Abstract
β-Glucans are well known for their immunomodulatory capacities in humans and mice. For this reason, together with the European ban on growth-promoting antibiotics, β-glucans are intensively used in pig feed. However, as shown in the present study, there is much variation in the stimulatory capacities of β-glucans from different sources. Since dendritic cells (DCs) are the first cells that are encountered after an antigen is taken up by the intestinal epithelial cell barrier, we decided to investigate the effect of two concentrations (5 and 10 μg/ml) of five commercial β-glucan preparations, differing in structure and source, on porcine monocyte-derived dendritic cells (MoDCs). Although all β-glucans gave rise to a significant reduction of the phagocytic activity of DCs, only Macrogard induced a significant phenotypic maturation. In addition to Macrogard, zymosan, another β-glucan derived from Saccharomyces cerevisiae, and curdlan also significantly improved the T-cell-stimulatory capacity of MoDCs. Most interesting, however, is the cytokine secretion profile of curdlan-stimulated MoDCs, since only curdlan induced significant higher expression levels of interleukin-1β (IL-1β), IL-6, IL-10, and IL-12/IL-23p40. Since the cytokine profile of DCs influences the outcome of the ensuing immune response and thus may prove valuable in intestinal immunity, a careful choice is necessary when β-glucans are used as dietary supplement.
INTRODUCTION
Dendritic cells (DCs) are the directors of the immune system and form the messengers between the innate and the adaptive immune system. Immature DCs identify pathogens by recognizing signatures present in microbes, so-called pathogen-associated molecular patterns (PAMPs), through the expression of pattern recognition receptors (PRRs). Recognition of PAMPs by these PRRs results in the activation and maturation of DCs. This maturation process is associated with a loss of phagocytic activity and an upregulation of major histocompatibility complex (MHC) and costimulatory molecules, such as CD80, CD86, and CD40. In addition to functional and phenotypic changes, the exposure of DCs to microbial components results in the production of cytokines that modulate the T-cell polarization and their functions. Upon interaction with DCs, CD4+ T cells can differentiate into a variety of effector subsets, including Th1 and Th2 cells, the more recently identified Th17 cells, and regulatory T cells (15, 21, 43). Furthermore, DCs have been shown to trigger B-cell growth and differentiation (9, 16). DCs are thus capable of modulating the nature of immune responses, which in turn are dependent on the type of PRR that is activated. Phagocytes, such as DCs, express a wide variety of PRRs, such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors, and C-type lectin receptors on their cell surfaces (18, 28, 34). Dectin-1, a C-type lectin receptor and the most important PRR recognizing β-glucans, is expressed by various antigen-presenting cells, including immature DCs (ImDCs) and macrophages (41).
β-Glucans are one of the most abundant forms of polysaccharides found inside the cell wall of bacteria, fungi, and yeasts (23). All β-glucans are glucose polymers linked by a 1,3 linear β-glycosidic chain core and, depending on the source, they differ in length and branching structures. β-Glucans have a number of beneficial effects on the immune system, making them interesting for the development of β-glucan-based therapeutics and food supplements (6, 42). In humans, β-glucans are regularly used as prebiotic supplement to increase the stability of the gut flora, to augment innate immune responses, and to orchestrate healthy immune responses (39). In the pig industry, β-glucans are also applied as dietary supplements. However, the molecular mechanisms through which different β-glucans exert their effects are not well known (38). Nevertheless, it is important to know and understand the effect of different β-glucans on the immune system to use β-glucans efficiently in practice. In a previous study, we tested the direct effect of different β-glucan preparations on porcine monocytes, neutrophils, and lymphocytes (31). In the present study, we focused on the effects they have on DC maturation since these cells are the most important antigen-presenting cells and key players in the initiation and stimulation of the adaptive immunity.
MATERIALS AND METHODS
β-Glucans and LPS.
Laminarin, curdlan, zymosan, and the β-glucan purified from Euglena gracilis were purchased from Sigma (Bornem, Belgium), as was lipopolysaccharide (LPS; serotype O55:B5), which served as a control. Macrogard, which is currently used as a dietary supplement in the pig industry, was kindly provided by Biotec Pharmacon ASA (Norway). A description and comparison of the carbohydrate structures, as well as the preparation and storage of these β-glucans, has been published (31). The endotoxin concentration present in each β-glucan preparation was determined by a chromogenic Limulus amebocyte lysate test (Cambrex BioScience, Inc., Walkersville, MD) and, with the exception of curdlan (47 endotoxin units/μg of β-glucan), were consistently lower than 0.5 endotoxin units/μg of β-glucan.
Generation of monocyte-derived DCs.
