Abstract
The human papillomavirus type 16/18 (HPV-16/18) AS04-adjuvanted cervical cancer vaccine is licensed for females aged 10 years and above and is therefore likely to be coadministered with other licensed vaccines, such as hepatitis B. In this randomized, open-label study, we compared the immunogenicity of the hepatitis B vaccine administered alone (HepB group) or with the HPV-16/18 AS04-adjuvanted vaccine (HepB+HPV group) in healthy women aged 20 to 25 years (clinical trial NCT00637195). The hepatitis B vaccine was given at 0, 1, 2, and 12 months (an accelerated schedule which may be required by women at high risk), and the HPV-16/18 vaccine was given at 0, 1, and 6 months. One month after the third dose of hepatitis B vaccine, in the according-to-protocol cohort (n = 72 HepB+HPV; n = 76 HepB), hepatitis B seroprotection rates (titer of ≥10 mIU/ml) were 96.4% (95% confidence interval [CI], 87.5 to 99.6) and 96.9% (CI, 89.2 to 99.6) in the HepB+HPV and HepB groups, respectively, in women initially seronegative for anti-hepatitis B surface antigen (HBs) and anti-hepatitis B core antigen (HBc). Corresponding geometric mean titers of anti-HBs antibodies were 60.2 mIU/ml (CI, 40.0 to 90.5) and 71.3 mIU/ml (CI, 53.9 to 94.3). Anti-HBs antibody titers rose substantially after the fourth dose of hepatitis B vaccine. All women initially seronegative for anti-HPV-16 and anti-HPV-18 antibodies seroconverted after the second HPV-16/18 vaccine dose and remained seropositive up to 1 month after the third dose. Both vaccines were generally well tolerated, with no difference in reactogenicity between groups. In conclusion, coadministration of the HPV-16/18 AS04-adjuvanted vaccine did not affect the immunogenicity or safety of the hepatitis B vaccine administered in an accelerated schedule in young women.
INTRODUCTION
Hepatitis B is a serious disease that can be fatal or lead to chronic liver disease, including hepatocellular carcinoma and liver cirrhosis. Approximately 2 billion people worldwide are infected with the hepatitis B virus (HBV), of whom approximately 350 million are currently suffering from a chronic HBV infection (30). About 1 million of these chronically infected patients die each year of HBV-related liver disease (30).
The World Health Organization (WHO) recommends integration of hepatitis B vaccination into national infant immunization programs, and 92% of countries had included the vaccine in routine immunization programs by 2008 (www.who.int/immunization_monitoring/diseases/hepatitis/en/index.html). However, some northern European countries with low carrier rates offer vaccination only to high-risk groups (35). The standard schedule for vaccination against hepatitis B consists of three doses given at 0, 1, and 6 months. Where rapid protection is required (e.g., in high-risk groups or travelers), an accelerated schedule of either 0, 1, and 2 months or 0, 7, and 21 days can be adopted (28). Both of these schedules require a fourth vaccine dose at 1 year after the first administration.
A number of hepatitis B vaccines are currently on the market, both as single-antigen formulations and in combination with other antigens (18, 19). They are widely used in routine immunization programs as well as for travelers and high-risk groups. The recombinant hepatitis B vaccine from GlaxoSmithKline (GSK) Biologicals has been available since the mid-1980s; it is well tolerated, with high immunogenicity and protective efficacy, offering protection for up to 20 years (25). In addition, it can be administered in a variety of vaccination schedules, providing considerable flexibility.
Cervical cancer is the second most common cancer in women worldwide (7). Human papillomavirus (HPV) infection is well established as the necessary cause of the disease (36). Fifteen HPV types have been identified as oncogenic (20), with HPV-16 and HPV-18 being the two most frequent types, associated with approximately 70% of cervical cancer cases worldwide (2).
Two HPV vaccines are currently available, an HPV-16/18 vaccine from GSK Biologicals and an HPV-6/11/16/18 vaccine from Merck & Co. Extensive clinical trial programs of both vaccines have shown that they are highly immunogenic, provide protection against vaccine and some nonvaccine oncogenic HPV types (cross-protection) together with associated cytohistological lesions, and are generally well tolerated (3, 5, 8, 12, 21, 23, 29, 32). The HPV-16/18 cervical cancer vaccine from GSK Biologicals is formulated with the Adjuvant System AS04 (comprising aluminum hydroxide and 3-O-desacyl-4′ monophosphoryl lipid A) (10). The AS04-adjuvanted vaccine produces a greater immune response, with higher and more sustained antibody titers and a higher frequency of memory B-cells, than HPV vaccines adjuvanted with aluminum (6, 11).
It is likely that some young women will need to receive the HPV-16/18 vaccine and the hepatitis B vaccine at the same time. Those at high risk of hepatitis B infection, such as travelers to areas of endemicity, drug users, or sex workers, will probably receive the vaccine in an accelerated schedule. The objectives of the present study were to evaluate the immunogenicity and safety of the hepatitis B vaccine from GSK Biologicals in an accelerated schedule when the vaccine is coadministered with the HPV-16/18 vaccine. The immune response after the third dose of the hepatitis B vaccine administered in the approved accelerated schedule was the main point of interest in this study, as vaccination against hepatitis B requires at least three vaccine doses to reach optimal levels of seroprotection.
MATERIALS AND METHODS
Study objectives.
The primary objective was to evaluate the immune response against HBV in terms of seroprotection rate and geometric mean titers (GMTs) 1 month after the third dose of the hepatitis B vaccine (at month 3 of the study). Secondary objectives included evaluation of the immune response against HBV in terms of seroconversion rate, seroprotection rate, and GMTs after the second, third (seroconversion rate only), and fourth hepatitis B vaccine doses; the immune response against HPV-16 and HPV-18 after the second and third HPV-16/18 vaccine doses; and reactogenicity and safety of the vaccines administered.
Study design and participants.
