 × 107 TCID50 of avian influenza H9N2 virus strain JS-1 (A/Chicken/Jiangsu/JS-1/2002) in 0.1 ml. Lungs were collected from six chickens from each group at 1, 3, and 5 days after virus challenge (Table 1). All lung samples were stored at −80°C. Viral copy numbers in lungs were determined by using real-time PCR. An RNeasy RNA extraction kit (Invitrogen, Norway) was used to prepare total RNA from the lung samples. The RNA was reverse transcribed to cDNA by using the reverse transcription system from Promega (Germany). A 2-μl portion of cDNA was used to amplify the HA gene by real-time PCR using one pair of PCR primers: HA- forward, 5′-CTACTGTTGGGAGGAAGAGAATGGT-3′, and HA-reverse, 5′-TGGGCGTCTTGAATAGGGTAA-3′. PCR primers were designed based on the HA gene sequence of avian influenza H9N2 virus strain JS-1 (A/Chicken/Jiangsu/JS-1/2002) in GenBank (accession no. AY364228). The amplification was performed by using SYBR green (ABI, Warrington, United Kingdom) according to the method described previously (24), with some modifications. The standard curve for real-time PCR quantification was constructed using the HA gene in the vector pET32a-HA (H9N2), a gift from Qisheng Zheng (Institute of Veterinary Science, Jiangsu Academy of Agricultural Sciences). The pretreatment of the reaction mixture was carried out at 94°C for 10 min, and then the mixture was subjected to 40 cycles of amplification at 95°C for 15 s and at 60°C for 30 s.
Statistical analysis.
Antibody titers, cytokine levels, percentages reflecting lymphocyte proliferation, percentages of CD3+, CD3+ CD4+, and CD3+ CD8+ cells in the peripheral blood, and numbers of viral copies in chicken lungs were recorded as means ± standard deviations (SD). Bonferroni correction multiple-comparison tests were used to evaluate any differences between groups. Differences between means were considered significant at a P of <0.05 or a P of <0.01.
RESULTS
BP5 stimulates significant antigen-specific immune responses.
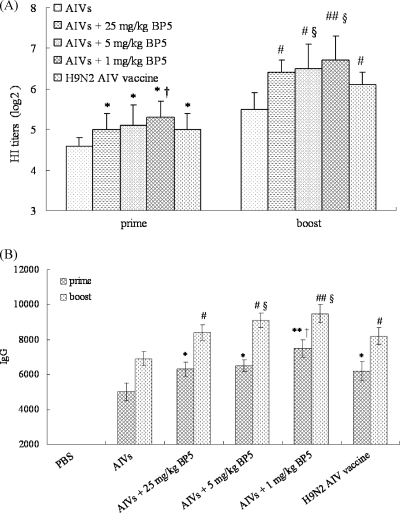
To test antigen-specific immune responses to immunization, chickens were immunized two times with a mixture of BP5 and inactivated avian influenza viruses (AIVs) or a commercial AIV (H9N2) vaccine (positive controls) or with PBS (negative control). Chickens coimmunized with inactivated AIVs and BP5 produced significantly higher hemagglutination inhibition (HI) antibody titers (Fig. 1A) (after priming with 25, 5, and 1 mg/kg [P < 0.05 {*}] and boosting with 25 and 5 mg/kg [P < 0.05 {#}] and 1 mg/kg [P < 0.01 {##}]) and anti-HA antibody (IgG) titers (Fig. 1B) (after priming with 25 and 5 mg/kg [P < 0.05 {*}] and 1 mg/kg [P < 0.01 {**}] and boosting with 25 and 5 mg/kg [P < 0.05 {#}] and 1 mg/kg [P < 0.01 {##}]) than those immunized with inactivated AIVs alone. Compared to chickens immunized with the commercial H9N2 AIV vaccine (with a combination of oil and water [oil/water] as an adjuvant), chickens coadministered inactivated AIVs and BP5 also produced significantly higher HI (Fig. 1A) and IgG (Fig. 1B) antibody titers (after priming with 1 mg/kg [P < 0.05 {†}] and boosting with 5 mg/kg and 1 mg/kg [P < 0.05 {§}]).