Porcine monocyte-derived DCs (MoDCs) were generated from peripheral blood mononuclear cells (PBMC). Briefly, peripheral blood was collected on heparin from the jugular vein of four Belgian Landrace pigs that were 8 to 12 weeks old; the PBMC were then isolated by density gradient centrifugation on a LymphoPrep (Nycomed Pharma AS, Life Technologies, Merelbeke, Belgium). CD172a+ cells were isolated from the PBMC fraction by positive magnetic activated cell separation (Miltenyi-Biotec, Bergisch Gladbach, Germany) using anti-CD172a monoclonal antibody (MAb; 74-12-15A [24]) and anti-mouse IgG microbeads, together with LS separation columns (Miltenyi-Biotec). The obtained cells were cultured in 24-well plates at a density of 5.105 cells in phenol-red free Dulbecco modified Eagle medium (DMEM; Gibco, Merelbeke, Belgium) containing 10% fetal calf serum (FCS; Greiner), penicillin (100 IU/ml; Gibco), streptomycin (100 μg/ml; Gibco), recombinant porcine granulocyte-macrophage colony-stimulating factor (rpGM-CSF) (14), and recombinant porcine interleukin-4 (rpIL-4; R&D Systems, Minneapolis, MN), followed by incubation at 37°C in a humidified atmosphere at 5% CO2 to generate MoDCs as previously described (5). After 3 days, the cultures were supplemented with fresh cytokines. On day 4, cells were stimulated for 24 h with 5 or 10 μg of the different β-glucans or LPS/ml.
Phenotyping of MoDCs.
The surface expression of various DC maturation markers after stimulation was assessed by flow cytometry (FACSCanto). Upon stimulation with 5 and 10 μg of the different β-glucans/ml or 1 or 10 μg of LPS/ml, the MoDCs were harvested, washed with RPMI 1640 plus 1% FCS, and labeled with a primary mouse MAb for 20 min at 4°C. The following primary MAbs were used to identify the maturation markers—anti-MHC-II MSA3 (20), anti-CD40 G28-5 (3), and a human CTLA4-muIg fusion protein (Ancell, Bayport, MN)—to detect the expression of CD80 and CD86. Cells stained with isotype-matched irrelevant MAbs were used as a negative control. After incubation, cells were washed and stained with fluorescein isothiocyanate-conjugated F(ab′)2 fragments of sheep anti-mouse IgG antibodies (Sigma) for another 20 min at 4°C. Next, the cells were washed, and propidium iodide was added to the cells to exclude dead cells from the flow cytometer analysis. The data were acquired on a FACSCanto flow cytometer with a minimum event count of 20,000 and analyzed using FACSDiva software (Becton Dickinson, Erembodegem, Belgium).
Antigen uptake.
The phagocytic activity of β-glucan-stimulated MoDCs was evaluated with ovalbumin-dQ (ova-dQ; Invitrogen/Molecular Probes). Upon incubation with the different β-glucans (5 or 10 μg/ml) or LPS (1 or 10 μg/ml) for 24 h, the MoDCs were harvested, washed with RPMI 1640 plus 1% FCS, and incubated for 1 h with 10 μg of ova-dQ/ml at 37°C in a humidified atmosphere at 5% CO2. To analyze the background fluorescence, the uptake at 4°C was measured. The uptake of ova-dQ by stimulated MoDCs was analyzed by flow cytometry as described above.
Allogeneic mixed leukocyte reaction.
Mixed leukocyte reactions were performed in 96-well round-bottom culture plates (Nunc) with CD172a-depleted cells (2 × 105 cells/well) as responder cells. Allogeneic MoDCs, stimulated for 24 h with the different β-glucans (5 or 10 μg/ml) or LPS (1 or 10 μg/ml), were added to the cultures as stimulator cells at a ratio of 1:30. Cocultures were performed in triplicate and maintained in DMEM, 10% FCS, 1% penicillin-streptomycin, and 50 μM 2-mercaptoethanol at 37°C in a humidified atmosphere at 5% CO2. After 5 days of culture, the cells were pulse-labeled with 1 μCi of [3H]methyl-thymidine (Amersham ICN, Bucks, United Kingdom) per well, and 18 h later the cells were harvested onto glass fiber filters (Perkin-Elmer Life Sciences, Brussels, Belgium). The radioactivity incorporated into the DNA was measured by using a β-scintillation counter (Perkin-Elmer). The results are presented as the mean counts per minute (cpm).
Cytokine ELISA.
MoDCs were stimulated with β-glucans as mentioned above and the culture supernatant was harvested after 24 h and stored at −20°C. The concentrations of IL-1β, IL-8, IL-12p40, IL-6, tumor necrosis factor alpha (TNF-α), and IL-10 were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer's recommended protocols. The cytokine concentrations were calculated using DeltaSOFT JV 2.1.2 software (BioMetallics, Princeton, NJ) with a four-parameter curve-fitting algorithm.