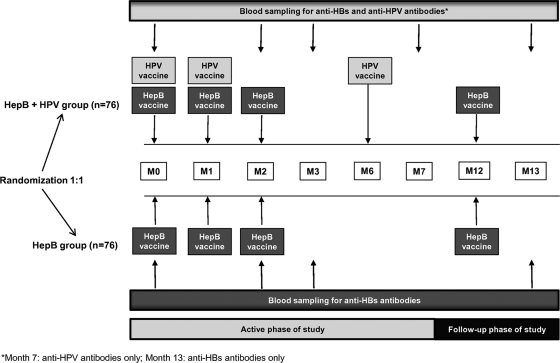
This was a phase IIIb, randomized, controlled, open-label study with two parallel treatment groups, conducted in two study centers in Belgium (clinical trial NCT00637195). The study included an active phase (month 0 to month 7), with a follow-up phase to month 13 (Fig. 1).
Fig. 1.
Study design. M, month.
Healthy women aged 20 to 25 years were included in the study. Women were excluded if they had received immunosuppressive or other immune-modifying drugs for more than 14 days within the previous 6 months or had a history of hepatitis B infection, exposure to hepatitis B within the previous 6 weeks before the start of the study, or previous vaccination against hepatitis B; they were also excluded if they had any immunosuppressant or immunodeficient condition, cancer or autoimmune disease, history of allergic disease likely to be exacerbated by a vaccine component, known acute or chronic clinically significant neurologic, hepatic or renal functional abnormality, or acute disease at enrollment. Women were required to use adequate contraceptive methods and must not have been pregnant or breastfeeding.
Women were randomized (1:1 ratio) to receive the hepatitis B vaccine given at 0, 1, 2, and 12 months and the HPV-16/18 vaccine given at 0, 1, and 6 months (HepB+HPV group) or the hepatitis B vaccine alone given at 0, 1, 2, and 12 months (HepB group) (Fig. 1). A randomization blocking scheme was used, with the randomization list generated at GSK Biologicals using a standard Statistical Analysis System (SAS) program. Treatment allocation at each study center was performed using an Internet-based randomization system with an algorithm using a minimization procedure accounting for center.
Both the hepatitis B and HPV vaccines were supplied by GSK Biologicals, Belgium. Each dose of the hepatitis B vaccine (Engerix-B) consisted of 20 μg of hepatitis B surface antigen (HBsAg) produced by recombinant yeast cells (Saccharomyces cerevisiae), formulated with 500 μg of aluminum salt. Each dose of the HPV-16/18 vaccine (Cervarix) consisted of 20 μg each of HPV-16 and HPV-18 L1 proteins, self-assembled as virus-like particles (VLPs) and formulated with the Adjuvant System AS04 (comprising 500 μg of aluminum hydroxide and 50 μg of the immunostimulatory molecule 3-O-desacyl-4′ monophosphoryl lipid A). Both vaccines were supplied as individual prefilled syringes (1 ml per dose for the hepatitis B vaccine and 0.5 ml per dose for the HPV-16/18 vaccine) and were to be administered intramuscularly into the deltoid muscle of the nondominant arm, except when the HPV-16/18 vaccine and the hepatitis B vaccine were coadministered, in which case the hepatitis B vaccine was administered in the dominant arm.
The study was conducted in accordance with the Declaration of Helsinki (1996) and the International Conference on Harmonisation Good Clinical Practice guidelines. The protocol and informed consent forms were approved by the Independent Ethics Committee or Institutional Review Board of each study center. Written informed consent was obtained from each woman. At the end of the study, vaccination with the HPV-16/18 AS04-adjuvanted vaccine was offered to all women in the HepB group.
Study procedures. (i) Immunogenicity.
Blood samples were collected at months 0, 2, 3, 7, and 13 in the HepB+HPV group and at months 0, 2, 3, and 13 in the HepB group (Fig. 1). Hepatitis B-related antibodies (anti-HBs and anti-hepatitis B core antigen [HBc]) were measured in both study groups. Anti-HBs antibodies were measured by enzyme-linked immunosorbent assay (ELISA) at months 0, 2, 3, and 13 (4). Seroconversion was defined as an antibody concentration of ≥3.3 mIU/ml (the assay threshold) in women who were seronegative at baseline; seroprotection was defined as an antibody concentration of ≥10 mIU/ml. Anti-HBc antibodies were measured qualitatively (present or absent) by a microparticle enzyme immunoassay (MEIA; Axsym Core/Abbott Laboratories) at month 0. Anti-HPV-16 and anti-HPV-18 antibodies were measured by ELISA using type-specific VLPs as coating antigens (serum-standardized protocol as previously published [22, 27]) at months 0, 2, 3, and 7 in the HepB+HPV study group. Seroconversion was defined as a titer of ≥8 ELISA units (EU)/ml for anti-HPV-16 and ≥7 EU/ml for anti-HPV-18 (the assay thresholds) in women who were seronegative at baseline.
(ii) Reactogenicity and safety.
Solicited adverse events (AEs) occurring within 7 days after each vaccination were recorded on diary cards. Solicited local AEs included pain, redness, and swelling. Solicited general AEs included fever, headache, fatigue, gastrointestinal symptoms (nausea, vomiting, diarrhea, and abdominal pain), arthralgia, myalgia, rash, and urticaria. In addition to diary card data, urticaria or rash that appeared within 30 min of each vaccine dose was recorded by the investigator. The greatest surface diameters of areas of redness or swelling were recorded. Fever was defined as an oral/axillary temperature of ≥37.5°C. All other solicited AEs were graded on a scale of 0 to 3. Grade 3 events were defined as preventing normal activity, an area of redness or swelling of >50 mm, or temperature of >39°C. Unsolicited AEs were reported from day 0 to day 29 after each vaccination. All solicited local AEs were considered related to vaccination. Investigators assessed the likely causality of all other AEs.