Fig. 1.
Effects of adding BP5 to the inactivated AIVs on the levels of antigen-specific HI titers (A) and anti-HA IgG antibodies (B). Chicken sera were collected on days 21 and 28 postimmunization, and the serum HI titers and IgG titers were analyzed by HI assay and by ELISA, respectively. The data presented are means ± SD of results from three replicates. *, P < 0.05, and **, P < 0.01 (prime); #, P < 0.05, and ##, P < 0.01 (boost), compared to chickens immunized with inactivated AIVs alone. †, P < 0.05 (prime); §, P < 0.05 (boost), compared to chickens immunized with the commercial H9N2 AIV vaccine.
BP5 increases the production of both Th1- and Th2-type cytokines.
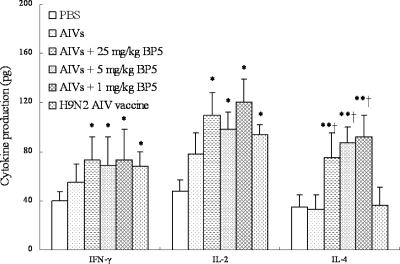
We then tested the levels of Th1 (IL-2 and IFN-γ) and Th2 (IL-4) cytokines upon coimmunization with inactivated AIV and BP5 in chickens. Compared with restimulation with inactivated AIVs alone, coimmunization with inactivated AIVs and BP5 remarkably increased the levels of both Th1-type (IL-2 and IFN-γ [P < 0.05]) and Th2-type (IL-4 [P < 0.01]) cytokines in chickens, whereas only Th1-type cytokines increased with commercially inactivated H9N2 AIV vaccine restimulation (Fig. 2).
Fig. 2.
Effect of adding different doses of BP5 to inactivated AIVs on Th1/Th2 cytokine production in chicken sera. Chickens were immunized two times, and chicken sera were collected on day 28 postimmunization. Cytokine release was measured by using a sandwich ELISA method and commercial ELISA kits. The data presented are means ± SD of results from four replicates. *, P < 0.05; **, P < 0.01, compared to chickens immunized with AIVs alone. †, P < 0.01, compared to chickens immunized with the commercial H9N2 AIV vaccine.
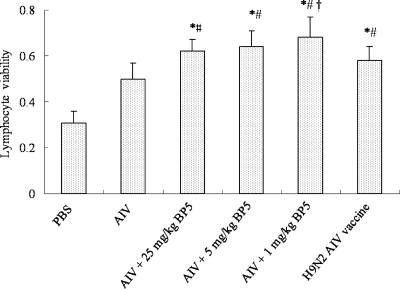
BP5 significantly enhances peripheral blood lymphocyte proliferation.
To investigate the effects of BP5 on peripheral lymphocyte proliferation, we collected peripheral lymphocytes from chickens treated with different dosages of BP5 coadministered with inactivated AIV and treated them with recombinant JS-1 (A/Chicken/Jiangsu/JS-1/2002, H9N2 AIV) HA protein in vitro. When chickens were immunized with inactivated AIVs and BP5, a significant proliferative response was observed (Fig. 3; *, P < 0.05, compared with chickens immunized with the inactivated AIVs alone; #, P < 0.01, compared with chickens immunized with PBS; †, P < 0.05, compared with chickens immunized with the commercially prepared H9N2 AIV vaccine with oil/water as an adjuvant). The data showed that chickens immunized with a combination of BP5 and inactivated AIVs also induce the highest AIV-specific cellular proliferation, in addition to the humoral responses described above.
Fig. 3.
BP5 significantly stimulates chicken peripheral blood lymphocyte proliferation. Chickens were immunized two times, and chicken peripheral blood lymphocytes were collected on day 28 postimmunization. Proliferative response was evaluated by MTT assay. Data are the means ± SD of results from four separate experiments. *, P < 0.05, compared to results with PBS alone; #, P < 0.05, compared to results with AIVs alone; †, P < 0.05, compared to chickens immunized with the commercial H9N2 AIV vaccine.