Statistics.
All experiments were performed with cells from four different pigs. Statistical analyses were performed using SPSS16. One-way analysis of variance with a least-significant-difference post hoc test was performed. Levene's test was used to assess the homogeneity of the variances. A P value of <0.05 was considered statistically significant.
RESULTS
β-Glucans enhance the upregulation of DC activation markers.
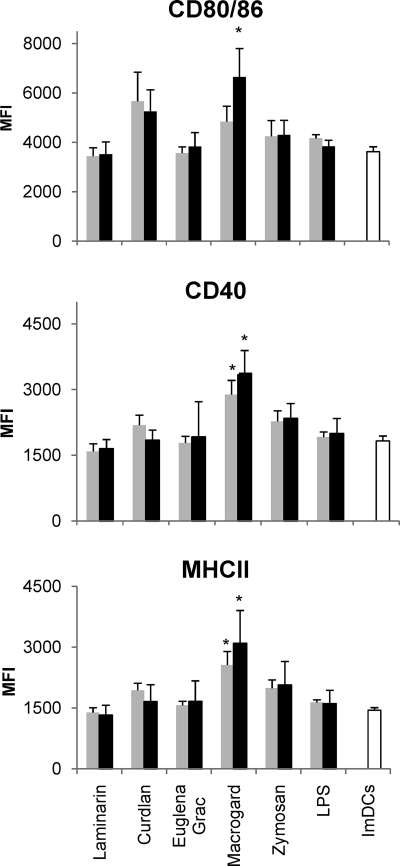
Optimal antigen presentation and subsequent T-cell responses require DC maturation. This process includes the upregulation of costimulatory molecules and MHC class II (MHC-II). To investigate the effect of β-glucans on the phenotypic DC maturation, immature MoDCs were stimulated for 24 h with 5 or 10 μg of the different β-glucan preparations/ml or 1 or 10 μg of LPS/ml, and the cell surface expression of CD80/86, CD40, and MHC-II was assessed by flow cytometry. Figure 1 shows that Macrogard, at both 5 and 10 μg/ml, induced a significant upregulation of MHC-II and CD40 expression, compared to the untreated immature cells (ImDCs) (P < 0.01), whereas CD80/86 expression was only significantly upregulated at a dose of 10 μg/ml (P < 0.01). Both doses of LPS (1 and 10 μg/ml) failed to induce an increased marker expression.
Fig. 1.
Analysis of the expression of CD80/86 (top panel), CD40 (middle panel), and MHCII (bottom panel) after stimulation of immature MoDCs with 5 μg ( ) or 10 μg (▪) of β-glucan/ml or LPS (1 and 10 μg/ml). The expression of the maturation markers was assayed by flow cytometry. The data are shown as the means ± the standard errors of the mean (SEM) for four pigs. Asterisks (*) indicate a significant difference between β-glucan-stimulated MoDCs and immature MoDCs (ImDCs) (P < 0.01).
) or 10 μg (▪) of β-glucan/ml or LPS (1 and 10 μg/ml). The expression of the maturation markers was assayed by flow cytometry. The data are shown as the means ± the standard errors of the mean (SEM) for four pigs. Asterisks (*) indicate a significant difference between β-glucan-stimulated MoDCs and immature MoDCs (ImDCs) (P < 0.01).
β-Glucan treatment downregulates the phagocytic capacity of MoDCs.
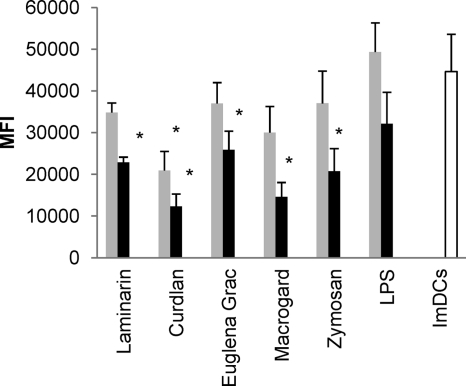
To evaluate DC maturation other than analyzing the phenotype switch associated with DC activation, functional maturation of the DCs upon treatment can be assessed. In terms of phagocytosis, it is known that ImDCs can efficiently engulf antigens but, upon maturation, DCs lose this phagocytic ability. In our studies, the phagocytic capacity of β-glucan-stimulated MoDCs was determined through the uptake of ova-dQ (Fig. 2). At 10 μg/ml, all β-glucans significantly reduced the capacity of MoDCs to endocytose antigen compared to the untreated MoDCs (for all β-glucans P < 0.01 except for the β-glucan from Euglena gracilis [P = 0.02]). Stimulation of MoDCs with 5 μg of curdlan/ml also significantly reduced their phagocytic ability (P = 0.005). Although there was a reduced, but not significant uptake of ova-dQ after stimulation of MoDCs with 10 μg of LPS/ml, no effect was seen at the lowest concentration.