Serious adverse events (SAEs), medically significant conditions, new-onset chronic diseases (NOCDs), new-onset autoimmune disorders (NOADs), and pregnancies and their outcomes were recorded throughout the study. Medically significant conditions were defined as AEs prompting either emergency room visits or physician visits that were not routine or related to common diseases or as SAEs that were not related to common diseases. Based on a prespecified list, the clinical database was searched for all potential NOCDs and reviewed in a blind manner by a GSK physician prior to data analysis. An event was considered to be a potential NOCD if it had not been recorded in the previous medical history of the subject (i.e., new onset) and/or if symptoms were characteristic of an NOCD. A separate list, restricted to potential autoimmune events, was also used by a GSK safety physician to identify NOADs.
Statistics and endpoints.
The primary endpoints of the study were seroprotection rates and GMTs for anti-HBs antibodies 1 month after the third dose of the hepatitis B vaccine. Secondary endpoints were as follows: (i) seroconversion rates and GMTs for anti-HPV-16 and anti-HPV-18 antibodies 1 month after the second and third doses of the HPV-16/18 vaccine; (ii) seroconversion rates for anti-HBs antibodies 1 month after the third dose of the hepatitis B vaccine; (iii) seroconversion rates, seroprotection rates, and GMTs for anti-HBs antibodies 1 month after the second and fourth doses of the hepatitis B vaccine; and (iv) occurrence of solicited and unsolicited AEs, SAEs, and medically significant conditions.
A sample size of 70 evaluable women in the HepB+HPV group was needed to show with at least 80% power that the seroprotection rate at month 3 for anti-HBs antibodies was not less than 90% (assuming a seroprotection rate of 98%). The power calculation was based on a two-sided exact test for one binomial proportion with an alpha of 5% using the Power Analysis and Sample Size (PASS) 2005 program. It was assumed that 8% of women may be unevaluable at month 3 due to withdrawals, seropositivity at baseline, or other reasons, and therefore a total of 76 women per group were to be enrolled.
The primary analysis of anti-HBs and anti-HPV antibodies was conducted in the according-to-protocol (ATP) cohort for the active phase of the study (ATP active-phase). This cohort included women who met all eligibility criteria and complied with the protocol up to month 7 of the study. Data are also reported for anti-HBs antibodies following the fourth dose of hepatitis B vaccine in a second ATP cohort (ATP HepB fourth-dose) which included women who met all eligibility criteria and complied with the protocol up to month 13.
Seroconversion rates and seroprotection rates with exact 95% confidence intervals (CI) and GMTs with 95% CI for anti-HBs were calculated in women with undetectable anti-HBs and anti-HBc titers at baseline. Seroconversion rates with exact 95% CI and GMTs with 95% CI for anti-HPV-16 and anti-HPV-18 were calculated according to prevaccination status (i.e., seropositive or seronegative at baseline for the corresponding HPV type). The 95% CI for GMTs was obtained within each group separately. The 95% CI for the mean of log-transformed titer was first obtained assuming that log-transformed values were normally distributed with unknown variance. The 95% CI for the GMTs was then obtained by exponential transformation of the 95% CI for the mean of log-transformed titer. The exact 95% CI for a proportion within a group was calculated from Proc StatXact.
Solicited and unsolicited symptoms following the first three doses of each vaccine (i.e., up to month 7) are reported for the total vaccinated cohort (TVC active-phase). The cohort included all women who had at least one vaccine dose documented for unsolicited symptoms. Solicited and unsolicited symptoms following the fourth dose of hepatitis B vaccine are reported for the TVC up to month 13 (TVC HepB fourth-dose). The cohort included all women who received the fourth hepatitis B vaccine dose at month 12. Serious adverse events, medically significant AEs, NOCDs, and NOADs occurring throughout the study are also reported for this cohort. Data were analyzed descriptively. The exact 95% CI for a proportion within a group was calculated from Proc StatXact.
RESULTS
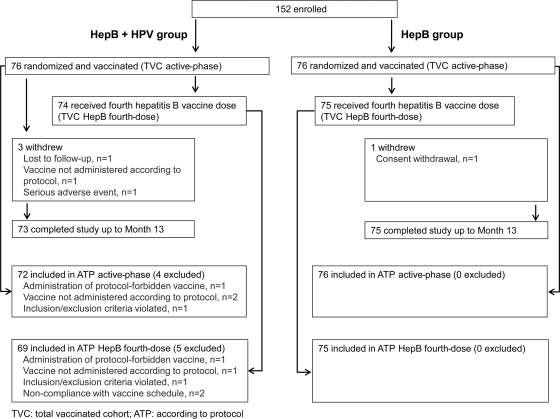
The first woman was enrolled in March 2008, and the last visit of the last study participant took place in June 2009. A total of 152 women were enrolled, 76 in each group. All were vaccinated and comprised the TVC active-phase (Fig. 2). The ATP active-phase cohort included 72 women in the HepB+HPV group and 76 women in the HepB group. Reasons for exclusion are shown in Fig. 2. A total of 74 women in the HepB+HPV group and 75 women in the HepB group received the fourth hepatitis B vaccine dose and comprised the TVC HepB fourth-dose cohort; 69 and 75 women comprised the ATP HepB fourth-dose cohort in the HepB+HPV and HepB groups, respectively (Fig. 2). A total of 73 and 75 women completed the study to month 13 in the HepB+HPV and HepB groups, respectively.
Fig. 2.
Subject disposition.
The mean (standard deviation) age of the women was 22.2 (1.42) years in the TVC active-phase, and most (96.7%) were of white Caucasian/European heritage. Demographic characteristics were similar in the other cohorts and between groups.
Immunogenicity: anti-HB antibodies.