BP5 stimulates both CD4+ and CD8+ T cells.
The percentages of overall CD3+ T cells and their subsets (CD4+ T cells [CD3+ CD4+] and CD8+ T cells [CD3+ CD8+]) in the peripheral blood lymphocyte populations were significantly increased in the chickens immunized with a mixture of inactivated AIV and BP5 (5 mg/kg, P < 0.05; 1 mg/kg, P < 0.01) compared with those in chickens immunized with inactivated AIV alone (Table 2). However, CD8+ T cells were only moderately affected by administration of the commercial H9N2 AIV vaccine. This indicated that BP5 has an adjuvant activity in that it promotes the AIV vaccine by stimulating not only CD4+ T cell proliferation but also CD8+ T cell proliferation.
Table 2.
Flow cytometric analysis of CD3+ T cells and their subsets CD3+ CD4+ and CD3+ CD8+ T cells from the peripheral blood lymphocytes of immunized chickensa
| Treatment | % of peripheral blood lymphocytes of type: |
||
|---|---|---|---|
| CD3+ | CD3+ CD4+ | CD3+ CD8+ | |
| PBS | 35.15 ± 2.14 | 14.36 ± 1.89 | 9.22 ± 1.51 |
| AIVs | 45.99 ± 1.23 | 20.87 ± 2.13 | 13.68 ± 2.12 |
| AIVs + 25 mg/kg BP5 | 48.78 ± 2.86 | 20.54 ± 1.38 | 16.87 ± 1.89 |
| AIVs + 5 mg/kg BP5 | 58.82 ± 2.48* | 26.89 ± 1.56* | 22.67 ± 1.78*† |
| AIVs + 1 mg/kg BP5 | 61.46 ± 1.61**† | 30.44 ± 2.24** | 25.01 ± 1.53**† |
| H9N2 AIV vaccine | 56.87 ± 2.31* | 28.57 ± 1.79** | 16.51 ± 1.35 |
Chickens were sacrificed on day 28 after first immunization, and the peripheral blood lymphocytes were collected for immunophenotyping. The data presented are means ± SD of results from four replicates.
, P < 0.05, and
, P < 0.01, compared with chickens immunized with the inactivated AIVs alone.
, P < 0.05, compared with chickens immunized with the commercial H9N2 AIV vaccine.
BP5 significantly promotes immune protection against H9N2 AIV challenge.
To verify that a killed vaccine in combination with BP5 can provide better protection against H9N2 AIV infection, we applied a real-time PCR assay using SYBR green 1 for detection of AIV copies in the lungs of chickens on days 1, 3, and 5 after H9N2 AIV challenge. In the assay, the dissolution curve showed that the HA primer had a good specificity, and the standard curve results showed that the amplification efficiency of the HA primer, which could be used for detection of virus in lung samples, was 99.89% (data not shown). As shown in Table 3, numbers of lung viral copies were significantly reduced in the chickens coimmunized with inactivated AIVs and BP5 compared to those in the chickens immunized with inactivated AIV alone on days 1, 3, and 5 after H9N2 AIV challenge (25 mg/kg and 5 mg/kg, P < 0.05; 1 mg/kg, P < 0.01). Compared to the number of lung viral copies in the chickens immunized with the commercial H9N2 AIV vaccine (with oil/water as an adjuvant), lung viral copies were also reduced significantly in the chickens coadministered inactivated AIVs and BP5 (1 mg/kg, P < 0.05) (Table 3).
Table 3.