Fig. 2.
Analysis of the phagocytic activity of β-glucan-stimulated MoDCs. Immature MoDCs were stimulated with 5 μg ( ) or 10 μg (▪) of β-glucan/ml or LPS (1 and 10 μg/ml), and the uptake of ova-dQ was assayed by flow cytometry. Mean fluorescence intensity (MFI) values were calculated by subtracting the MFI values obtained at 4°C from those obtained at 37°C. The data are shown as the means ± the SEM for four pigs. Asterisks (*) indicate a significant difference between β-glucan-stimulated MoDCs and immature MoDCs (ImDCs) (for all β-glucans P < 0.01 except for the β-glucan from Euglena gracilis).
) or 10 μg (▪) of β-glucan/ml or LPS (1 and 10 μg/ml), and the uptake of ova-dQ was assayed by flow cytometry. Mean fluorescence intensity (MFI) values were calculated by subtracting the MFI values obtained at 4°C from those obtained at 37°C. The data are shown as the means ± the SEM for four pigs. Asterisks (*) indicate a significant difference between β-glucan-stimulated MoDCs and immature MoDCs (ImDCs) (for all β-glucans P < 0.01 except for the β-glucan from Euglena gracilis).
β-Glucan-stimulated MoDCs induce T-cell proliferation.
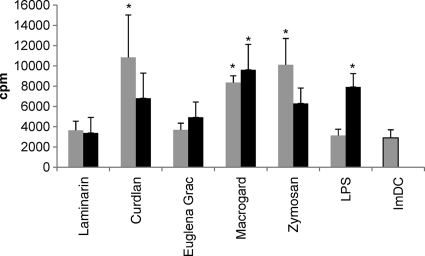
As a result of maturation, MoDCs become potent stimulators of immune responses, resulting in an increased T-cell stimulatory capacity. In order to determine whether MoDCs stimulated with β-glucans are able to induce T-cell proliferation, β-glucan-stimulated MoDCs were cocultured with CD172a-depleted cells (Fig. 3). When stimulated at 5 μg/ml, Macrogard-, curdlan-, and zymosan-stimulated MoDCs significantly increased T-cell proliferation compared to ImDCs (P < 0.01). MoDCs stimulated with a higher dose of curdlan and zymosan (10 μg/ml) tended to induce a weaker T-cell proliferation than after stimulation with 5 μg/ml, whereas MoDCs stimulated with 10 μg of Macrogard/ml significantly increased T-cell proliferation more than after stimulation with 5 μg/ml (P < 0.01). Stimulation of MoDCs with the higher dose of LPS (10 μg/ml) also gave a significantly stronger T-cell proliferation (P < 0.001) than the lower dose (1 μg/ml) compared to the ImDCs.
Fig. 3.
Analysis of the ability of β-glucan-stimulated MoDCs to enhance T-cell proliferation. Porcine MoDCs were left untreated or stimulated for 24 h with 5 μg ( ) or 10 μg (▪) of different β-glucans/ml or LPS (1 or 10 μg/ml) and then added to CD172a-depleted PBMC (lymphocytes). After 5 days, the cultures were pulsed with 1 μCi of [3H]methyl-thymidine. T-cell proliferation was measured after an additional coculture of 18 h. The data are shown as means ± the SEM for four pigs. Asterisks (*) indicate a significant difference between β-glucan-stimulated conditions and the untreated condition (ImDCs) (P < 0.01 except for LPS [10 μg/ml]; P < 0.05). The proliferative responses of the CD172a− lymphocytes (i.e., no MoDCs were added) was less than 300 cpm.
) or 10 μg (▪) of different β-glucans/ml or LPS (1 or 10 μg/ml) and then added to CD172a-depleted PBMC (lymphocytes). After 5 days, the cultures were pulsed with 1 μCi of [3H]methyl-thymidine. T-cell proliferation was measured after an additional coculture of 18 h. The data are shown as means ± the SEM for four pigs. Asterisks (*) indicate a significant difference between β-glucan-stimulated conditions and the untreated condition (ImDCs) (P < 0.01 except for LPS [10 μg/ml]; P < 0.05). The proliferative responses of the CD172a− lymphocytes (i.e., no MoDCs were added) was less than 300 cpm.
β-Glucans stimulate cytokine secretion by MoDCs.