Seventeen women (23.6%) in the HepB+HPV group and 11 women (14.5%) in the HepB group were already seropositive for anti-HBs antibodies before vaccination. Ten (13.9%) and seven (9.2%) women were seroprotected before vaccination. One participant in the HepB group was seropositive for anti-HBc antibodies. In women who were seronegative for both anti-HBs and anti-HBc before vaccination, seroprotection rates were 96.4% (95% CI, 87.5 to 99.6) and 96.9% (95% CI, 89.2 to 99.6) in the HepB+HPV and HepB groups, respectively, at month 3, i.e., 1 month after the third dose of hepatitis B vaccine (a coprimary endpoint) (Table 1). High rates of seroconversion and seroprotection were also seen at other time points (Table 1).
Table 1.
Seroconversion and seroprotection rates for anti-HBs antibodies in women initially seronegative before vaccinationa
| Antibody titer group and time point | HepB+HPV group |
HepB group |
||
|---|---|---|---|---|
| No. of women | Rate (% [95% CI]) | No. of women | Rate (% [95% CI]) | |
| Seroconversion (titer ≥3.3 mIU/ml) | ||||
| Prevaccination | 55 | 0.0 (0.0–6.5) | 64 | 0.0 (0.0–5.6) |
| Month 2 | 55 | 83.6 (71.2–92.2) | 64 | 84.4 (73.1–92.2) |
| Month 3 | 55 | 100 (93.5–100) | 64 | 98.4 (91.6–100) |
| Month 13 | 52 | 100 (93.2–100) | 63 | 100 (94.3–100) |
| Seroprotection (titer ≥10 mIU/ml) | ||||
| Prevaccination | 55 | 0.0 (0.0–6.5) | 64 | 0.0 (0.0–5.6) |
| Month 2 | 55 | 58.2 (44.1–71.3) | 64 | 70.3 (57.6–81.1) |
| Month 3 | 55 | 96.4 (87.5–99.6) | 64 | 96.9 (89.2– 99.6) |
| Month 13 | 52 | 100 (93.2–100) | 63 | 100 (94.3–100) |
Data are from women who were seronegative for anti-HBs and anti-HBc before vaccination. Data are shown for the ATP active-phase cohort for the prevaccination, month 2, and month 3 time points and for the ATP HepB fourth-dose cohort for the month 13 time point.
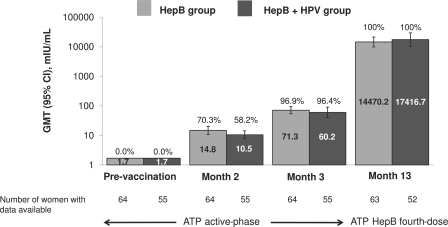
Geometric mean titers at month 3 (a coprimary endpoint) were similar in both study groups: 60.2 mIU/ml (95% CI, 40.0 to 90.5) in the HepB+HPV group and 71.3 mIU/ml (95% CI, 53.9 to 94.3) in the HepB group (Fig. 3). Titers rose more than 200-fold after the fourth dose of hepatitis B vaccine to 17,416.7 mIU/ml (95% CI, 10,294.3 to 29,466.9) and 14,470.2 mIU/ml (95% CI, 9,824.6 to 21,312.4) in the HepB+HPV and HepB groups, respectively (Fig. 3).
Fig. 3.
Seroprotection rates (values above the bars) and GMTs (values inside the bars) for anti-HBs antibodies in women initially seronegative before vaccination. Data are shown for the ATP active-phase cohort for prevaccination, month 2, and month 3 time points and for the ATP HepB fourth-dose cohort at the month 13 time point.
Immunogenicity: anti-HPV-16 and anti-HPV-18 antibodies.
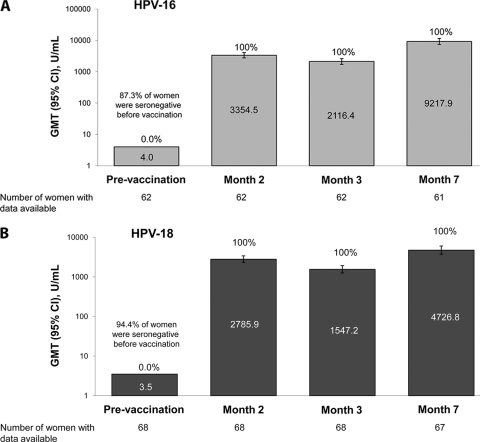
Before vaccination, 87.3% and 94.4% of women were seronegative for HPV-16 and HPV-18, respectively. All initially seronegative women had seroconverted for both HPV-16 and HPV-18 at 1 month after the second vaccine dose (month 2) and remained seropositive at 1 month after the third dose (month 7). One month after the third vaccination, GMTs were 9,217.9 EU/ml (95% CI, 7,396.6 to 11,487.7) for anti-HPV-16 antibodies and 4,726.8 EU/ml (95% CI, 3,718.5 to 6,008.4) for anti-HPV-18 antibodies in women seronegative at baseline for the corresponding HPV type (Fig. 4).
Fig. 4.
Seroconversion rates (values above the bars) and GMTs (values inside the bars) for anti-HPV-16 (A) and anti-HPV-18 (B) antibodies in women initially seronegative before vaccination (ATP active-phase cohort, HepB+HPV group).
Reactogenicity and safety. (i) Reactogenicity following the first three doses of each vaccine.
Overall, solicited symptoms (local and general) were reported during the 7-day postvaccination period following 83.5% of all vaccine doses during the active phase of the study (the first three hepatitis B vaccine doses and all three HPV-16/18 vaccine doses) in the HepB+HPV group. Grade 3 solicited symptoms were reported after 4.0% of doses in this group. In the HepB group, solicited symptoms were reported after 76.1% of doses during the 7-day postvaccination period; grade 3 solicited symptoms were reported after 6.6% of doses.