Detection of AIV copies in the lungs of H9N2 AIV-challenged chickens by a SYBR green 1 real-time PCRa
| Group | No. of lung viral copies (log 10)/ml PBS on day postchallenge: |
||
|---|---|---|---|
| 1 | 3 | 5 | |
| PBS | 8.6 ± 0.015 | 7.2 ± 0.036 | 4.5 ± 0.045 |
| AIVs | 7.1 ± 0.023 | 5.5 ± 0.024 | 3.1 ± 0.054 |
| AIVs + 25 mg/kg BP5 | 5.7 ± 0.034* | 4.4 ± 0.028* | 1.9 ± 0.027* |
| AIVs + 5 mg/kg BP5 | 5.4 ± 0.042* | 4.0 ± 0.064* | 1.5 ± 0.039* |
| AIVs + 1 mg/kg BP5 | 5.0 ± 0.041**† | 3.1 ± 0.055**† | 0.9 ± 0.034**† |
| H9N2 AIV Vaccine | 6.0 ± 0.031* | 4.3 ± 0.034* | 2.2 ± 0.035* |
Lung samples from individual chickens in each group were collected on days 1, 3, and 5 postchallenge. Each lung sample was diluted to 1 ml with PBS. The titers are presented as numbers of copies per ml PBS. The data presented are means ± SD of results from five replicates.
, P < 0.05, and
, P < 0.01, compared to chickens immunized with AIVs alone.
, P < 0.05, compared with chickens immunized with the commercial H9N2 AIV vaccine.
DISCUSSION
Many adjuvant approaches have been evaluated for use in vaccines. However, since most of the adjuvants used in conjugation with antigen have unacceptable levels of side effects, such as toxicity and adverse site reactions, only a few of them are used clinically (26, 35). Aluminum-based mineral salts (aluminum adjuvant; alum) have commonly been used in many veterinary and human vaccines because of their safety (1), but they induce antibody production weakly and are poor at eliciting cell-mediated immune responses (3), which are significant drawbacks for their use in vaccines against intracellular parasites and some viruses. The oil-based adjuvants, which are common in veterinary vaccines, in contrast, are limited by their induction of side effects and adverse site reactions (5, 18, 34). Thus, research to find new and optimal adjuvant candidates for vaccine formulations has been described in many publications. In some of these publications, research on some small peptide immunostimulants used for vaccine adjuvant strategies has also been reported (2, 8, 36).
In our previous study, we isolated and purified a novel bursa pentapeptide, BP5, which was capable of enhancing antigen-specific humoral and cell-mediated immune responses in mice (19). In the present study, we found that a simple mixture of inactivated H9N2 AIVs and BP5 also enhanced humoral and cell-mediated immune responses in chickens. When coinjected with the model antigen (an inactivated avian influenza virus [AIV], A/Duck/Jiangsu/NJ08/05 [AIV H9N2 subtype]), BP5 induced higher levels of antigen-specific hemagglutination inhibition (HI) antibody titers and antigen-specific HA antibody (IgG) titers in chickens than were induced in chickens immunized with inactivated avian influenza virus alone. Furthermore, chickens coadministered inactivated AIVs and proper concentrations of BP5 (used as an adjuvant) produced significantly higher HI and IgG antibody titers than chickens immunized with the commercial H9N2 AIV vaccine (prepared with oil/water as an adjuvant). In some literature, it has been reported that a single administration of commercial H9N2 AIV vaccine in oil emulsion induced higher HI antibody titers (about 9 log2) 3 weeks after vaccination than the control (16), whereas in other literature, it has been reported that oil adjuvant H9N2 AIV vaccine produced HI antibody titers that were less than 6 log2 2 weeks after the first vaccination, less than 7.0 log2 3 weeks after the first vaccination, and less than 8.0 log2 3 weeks after the second vaccination (17). It is well known that various factors, like source of erythrocytes, type of diluent, incubation temperature, and incubation period, affect hemagglutination activity, and thereby, they affect the HI titers (12). In view of this, the data for HI antibody titers obtained from this study are generally consistent with the data reported by Lee et al. (17). Although the HI antibody titers induced by the commercial AIV vaccine and by BP5 adjuvant-inactivated AIVs were not very high in this study, BP5 adjuvant-inactivated AIVs induced higher HI antibody titers than oil adjuvant commercial AIV vaccine. This suggested that BP5 has an effective adjuvant activity in vaccines that enhances antigen-specific humoral immune responses.