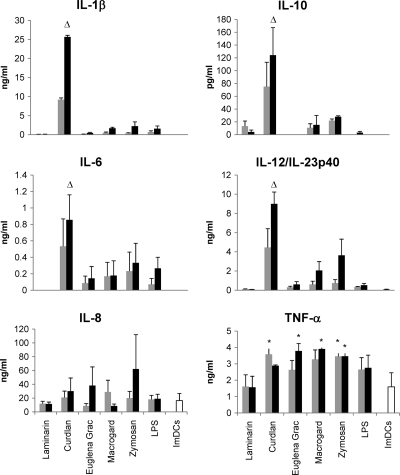
Cytokine expression is another parameter to evaluate the functional maturation of DCs and is responsible for the differentiation and polarization of T cells. To investigate whether the observed phenotypic and functional maturation of MoDCs induced after β-glucan treatment correlates with a certain cytokine expression profile, the IL-1β, IL-6, IL-8, IL-12/IL-23p40, TNF-α, and IL-10 concentrations were determined in the culture supernatant of β-glucan-stimulated MoDCs (Fig. 4). Compared to ImDCs, stimulation with all β-glucans, except laminarin, could significantly increase TNF-α secretion. Interestingly, whereas the β-glucan from Euglena gracilis and Macrogard induced a significantly higher TNF-α production at 10 μg/ml and zymosan induced a significantly higher production at both concentrations, curdlan only induced a significant increase in TNF-α secretion at the lowest concentration (5 μg/ml) (P < 0.05). In contrast, only stimulation with 10 μg of curdlan/ml resulted in a significantly higher production of IL-1β, IL-6, IL-10, and IL-12/IL-23p40 compared to ImDCs (P < 0.05) and all of the other stimulated MoDCs (P < 0.01). Macrogard and zymosan also enhanced the production of these cytokines, while zymosan and, to a lesser degree, the β-glucan from Euglena gracilis and curdlan gave rise to IL-8 secretion, but these increases were not significant.
Fig. 4.
Cytokine expression pattern of MoDCs treated with different β-glucans. MoDCs were left untreated (ImDCs) or stimulated with 5 μg ( ) and 10 μg (▪) of different β-glucans/ml or LPS (1 or 10 μg/ml). After 24 h, the culture supernatant was harvested, and the IL-1β, IL-6, IL-8, IL-10, IL-12/IL-23p40, and TNF-α cytokine concentrations were measured using commercially available ELISA kits. The data are shown as means ± the SEM for four pigs. Asterisks (*) indicate a significant difference (P < 0.05) between β-glucan-stimulated MoDCs and immature MoDCs, while a triangle (▵) indicates a significant difference (P < 0.01) between MoDCs stimulated with 10 μg of curdlan/ml and all of the other β-glucan-stimulated (10 μg/ml) MoDCs or immature MoDCs.
) and 10 μg (▪) of different β-glucans/ml or LPS (1 or 10 μg/ml). After 24 h, the culture supernatant was harvested, and the IL-1β, IL-6, IL-8, IL-10, IL-12/IL-23p40, and TNF-α cytokine concentrations were measured using commercially available ELISA kits. The data are shown as means ± the SEM for four pigs. Asterisks (*) indicate a significant difference (P < 0.05) between β-glucan-stimulated MoDCs and immature MoDCs, while a triangle (▵) indicates a significant difference (P < 0.01) between MoDCs stimulated with 10 μg of curdlan/ml and all of the other β-glucan-stimulated (10 μg/ml) MoDCs or immature MoDCs.
DISCUSSION
For humans and mice, there is an extensive amount of information regarding β-glucans and their immunomodulatory effects. However, the effects are not always consistent and it is not clear whether these differences are due to the use of different β-glucan preparations or due to variation among experimental models. In the pig industry, dietary β-glucan supplementation is often used, especially during periods of stress and immune challenge such as weaning. However, the beneficial effect of β-glucan supplementation may be influenced by several factors, such as structural features and the dose of β-glucan used in the diets. In a previous study, we described the dose effect of different β-glucan preparations on porcine leukocytes (31). In the present study, we focused on the action of different β-glucans on porcine dendritic cells. DCs have not only been described to sense the luminal environment at epithelial surfaces and sample luminal pathogens but have also been shown to be key players in the initiation and differentiation of the immune response (15, 17, 26). Although intestinal DCs are presumed to be the in vivo target for β-glucan activity, they are not easily accessible for in vitro studies. In contrast, MoDCs are a well-established DC model in swine research (3, 25).