The incidences of solicited local symptoms at the hepatitis B vaccination site were similar regardless of coadministration of the HPV-16/18 vaccine. Pain at the injection site was the most frequent solicited local symptom, reported at the hepatitis B vaccine injection site after 53.9% of doses in the HepB+HPV group and after 58.4% of doses in the HepB group (Table 2). Pain was experienced more frequently at the HPV-16/18 vaccine injection site, reported after 89.0% of doses (Table 2). Redness and swelling also occurred less often at the hepatitis B vaccine injection site than at the HPV-16/18 vaccine injection site (Table 2). Few grade 3 local symptoms occurred at either injection site (Table 2). In the HepB+HPV group, grade 3 pain occurred following 0.4% of doses at the hepatitis B vaccine injection site and following 3.1% of doses at the HPV-16/18 vaccine injection site. In the HepB group, grade 3 pain occurred following 0.9% of doses.
Table 2.
Percentage of vaccine doses followed by a solicited local symptom during the 7-day postvaccination perioda
| Symptom and grade | HepB+HPV group |
HepB group (n = 226)b |
||||
|---|---|---|---|---|---|---|
| HepB (n = 228)b |
HPV-16/18 (n = 227)b |
|||||
| No. of doses | % of doses (95% CI) | No. of doses | % of doses (95% CI) | No. of doses | % of doses (95% CI) | |
| Pain | ||||||
| All | 123 | 53.9 (47.2–60.5) | 202 | 89.0 (84.2–92.7) | 132 | 58.4 (51.7–64.9) |
| Grade 3 | 1 | 0.4 (0.0–2.4) | 7 | 3.1 (1.2–6.3) | 2 | 0.9 (0.1–3.2) |
| Redness | ||||||
| All | 23 | 10.1 (6.5–14.8) | 44 | 19.4 (14.5–25.1) | 20 | 8.8 (5.5–13.3) |
| Grade 3 | 0 | 0.0 (0.0–1.6) | 0 | 0.0 (0.0–1.6) | 0 | 0.0 (0.0–1.6) |
| Swelling | ||||||
| All | 14 | 6.1 (3.4–10.1) | 43 | 18.9 (14.1–24.7) | 14 | 6.2 (3.4–10.2) |
| Grade 3 | 1 | 0.4 (0.0–2.4) | 2 | 0.9 (0.1–3.1) | 1 | 0.4 (0.0–2.4) |
Includes the first three doses of HepB vaccine and all three doses of HPV-16/18 vaccine; TVC active phase.
n, total number of doses.
The incidences of solicited general symptoms reported on diary cards were also similar regardless of coadministration of the HPV-16/18 vaccine (Table 3). Fatigue was the most common solicited general symptom, occurring after 38.6% and 42.5% of vaccine doses in the HepB+HPV group and the HepB group, respectively. Grade 3 solicited general symptoms occurred rarely (Table 3). There were no grade 3 cases of arthralgia, rash, or urticaria reported on diary cards. Only one case of urticaria/rash was reported by the investigator within 30 min of vaccination, in a woman in the HepB+HPV group.
Table 3.
Percentage of vaccine doses followed by a solicited general symptom during the 7-day postvaccination perioda
| Symptom and grade | HepB+HPV group (n = 303)b |
HepB group (n = 226)b |
||
|---|---|---|---|---|
| No. of doses | % of doses (95% CI) | No. of doses | % of doses (95% CI) | |
| Arthralgia | ||||
| All | 11 | 3.6 (1.8–6.4) | 14 | 6.2 (3.4–10.2) |
| Grade 3 | 0 | 0.0 (0.0–1.2) | 0 | 0.0 (0.0–1.6) |
| Fatigue | ||||
| All | 117 | 38.6 (33.1–44.4) | 96 | 42.5 (35.9–49.2) |
| Grade 3 | 3 | 1.0 (0.2–2.9) | 5 | 2.2 (0.7–5.1) |
| Fever | ||||
| All | 10 | 3.3 (1.6–6.0) | 5 | 2.2 (0.7–5.1) |
| Grade 3 | 0 | 0.0 (0.0–1.2) | 1 | 0.4 (0.0–2.4) |
| Gastrointestinal | ||||
| All | 33 | 10.9 (7.6–15.0) | 42 | 18.6 (13.7–24.3) |
| Grade 3 | 1 | 0.3 (0.0–1.8) | 5 | 2.2 (0.7–5.1) |
| Headache | ||||
| All | 90 | 29.7 (24.6–35.2) | 70 | 31.0 (25.0–37.4) |
| Grade 3 | 1 | 0.3 (0.0–1.8) | 7 | 3.1 (1.3–6.3) |
| Myalgia | ||||
| All | 41 | 13.5 (9.9–17.9) | 48 | 21.2 (16.1–27.2) |
| Grade 3 | 0 | 0.0 (0.0–1.2) | 2 | 0.9 (0.1–3.2) |
| Rash | ||||
| All | 3 | 1.0 (0.2–2.9) | 7 | 3.1 (1.3–6.3) |
| Grade 3 | 0 | 0.0 (0.0–1.2) | 0 | 0.0 (0.0–1.6) |
| Urticaria | ||||
| All | 1 | 0.3 (0.0–1.8) | 3 | 1.3 (0.3–3.8) |
| Grade 3 | 0 | 0.0 (0.0–1.2) | 0 | 0.0 (0.0–1.6) |
Includes the first three doses of hepatitis B vaccine and all three doses of HPV-16/18 vaccine; TVC active-phase.
n, total number of doses.
The percentage of doses followed by an unsolicited AE within 30 days of vaccination was 33.7% (95% CI, 28.4 to 39.3) in the HepB+HPV group and 31.6% (95% CI, 25.6 to 38.0) in the HepB group. The most common unsolicited symptom was headache, occurring after 6.3% of doses in the HepB+HPV group and 7.0% in the HepB group. Overall, 5.6% and 4.8% of vaccine doses in the HepB+HPV and HepB groups, respectively, were followed by unsolicited AEs considered related to vaccination.
(ii) Reactogenicity following the fourth dose of hepatitis B vaccine.