In addition to humoral responses, cellular immunity plays an important role in fighting influenza virus infections (14). In this study, cell-mediated immunity was evaluated in vaccinated chickens through cytokine analysis and in vitro proliferation assay of peripheral blood splenocytes pre- and postimmunization. Currently, special attention is being given to adjuvants capable of efficiently promoting a Th1-type immune response, which is considered the best correlate of a protective immune response to infections (32). However, the most powerful Th1-promoting adjuvants exhibit some toxicity, which limits their clinical use (27). The most remarkable finding reported in the present study is the demonstration that BP5, coadministered with inactivated AIVs, represents an unexpectedly powerful adjuvant, not only inducing the production of Th1-type cytokines (IL-2 and IFN-γ) but also inducing the production of Th2-type cytokines (IL-4). Moreover, in vivo/ex vivo, using MTT incorporation to measure cell proliferation and flow cytometric analysis to measure immunophenotyping of T lymphocytes, significant increases in peripheral blood lymphocyte proliferation and in the sizes of CD3+ T cell populations, including CD3+ CD4+ and CD3+ CD8+ T cell populations, were found in chickens coadministered inactivated AIVs and BP5. In contrast, although the levels of cytokines in sera and the levels of peripheral blood lymphocyte proliferation and CD3+ T cell populations were increased in chickens immunized with a commercial, inactivated AIV vaccine (prepared with oil/water as an adjuvant), levels of only Th1-type cytokines increased, and the CD8+ T cells were only moderately affected. These results indicate that BP5 has the potential to affect cell-mediated responses and balance Th1- and Th2-type immune responses when used as an adjuvant.
To further evaluate the influence of BP5 as an adjuvant on the immunity protection provided by inactivated AIVs against avian influenza virus infection, chickens were challenged intranasally with avian influenza H9N2 virus strain JS-1 (A/Chicken/ Jiangsu/JS-1/2002) on day 28 after they had been coimmu- nized with inactivated AIVs and BP5. At 2 days postchallenge, the nonvaccinated chickens that received the challenge virus were mildly depressed. No other clinical signs were observed in that group or any of the other groups, which is typical of low-pathogenicity AIVs in chickens (15, 33). At 5 days postchallenge, only the nonvaccinated challenged group had mild, grossly detectable lesions in both the respiratory and gastrointestinal tract. As JS-1 H9N2 virus is a low-pathogenicity avian influenza virus and all challenged chickens survived the infections, we used SYBR green 1-based real-time PCR to assess the extent of virus infection, monitoring the protection level of inactivated AIVs after they were coadministered with BP5. We detected the challenge virus in the lungs of the challenged chickens on days 1, 3, and 5. Our data indicated that viral replication (viral shedding) occurred and that virus shedding could be more efficiently blocked or reduced after a homologous vaccine was coadministered with BP5, which was used to vaccinate chickens against the challenge virus. This result suggests that BP5 has the potential to be used in vaccine formulations to provide improved protection against H9N2 AIV infection in poultry.
Several small peptides have been synthesized in an effort to discover an idealized peptide sequence with significant immunological adjuvant activity (7, 8). Our previous study revealed that B lymphocyte proliferation induced by BP5 is mediated by reactive oxygen species generated from thiol auto-oxidation of Cys in BP5 (19). We presume that Cys plays an important role in the immune functions of BP5. Thus, analogs of BP5, such as Gly-Lys-Asp-Val-Tyr, Ala-Lys-Asp-Val-Tyr, and Glu-Lys-Asp-Val-Tyr, were also synthesized and used to evaluate their immune activities in mice and chickens. In the assays, no significant immune adjuvant activities of these peptides were detected (data not shown). This suggests that the specific immune inducer properties of BP5 are associated with its special amino acid sequence. Further research on the relationship between the structure and the immune activity of BP5 will contribute new insights into the mechanisms of adjuvant activity and may lead to the development of a practical application in vaccine design. Further studies are also needed to further compare the effects of BP5 and other adjuvants.