The results of the present study demonstrate that there is a large variation in terms of DC maturation-inducing properties between the different β-glucans. All particulate commercial β-glucan preparations affect one or more characteristics typical for maturation, and this is partly caused by their particulate nature. However, Macrogard, zymosan, and curdlan affect maturation more than the glucan from Euglena gracilis. Macrogard and zymosan are both β-(1,3)-(1,6)-glucans isolated from Saccharomyces cerevisiae. Although both preparations contain small amounts of mannose units, possibly having an additional stimulating effect through the mannose receptor (22, 33), Macrogard contains proportionally more glucans than zymosan (31). Since Macrogard-stimulated DCs are more activated than after stimulation with zymosan, these results demonstrate that β-(1,3)-(1,6)-glucans and their recognition by one or several β-glucan receptors are the determining factor for the DC stimulatory capacity. The immunomodulatory effect of β-glucans has been attributed to dectin-1-mediated signaling, which is the primary PRR for β-glucans (4). In human and murine DCs, dectin-1 is the major β-glucan receptor responsible for downstream signaling and the uptake of β-glucans by DCs (2). Recently, we demonstrated dectin-1 expression in porcine intestinal tissues (32) and, as in humans (40, 41), we found that dectin-1 mRNA transcripts are abundantly present in porcine MoDCs (data not shown). This allows us to assume that the recognition of β-glucans by porcine DCs is primarily mediated via dectin-1. However, in addition to dectin-1, other β-glucan receptors, such as complement receptor 3 (CR3) (30), lactosylceramide (44), and scavenger receptors (27), may be involved in the recognition of β-glucans and their immunostimulatory effects.
The presence of β-(1,6) branches contributes possibly to the stimulatory activity of β-glucans since Macrogard-stimulated MoDCs are much more mature than MoDCs stimulated with the unbranched β-(1,3)-glucan from Euglena gracilis. Curdlan, however, also an unbranched β-(1,3)-glucan, has much higher immunostimulating capacities than the glucan from Euglena gracilis. which is probably caused by the high LPS contamination (47 endotoxin units [EU]/μg). Based on the approximation that 1 EU correlates to 0.2 ng of LPS (35), we estimated the LPS contamination in curdlan to correspond to the administration of ∼10 ng of LPS/μg of β-glucan, which is 100 times less than the LPS control, representing a minor but possibly stimulatory dose. We had only LPS-contaminated curdlan to our disposal, but Ferwerda et al. (10) conducted their experiments with LPS-free curdlan. In human monocyte-derived macrophages, they investigated the synergistic effect of curdlan and LPS on TNF-α and IL-10 production (10). Both TNF-α and IL-10 production was increased, and they could inhibit the synergistic effect on TNF-α production, but not IL-10 production, by a neutralizing dectin-1 antibody. These results demonstrate that in human macrophages, at least for TNF-α production, curdlan exerts its effects through dectin-1 and that curdlan in combination with LPS induces a synergistic signaling between dectin-1 and TLR-4 (10). In addition to TNF-α and IL-10, it was shown that IL-6 and IL-23 production were also mediated through dectin-1 (19, 29), while the production of all of these cytokines was enhanced after coligation of dectin-1 and TLRs (7). In our results, we demonstrated that although most of the β-glucans could induce IL-1β, TNF-α, IL-6, IL-12/IL-23p40, and IL-10 production, the highest production of all of these cytokines was found after stimulation with LPS-contaminated curdlan, suggesting that also in the pig coligation of multiple pattern recognition receptors (PRRs) can occur and that possibly also dectin-1 is involved. The combination of β-glucans together with LPS and other PRR ligands gives a more accurate picture of the real situation in the intestine and should be considered when evaluating the effectiveness of a β-glucan preparation as a dietary component. However, in contrast to humans and mice and in line with earlier reports (8, 12, 25), porcine MoDCs are less responsive to LPS itself. LPS could not upregulate the expression of maturation markers and did not significantly increase the proinflammatory cytokines IL-1β, IL-6, and TNF-α or the Th1 cell-inducing IL-12, the chemoattractant IL-8, or the anti-inflammatory cytokine IL-10.
It is well known that the spectrum of cytokines produced by DCs modulates the polarization of the T-cell response and determines the outcome of an immune response (36). We found high secretion levels of TNF-α and IL-8 in the supernatant of all β-glucan-stimulated MoDCs. TNF-α and IL-8 serve both as chemoattractants for innate and adaptive immune cells such as neutrophils and T cells, respectively, underlining the role of DCs in linking innate and adaptive immunity (11). Furthermore, we demonstrated that stimulation of MoDCs with curdlan gave rise to high levels of the proinflammatory cytokines IL-6, IL-12/IL-23p40, and IL-1β, cytokines which can, together with TNF-α, direct the T cells toward a Th1 or Th17 response (37). However, it is also possible that the LPS contamination in the curdlan preparation could favor regulatory T cell (Treg) responses, characterized by high IL-10 levels. To test the qualitative T-cell responses, we measured IFN-γ, IL-10, and IL-17 production in the supernatant of β-glucan-stimulated DC/T-cell cocultures. However, after 5 to 7 days of coculture, we did not find any of those cytokines in the supernatant. This is in contrast to the report by Agrawal et al. (1), who found that a culture of curdlan (20 μg/ml)-stimulated DCs with naive CD4+ T cells sustained their differentiation toward both IL-17- and IFN-γ-producing Th cells (1). In another study, in which these cells were stimulated with much lower concentrations of curdlan (1 ng/ml), only IFN-γ and IL-17 production after restimulation with anti-CD3 was observed (13). It is possible that in our experiments T-cell-specific cytokines will also only be produced after restimulation with anti-CD3.