Table 4 shows the percentage of women who reported a solicited local or general symptom on their diary cards within the 7-day period following the fourth dose of hepatitis B vaccine. In addition, one potential case of investigator-reported urticaria within the first 30 min following vaccination was suspected in the HepB+HPV group. Reactogenicities were similar in both study groups and comparable with findings following the first three doses of vaccine.
Table 4.
Percentage of women reporting a solicited local or general symptom during the 7-day post-vaccination period following the fourth dose of hepatitis B vaccine (TVC HepB fourth-dose)
| Symptom | Symptom grade | HepB+HPV group (n = 74)a |
HepB group (n = 75)a |
||
|---|---|---|---|---|---|
| No. of women with symptom | % of total (95% CI) | No. of women with symptom | % of total (95% CI) | ||
| Local symptoms | |||||
| Pain | All | 36 | 48.6 (36.9–60.6) | 44 | 58.7 (46.7–69.9) |
| Grade 3 | 2 | 2.7 (0.3–9.4) | 0 | 0.0 (0.0–4.8) | |
| Redness | All | 8 | 10.8 (4.8–20.2) | 13 | 17.3 (9.6–27.8) |
| Grade 3 | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) | |
| Swelling | All | 12 | 16.2 (8.7–26.6) | 10 | 13.3 (6.6–23.2) |
| Grade 3 | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) | |
| General symptoms | |||||
| Arthralgia | All | 1 | 1.4 (0.0–7.3) | 4 | 5.3 (1.5–13.1) |
| Grade 3 | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) | |
| Fatigue | All | 26 | 35.1 (24.4–47.1) | 34 | 45.3 (33.8–57.3) |
| Grade 3 | 1 | 1.4 (0.0–7.3) | 0 | 0.0 (0.0–4.8) | |
| Fever | All | 3 | 4.1 (0.8–11.4) | 0 | 0.0 (0.0–4.8) |
| Grade 3 | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) | |
| Gastrointestinal | All | 3 | 4.1 (0.8–11.4) | 13 | 17.3 (9.6–27.8) |
| Grade 3 | 1 | 1.4 (0.0–7.3) | 0 | 0.0 (0.0–4.8) | |
| Headache | All | 21 | 28.4 (18.5–40.1) | 25 | 33.3 (22.9–45.2) |
| Grade 3 | 1 | 1.4 (0.0–7.3) | 0 | 0.0 (0.0–4.8) | |
| Myalgia | All | 4 | 5.4 (1.5–13.3) | 18 | 24.0 (14.9–35.3) |
| Grade 3 | 1 | 1.4 (0.0–7.3) | 1 | 1.3 (0.0–7.2) | |
| Rash | All | 1 | 1.4 (0.0–7.3) | 0 | 0.0 (0.0–4.8) |
| Grade 3 | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) | |
| Urticaria | All | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) |
| Grade 3 | 0 | 0.0 (0.0–4.9) | 0 | 0.0 (0.0–4.8) | |
n, total number women.
The percentage of women reporting an unsolicited AE within 30 days of vaccination was 27.0% (95% CI, 17.4 to 38.6%) in the HepB+HPV group and 29.3% (95% CI, 19.4 to 41.0%) in the HepB group. The most common events were cystitis and oropharyngeal pain. Unsolicited symptoms considered related to vaccination were reported by 4.1% and 12.0% of women in the HepB+HPV and HepB groups, respectively.
(iii) Overall safety up to month 13.
No SAEs were reported up to month 7 of the study. Four women reported five SAEs between month 7 and month 13. One woman in the HepB+HPV group experienced spontaneous abortion and endometriosis, and another experienced Graves-Basedow's disease. The woman with Graves-Basedow's disease discontinued the study because the investigator concluded that further vaccination was contraindicated. One woman in the HepB group experienced type 1 diabetes mellitus, and another experienced viral gastroenteritis. No SAEs were considered related to vaccination.
New-onset chronic diseases were reported during the whole follow-up period to month 13 in one woman in the HepB+HPV group (Graves-Basedow's disease [mentioned above]) and in three women in the HepB group (type 1 diabetes mellitus [mentioned above], allergic dermatitis, and urticaria). The cases of Graves-Basedow's disease and type 1 diabetes mellitus were also considered to be potential NOADs. No NOCDs or NOADs were considered related to vaccination. Medically significant conditions were reported up to month 13 in 50.0% and 42.7% of women in the HepB+HPV and HepB groups, respectively. The only medically significant condition reported by >5% of women was cystitis (8.1% of women in the HepB+HPV group and 8.0% of women in the HepB group).
No pregnancies were reported between months 0 to 7 of the study. One pregnancy reported between months 7 and 13 by a woman in the HepB+HPV group resulted in a spontaneous abortion. It was not considered related to vaccination, as mentioned above.
DISCUSSION
Overall, the immune responses to the hepatitis B vaccine were similar in both study groups, regardless of coadministration of the HPV-16/18 vaccine. The findings of the study indicate that the hepatitis B vaccine given in an accelerated schedule may be coadministered with the HPV-16/18 AS04-adjuvanted vaccine without compromising safety or immunogenicity.
The coprimary endpoints of the study were seroprotection rates and GMTs of anti-HBs antibodies at 1 month following administration of the third dose of the hepatitis B vaccine. High rates of seroprotection (>96%) were achieved in both groups and were similar to or somewhat higher than those observed in other studies of the same accelerated schedule 1 month after the third vaccine dose (14, 17). One month after the fourth dose of the hepatitis B vaccine, the seroprotection rate was 100% in both groups.
Geometric mean titers of anti-HBs antibodies were similar in both study groups although they tended to be slightly lower in the HepB+HPV group 1 month after the third vaccine dose. This trend was reversed 1 month after the fourth dose. These findings are unlikely to be clinically relevant, given the high seroprotection rates observed. Similar results were observed in a study of coadministration of the HPV-6/11/16/18 vaccine with a hepatitis B vaccine administered in a standard schedule (31). The authors of this study also concluded that the slightly lower GMTs in the group who received both vaccines were unlikely to be clinically significant. Antibody levels of ≥10 mIU/ml are generally accepted as offering protection against clinically significant hepatitis B infection (15, 18, 33), and geometric mean titers were well above this level in the present study in both study groups.