In summary, we demonstrated that BP5 enhanced the avian influenza virus-specific cell-mediated and humoral immune responses induced by inactivated AIVs. Furthermore, intramuscular immunization with a mixture of inactivated AIVs and BP5 enhanced protection against a homologous avian influenza virus challenge by reducing viral replication in chicken lungs. This study indicates that BP5 possesses adjuvant activities and that it may be used as a new experimental reagent for immuno-adjuvant uses.
ACKNOWLEDGMENT
The present study was supported by a grant from the National Agriculture Special Research Project (grant 200803020).
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Bowersock T. L., Martin S. 1999. Vaccine delivery to animals. Adv. Drug Deliv. Rev. 38:167–194 [DOI] [PubMed] [Google Scholar]
- 2. Charoenvit Y., Goel N., Whelan M., Rosenthal K. S., Zimmerman D. H. 2004. CEL-1000—a peptide with adjuvant activity for Th1 immune responses. Vaccine 22:2368–2373 [DOI] [PubMed] [Google Scholar]
- 3. Cox J. C., Coulter A. R. 1997. Adjuvants, a classification and review of their modes of action. Vaccine 15:248–256 [DOI] [PubMed] [Google Scholar]
- 4. Domingo E. 1997. Rapid evolution of viral RNA genomes. J. Nutr. 127:958S–961S [DOI] [PubMed] [Google Scholar]
- 5. Droual R., Bickford A. A., Cutler G. J. 1993. Local reaction and serological response in commercial layer chickens injected intramuscularly in the leg with oil-adjuvanted Mycoplasma gallisepticum bacterin. Avian Dis. 37:1001–1008 [PubMed] [Google Scholar]
- 6. Foss D. L., Murtaugh M. P. 2000. Mechanisms of vaccine adjuvanticity at mucosal surfaces. Anim. Health Res. Rev. 1:3–24 [DOI] [PubMed] [Google Scholar]
- 7. Fritz J. H., et al. 2004. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine 22:3274–3284 [DOI] [PubMed] [Google Scholar]
- 8. Gagnon L., DiMarco M., Kirby R., Zacharie B., Penney C. L. 2000. d-LysAsnProTyr tetrapeptide: a novel B-cell stimulant and stabilized bursin mimetic. Vaccine 18:1886–1892 [DOI] [PubMed] [Google Scholar]
- 9. Hilleman M. R. 1998. Six decades of vaccine development: a personal history. Nat. Med. 4:507–514 [DOI] [PubMed] [Google Scholar]
- 10. Hirst G. K. 1942. The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J. Exp. Med. 75:49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung C. M., et al. 2009. Gingyo-san enhances immunity and potentiates infectious bursal disease vaccination. Evid. Based Complement Alternat. Med. 2011:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hussain M., Mehmood M. D., Ahmad A., Shabbir M. Z., Yaqub T. 2008. Factors affecting hemagglutination activity of avian influenza virus subtype H5N1. J. Vet. Anim. Sci. 1:31–36 [Google Scholar]
- 13. Kong X., Hu Y., Rui R., Wang D., Li X. 2004. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int. Immunopharmacol. 4:975–982 [DOI] [PubMed] [Google Scholar]
- 14. Kreijtz J. H., et al. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25:612–620 [DOI] [PubMed] [Google Scholar]
- 15. Lee C. W., et al. 2000. Sequence analysis of the hemagglutinin gene of H9N2 Korean avian influenza virus and assessment of the pathogenic potential of isolate MS96. Avian Dis. 44:527–535 [PubMed] [Google Scholar]
- 16. Lee D. H., et al. 2011. H9N2 avian influenza virus-like particle vaccine provides protective immunity and a strategy for the differentiation of infected from vaccinated animals. Vaccine 29:4003–4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee D. H., et al. 2011. Inactivated H9N2 avian influenza virus vaccine with gel-primed and mineral oil-boosted regimen could produce improved immune response in broiler breeders. Poult. Sci. 90:1020–1022 [DOI] [PubMed] [Google Scholar]
- 18. Leenaars M., Koedam M. A., Hendriksen C. F., Claassen E. 1998. Immune responses and side effects of five different oil-based adjuvants in mice. Vet. Immunol. Immunopathol. 61:291–304 [DOI] [PubMed] [Google Scholar]