In summary, we have shown that β-glucans differing in structure, molecular weight, and origin have a varying effect on the maturation of porcine MoDCs. The β-glucans derived from Saccharomyces cerevisiae enhance DC maturation and DC-induced T-cell proliferation. Also, curdlan has a strong effect on the maturation of porcine MoDCs, which is probably caused by a costimulatory effect of the LPS contamination. These results, together with the results obtained in porcine leukocytes (31), demonstrate that different β-glucans can have varying effects on the host immune system and that a careful choice is needed when β-glucans are incorporated in the feed as a dietary supplement.
ACKNOWLEDGMENTS
This research was funded by a Ph.D. grant from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
We thank S. Inumaru (Institute of Animal Health, Ibaraki, Japan) for kindly providing rpGM-CSF and H. J. Rothkötter (Institute of Anatomy, Magdeburg, Germany) for the anti-CD40 hybridoma SN.
Footnotes
Published ahead of print on 13 July 2011.
REFERENCES
- 1. Agrawal S., Gupta S., Agrawal A. 2010. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One 5:e13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backer R., van Leeuwen F., Kraal G., den Haan J. M. 2008. CD8− dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur. J. Immunol. 38:370–380 [DOI] [PubMed] [Google Scholar]
- 3. Bimczok D., et al. 2007. Cholera toxin promotes the generation of semi-mature porcine monocyte-derived dendritic cells that are unable to stimulate T cells. Vet. Res. 38:597–612 [DOI] [PubMed] [Google Scholar]
- 4. Brown G. D., et al. 2003. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197:1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrasco C. P., et al. 2001. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology 104:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan G. C., Chan W. K., Sze D. M. 2009. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dennehy K. M., Willment J. A., Williams D. L., Brown G. D. 2009. Reciprocal regulation of IL-23 and IL-12 following coactivation of dectin-1 and TLR signaling pathways. Eur. J. Immunol. 39:1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devriendt B., Verdonck F., Summerfield A., Goddeeris B. M., Cox E. 2010. Targeting of Escherichia coli F4 fimbriae to Fcγ receptors enhances the maturation of porcine dendritic cells. Vet. Immunol. Immunopathol. 135:188–198 [DOI] [PubMed] [Google Scholar]
- 9. Dubois B., et al. 1998. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 161:2223–2231 [PubMed] [Google Scholar]
- 10. Ferwerda G., Meyer-Wentrup F., Kullberg B. J., Netea M. G., Adema G. J. 2008. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 10:2058–2066 [DOI] [PubMed] [Google Scholar]
- 11. Gesser B., et al. 1996. IL-8 induces T cell chemotaxis, suppresses IL-4, and upregulates IL-8 production by CD4+ T cells. J. Leukoc. Biol. 59:407–411 [DOI] [PubMed] [Google Scholar]
- 12. Guzylack-Piriou L., Piersma S., McCullough K., Summerfield A. 2006. Role of natural interferon-producing cells and T lymphocytes in porcine monocyte-derived dendritic cell maturation. Immunology 118:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higashi T., et al. 2010. Curdlan induces DC-mediated Th17 polarization via Jagged1 activation in human dendritic cells. Allergol. Int. 59:161–166 [DOI] [PubMed] [Google Scholar]
- 14. Inumaru S., et al. 1998. Expression of biologically active recombinant porcine GM-CSF by baculovirus gene expression system. Immunol. Cell Biol. 76:195–201 [DOI] [PubMed] [Google Scholar]
- 15. Iwasaki A., Medzhitov R. 2010. Regulation of adaptive immunity by the innate immune system. Science 327:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jego G., et al. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225–234 [DOI] [PubMed] [Google Scholar]
- 17. Lanzavecchia A., Sallusto F. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263–266 [DOI] [PubMed] [Google Scholar]
- 18. Lee M. S., Kim Y. J. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76:447–480 [DOI] [PubMed] [Google Scholar]
- 19. Leibund Gut-Landmann S., et al. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 [DOI] [PubMed] [Google Scholar]
- 20. Lunney J. K., et al. 1994. Overview of the First International Workshop to Define Swine Leukocyte Cluster of Differentiation (CD) Antigens. Vet. Immunol. Immunopathol. 43:193–206 [DOI] [PubMed] [Google Scholar]