All initially seronegative women who received the HPV-16/18 vaccine seroconverted for HPV-16 and HPV-18, and 100% remained so up to 1 month after the third vaccine dose. Peak GMT levels were similar to those observed in a large phase III study in which efficacy against cervical lesions associated with HPV-16/18 and some nonvaccine oncogenic types has been shown (23). Furthermore, antibody levels were similar to those observed at the same time point in a long-term study in which sustained immunogenicity and efficacy have been shown up to 6.4 years (12).
Two other studies have investigated coadministration of the HPV-16/18 AS04-adjuvanted vaccine with other vaccines, i.e., a combined hepatitis A and B vaccine and a diphtheria-tetanus-pertussis-inactivated polio vaccine. Coadministration of these vaccines did not indicate any interference with the immune response of either vaccine (9, 24).
In the present study, both vaccines had an acceptable safety profile. Few SAEs occurred, and none was considered related to vaccination. Two potential NOADs, Graves-Basedow's disease and type 1 diabetes mellitus, occurred during the study. It is worth noting that the investigators caring for these women considered that the conditions might have been present but undiagnosed prior to vaccination. The safety profiles of the hepatitis B vaccine were similar regardless of whether it was administered alone or with the HPV-16/18 vaccine. The hepatitis B vaccine used in the study has been widely available for many years and has a well-documented safety profile when used both in the standard schedule and the accelerated schedule (1, 16, 26). The HPV-16/18 vaccine also exhibited a safety profile similar to that observed in other studies (13, 22) and in pooled analyses (5, 29).
The study had some limitations. Data analyses were descriptive, and the study was not statistically powered to perform noninferiority or equivalence testing between the two study groups. In addition, there was no group who received the HPV-16/18 vaccine alone, meaning that the impact of coadministration of the hepatitis B vaccine on the immunogenicity of the HPV-16/18 vaccine could not be established in this study. However, in a previous study of coadministration of the HPV-16/18 and hepatitis A/B vaccines, the HPV-16/18 immune response was statistically noninferior for coadministration of the two vaccines compared with administration of the HPV-16/18 vaccine alone (24).
Although infant immunization against hepatitis B has now been introduced in many countries, a large proportion of the population remains unvaccinated because they were born before the mass vaccination program was introduced, and thus a considerable burden of disease may remain. Catch-up programs may be needed in some countries to hasten the spread of population-based immunity (33). The key target population for the HPV vaccine is young adolescent girls. However, many countries have also implemented catch-up programs in older girls and young women (34). Because the hepatitis B vaccine was introduced into infant immunization programs only relatively recently in many countries or has not yet been introduced, many young women receiving the HPV vaccine in a catch-up program may also require the hepatitis B vaccine. If young women receiving both vaccines are in a high-risk group for acquiring hepatitis B (e.g., travelers, drug users, or sex workers), they are likely to receive the vaccine in an accelerated schedule.
In conclusion, data from this study show that coadministration of the HPV-16/18 AS04-adjuvanted vaccine appears not to affect the immunogenicity and safety of the hepatitis B vaccine administered in adult women in an accelerated schedule. Women at high risk for hepatitis B who require the accelerated vaccination schedule need not delay either their hepatitis B or their HPV vaccination course.
ACKNOWLEDGMENTS
The study was funded by GlaxoSmithKline Biologicals, which was involved in all stages of the study/project conduct and analysis. GlaxoSmithKline Biologicals also took in charge all costs associated with the development and the publishing of the present publication. Engerix and Cervarix are trademarks of the GlaxoSmithKline group of companies.
M.-P.D., K.D., and D.D. are employees of GlaxoSmithKline Biologicals. G.L.-R. has been principal investigator of vaccine trials for several manufacturers, including GlaxoSmithKline Biologicals, for which his institution (Center for Vaccinology, Ghent University and Hospital) obtained research grants. He also received payment from GlaxoSmithKline Biologicals for consultancy and lectures on different vaccine topics. C.M. and F.D.B. are members of G.L.-R.'s research group. J.L., E.H., and L.L. conducted the study visits through fees provided by GlaxoSmithKline Biologicals to their institution (ImmuneHealth Clinical Unit), which conducts other vaccine trials sponsored by GlaxoSmithKline Biologicals.
We gratefully acknowledge and thank the study participants and the staff members of the study centers that participated in this study: Nathalie Lorent for her support as clinical investigator, Laurence Nuret and Dorothée Bossart for providing care to study patients as study nurses (Unité d'Investigation Clinique ImmuneHealth, CHU Tivoli, Belgium), and Jacques Francotte and his team for their important support for the recruitment (Head of Obstetrics Department, CHU Tivoli, Belgium).
We also acknowledge the GlaxoSmithKline Biologicals Clinical Study network: Anne-Sophie Perreaux for providing global study coordination; Elke Geyssens for providing local study coordination; Nathalie Durant for the clinical immunology; Nele Martens, Jacques Bruhwyler, and Matondo M'Baku for the clinical report scientific writing; and Marie Lebacq for statistical contributions.
We also thank Mary Greenacre (freelance) for providing writing assistance and Mélanie Muylaert (XPE Pharma & Science) and Denis Sohy (Business and Decision) for providing editorial assistance and manuscript coordination on behalf of GlaxoSmithKline Biologicals.