- 19. Li D. Y., et al. 2011. Immunomodulatory activities of a new pentapeptide (Bursopentin) from the chicken bursa of Fabricius. Amino Acids 40:505–515 [DOI] [PubMed] [Google Scholar]
- 20. Liu M. A. 1998. Vaccine developments. Nat. Med. 4:515–519 [DOI] [PubMed] [Google Scholar]
- 21. Maines T. R., et al. 2008. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol. Rev. 225:68–84 [DOI] [PubMed] [Google Scholar]
- 22. Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application of proliferation and cytotoxicity assays. J. Immunol. Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- 23. Mosmann T. R., Coffman R. L. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173 [DOI] [PubMed] [Google Scholar]
- 24. Ong W. T., Omar A. R., Ideris A., Hassan S. S. 2007. Development of a multiplex real-time PCR assay using SYBR Green 1 chemistry for simultaneous detection and subtyping of H9N2 influenza virus type A. J. Virol. Methods 144:57–64 [DOI] [PubMed] [Google Scholar]
- 25. Peiris M., et al. 1999. Human infection with influenza H9N2. Lancet 354:916–917 [DOI] [PubMed] [Google Scholar]
- 26. Petrovsky N., Aguilar J. C. 2004. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 82:488–496 [DOI] [PubMed] [Google Scholar]
- 27. Proietti E., et al. 2002. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169:375–383 [DOI] [PubMed] [Google Scholar]
- 28. Qiu Y., et al. 2007. Immunopotentiating effects of four Chinese herbal polysaccharides administered at vaccination in chickens. Poult. Sci. 86:2530–2535 [DOI] [PubMed] [Google Scholar]
- 29. Saito T., et al. 2001. Characterization of a human H9N2 influenza virus isolated in Hong Kong. Vaccine 20:125–133 [DOI] [PubMed] [Google Scholar]
- 30. Sasai K., et al. 1997. Analysis of splenic and thymic lymphocyte subpopulations in chickens infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 59:359–367 [DOI] [PubMed] [Google Scholar]
- 31. Schellack C., et al. 2006. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine 24:5461–5472 [DOI] [PubMed] [Google Scholar]
- 32. Singh M., O'Hagan D. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075–1081 [DOI] [PubMed] [Google Scholar]
- 33. Soda K., Asakura S., Okamatsu M., Sakoda Y., Kida H. 2011. H9N2 influenza virus acquires intravenous pathogenicity on the introduction of a pair of di-basic amino acid residues at the cleavage site of the hemagglutinin and consecutive passages in chickens. Virol. J. 8:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steiner J. W., Langer B., Schatz D. L. 1960. The local and systemic effects of Freund's adjuvant and its fractions. Arch. Pathol. 70:424–434 [PubMed] [Google Scholar]
- 35. Uto T., et al. 2007. Targeting of antigen to dendritic cells with poly(γ-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J. Immunol. 178:2979–2986 [DOI] [PubMed] [Google Scholar]
- 36. Wang C., et al. 2008. Bursin as an adjuvant is a potent enhancer of immune response in mice immunized with the JEV subunit vaccine. Vet. Immunol. Immunopathol. 122:265–274 [DOI] [PubMed] [Google Scholar]
- 37. Wareing M. D., Tannock G. A. 2001. Live attenuated vaccines against influenza; an historical review. Vaccine 19:3320–3330 [DOI] [PubMed] [Google Scholar]
- 38. Webster R. G., Thomas T. L. 1993. Efficacy of equine influenza vaccines for protection against A/Equine/Jilin/89 (H3N8)—a new equine influenza virus. Vaccine 11:987–993 [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization 1999. Influenza. Wkly. Epidemiol. Rec. 74:111 [Google Scholar]
- 40. Zheng Q. S., et al. 2005. Prokaryotic expression and the establishment of a putative indirect ELISA assay for the HA gene of avian influenza virus H9N2 subtype. Virol. Sin. 20:293–297 [PubMed] [Google Scholar]