- 21. Manicassamy S., Pulendran B. 2009. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCullough K. C., Summerfield A. 2009. Targeting the porcine immune system: particulate vaccines in the 21st century. Dev. Comp. Immunol. 33:394–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novak M., Vetvicka V. 2009. Glucans as biological response modifiers. Endocrinol. Metab. Immune Disord. Drug Targets 9:67–75 [DOI] [PubMed] [Google Scholar]
- 24. Pescovitz M. D., Lunney J. K., Sachs D. H. 1984. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 133:368–375 [PubMed] [Google Scholar]
- 25. Pilon C., et al. 2009. CD40 engagement strongly induces CD25 expression on porcine dendritic cells and polarizes the T cell immune response toward Th1. Mol. Immunol. 46:437–447 [DOI] [PubMed] [Google Scholar]
- 26. Pulendran B., Palucka K., Banchereau J. 2001. Sensing pathogens and tuning immune responses. Science 293:253–256 [DOI] [PubMed] [Google Scholar]
- 27. Rice P. J., et al. 2002. Human monocyte scavenger receptors are pattern recognition receptors for (1→3)-β-d-glucans. J. Leukoc. Biol. 72:140–146 [PubMed] [Google Scholar]
- 28. Robinson M. J., Sancho D., Slack E. C., Leibund Gut-Landmann S., Reis e Sousa C. 2006. Myeloid C-type lectins in innate immunity. Nat. Immunol. 7:1258–1265 [DOI] [PubMed] [Google Scholar]
- 29. Rogers N. C., et al. 2005. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C-type lectins. Immunity 22:507–517 [DOI] [PubMed] [Google Scholar]
- 30. Ross G. D., et al. 1985. Characterization of patients with an increased susceptibility to bacterial infections and a genetic deficiency of leukocyte membrane complement receptor type 3 and the related membrane antigen LFA-1. Blood 66:882–890 [PubMed] [Google Scholar]
- 31. Sonck E., Stuyven E., Goddeeris B., Cox E. 2010. The effect of beta-glucans on porcine leukocytes. Vet. Immunol. Immunopathol. 135:199–207 [DOI] [PubMed] [Google Scholar]
- 32. Sonck E., Stuyven E., Goddeeris B., Cox E. 2009. Identification of the porcine C-type lectin dectin-1. Vet. Immunol. Immunopathol. 130:131–134 [DOI] [PubMed] [Google Scholar]
- 33. Taylor P. R., et al. 2002. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169:3876–3882 [DOI] [PubMed] [Google Scholar]
- 34. Trinchieri G., Sher A. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7:179–190 [DOI] [PubMed] [Google Scholar]
- 35. U.S. Department of Health and Human Services 1987. Guideline on validation of the Limulus amebocyte lysate test as an end product endotoxin test for human and animal parenteral drugs, biological products, and medical devices. U.S. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Washington, DC [Google Scholar]
- 36. van Vliet S. J., den Dunnen J., Gringhuis S. I., Geijtenbeek T. B., van Kooyk Y. 2007. Innate signaling and regulation of dendritic cell immunity. Curr. Opin. Immunol. 19:435–440 [DOI] [PubMed] [Google Scholar]
- 37. Veldhoen M., Hocking R. J., Atkins C. J., Locksley R. M., Stockinger B. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179–189 [DOI] [PubMed] [Google Scholar]
- 38. Vetvicka V., Vetvickova J. 2007. Physiological effects of different types of beta-glucan. Biomed. Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 151:225–231 [DOI] [PubMed] [Google Scholar]
- 39. Vos A. P., M'Rabet L., Stahl B., Boehm G., Garssen J. 2007. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit. Rev. Immunol. 27:97–140 [DOI] [PubMed] [Google Scholar]
- 40. Willment J. A., et al. 2003. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 171:4569–4573 [DOI] [PubMed] [Google Scholar]
- 41. Willment J. A., et al. 2005. The human beta-glucan receptor is widely expressed and functionally equivalent to murine dectin-1 on primary cells. Eur. J. Immunol. 35:1539–1547 [DOI] [PubMed] [Google Scholar]
- 42. Zekovic D. B., Kwiatkowski S., Vrvic M. M., Jakovljevic D., Moran C. A. 2005. Natural and modified (1→3)-β-d-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 25:205–230 [DOI] [PubMed] [Google Scholar]
- 43. Zhou L., Chong M. M., Littman D. R. 2009. Plasticity of CD4+ T cell lineage differentiation. Immunity 30:646–655 [DOI] [PubMed] [Google Scholar]
- 44. Zimmerman J. W., et al. 1998. A novel carbohydrate-glycosphingolipid interaction between a β-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 273:22014–22020 [DOI] [PubMed] [Google Scholar]