Footnotes
Published ahead of print on 6 July 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Bock H. L., et al. 1995. Accelerated schedule for hepatitis B immunization. J. Travel Med. 2:213–217 [DOI] [PubMed] [Google Scholar]
- 2. Bosch F. X., et al. 2008. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26(Suppl. 10):K1–K16 [DOI] [PubMed] [Google Scholar]
- 3. Brown D. R., et al. 2009. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J. Infect. Dis. 199:926–935 [DOI] [PubMed] [Google Scholar]
- 4. Cambron P., Jacquet J.-M., Hoet B., Lievens M. 2009. Development and technical and clinical validation of a quantitative enzyme-linked immunosorbent assay for the detection of human antibodies to hepatitis B surface antigen in recipients of recombinant hepatitis B virus vaccine. Clin. Vaccine Immunol. 16:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Descamps D., et al. 2009. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum. Vaccin. 5:332–340 [DOI] [PubMed] [Google Scholar]
- 6. Einstein M. H., et al. 2009. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum. Vaccin. 5:705–719 [DOI] [PubMed] [Google Scholar]
- 7. Ferlay J., et al. (ed.) 2008. Globocan 2008: cancer incidence and mortality worldwide. IARC CancerBase no. 10 International Agency for Research on Cancer, Lyon, France [Google Scholar]
- 8. FUTURE II Study Group 2007. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 356:1915–1927 [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Sicilia J., et al. 2010. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine coadministered with combined diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine to girls and young women. J. Adolesc. Health 46:142–151 [DOI] [PubMed] [Google Scholar]
- 10. Garçon N., Chomez P., Van Mechelen M. 2007. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 6:723–739 [DOI] [PubMed] [Google Scholar]
- 11. Giannini S. L., et al. 2006. Enhanced humoral and memory B cell immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 24:5937–5949 [DOI] [PubMed] [Google Scholar]
- 12. GlaxoSmithKline Vaccine HPV-007 Study Group 2009. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 374:1975–1985 [DOI] [PubMed] [Google Scholar]
- 13. Harper D. M., et al. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757–1765 [DOI] [PubMed] [Google Scholar]
- 14. Hess G., Hingst V., Cseke J., Bock H. L., Clemens R. 1992. Influence of vaccination schedules and host factors on antibody response following hepatitis B vaccination. Eur. J. Clin. Microbiol. Infect. Dis. 11:334–340 [DOI] [PubMed] [Google Scholar]
- 15. Jack A. D., Hall A. J., Maine N., Mendy M., Whittle H. C. 1999. What level of hepatitis B antibody is protective? J. Infect. Dis. 179:489–492 [DOI] [PubMed] [Google Scholar]
- 16. Keating G. M., Noble S. 2003. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 63:1021–1051 [DOI] [PubMed] [Google Scholar]
- 17. Marsano L. S., et al. 1996. Comparison of a rapid hepatitis B immunization schedule to the standard schedule for adults. Am. J. Gastroenterol. 91:111–115 [PubMed] [Google Scholar]
- 18. Mast E. E., et al. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: immunization of infants, children, and adolescents. MMWR Recommend. Rep. 54(RR16):1–23 [PubMed] [Google Scholar]
- 19. Mast E. E., et al. 2006. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II: immunization of infants, children, and adolescents. MMWR Recommend. Rep. 55(RR16):1–31 [PubMed] [Google Scholar]
- 20. Muñoz N., et al. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518–527 [DOI] [PubMed] [Google Scholar]
- 21. Muñoz N., et al. 2010. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J. Natl. Cancer Inst. 102:325–339 [DOI] [PubMed] [Google Scholar]
- 22. Paavonen J., et al. 2007. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161–2170 [DOI] [PubMed] [Google Scholar]
- 23. Paavonen J., et al. 2009. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314 [DOI] [PubMed] [Google Scholar]
- 24. Pedersen C., et al. 2009. Co-administration of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine with hepatitis A/B vaccine in girls aged 9-15 years, abstr. 099. Abstr. 6th World Congress of World Society for Pediatric Infectious Diseases, Buenos Aires, Argentina, 18 to 22 November, 2009 [Google Scholar]
- 25. Poovorawan Y., et al. 2011. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J. Viral Hepat. 18:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rustgi V. K., Schleupner C. J., Krause D. S. 1995. Comparative study of the immunogenicity and safety of Engerix-B administered at 0, 1, 2 and 12 months and Recombivax HB administered at 0, 1, and 6 months in healthy adults. Vaccine 13:1665–1668 [DOI] [PubMed] [Google Scholar]
- 27. Schwarz T. F., et al. 2009. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine 22:581–587 [DOI] [PubMed] [Google Scholar]
- 28. Van Herck K., Leuridan E., Van Damme P. 2007. Schedules for hepatitis B vaccination of risk groups: balancing immunogenicity and compliance. Sex. Transm. Infect. 83:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verstraeten T., et al. 2008. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 26:6630–6638 [DOI] [PubMed] [Google Scholar]
- 30. Vryheid R. E., Kane M. A., Muller N., Schatz G. C., Bezabeh S. 2000. Infant and adolescent hepatitis B immunization up to 1999: a global overview. Vaccine 19:1026–1037 [DOI] [PubMed] [Google Scholar]
- 31. Wheeler C. M., et al. 2008. Safety and immunogenicity of co-administered quadrivalent human papillomavirus (HPV)-6/11/16/18 L1 virus-like particle (VLP) and hepatitis B (HBV) vaccines. Vaccine 26:686–696 [DOI] [PubMed] [Google Scholar]
- 32. Wheeler C. M., et al. 2009. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J. Infect. Dis. 199:936–944 [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization 2009. Hepatitis B vaccines. WHO Position Paper. Wkly. Epidemiol. Rec. 84:405–42019817017 [Google Scholar]
- 34. Wright T. C., et al. 2008. Age considerations when vaccinating against HPV. Gynecol. Oncol. 109:S40–S47 [DOI] [PubMed] [Google Scholar]
- 35. Zanetti A. R., Van Damme P., Shouval D. 2008. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 26:6266–6273 [DOI] [PubMed] [Google Scholar]
- 36. zur Hausen H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55–F78 [DOI] [PubMed] [Google Scholar]