Abstract
The vaccination program against the 2009 pandemic H1N1 influenza virus (2009 H1N1) provided a unique opportunity to determine if immune responses to the 2009 H1N1 vaccine were affected by a recent, prior vaccination against seasonal influenza virus. In the present study, we studied the immune responses to the 2009 H1N1 vaccine in subjects who either received the seasonal influenza virus vaccination within the prior 3 months or did not. Following 2009 H1N1 vaccination, subjects previously given a seasonal influenza virus vaccination exhibited significantly lower antibody responses, as determined by hemagglutination inhibition assay, than subjects who had not received the seasonal influenza virus vaccination. This result is compatible with the phenomenon of “original antigenic sin,” by which previous influenza virus vaccination hampers induction of immunity against a new variant. Our finding should be taken into account for future vaccination programs against pandemic influenza virus outbreaks.
INTRODUCTION
Only 2 months after a novel swine-origin influenza A (H1N1) virus had been identified (2, 7), the first influenza virus pandemic of this century was declared by the World Health Organization (WHO) (20). Global spread of the 2009 pandemic H1N1 influenza virus (2009 H1N1) led to the urgent need for development of effective vaccines and clinical trials to evaluate their safety profiles and efficacy (4, 10, 14, 17, 21). As preexisting immunity to a recent seasonal H1N1 influenza virus strain [A/Brisbane/59/2007 (H1N1)] conferred only a limited cross-protection to 2009 H1N1 (11, 16), the U.S. Centers for Disease Control and Prevention made a nationwide effort to encourage more people to get the 2009 H1N1 vaccine (1, 3). However, the potential effect of previous seasonal influenza virus vaccination on the efficacy of the 2009 H1N1 vaccine was not considered during the nationwide vaccination program.
The effects of a prior exposure to influenza virus on the efficacy of a subsequent vaccination against a variant strain are poorly understood. One published report addressed whether a previous vaccine against seasonal influenza virus might affect the response to subsequent 2009 H1N1 vaccination, albeit in a nonhuman setting. Using a ferret model, it was found that animals primed with the seasonal influenza virus vaccine showed an enhanced response to MF59-adjuvanted 2009 H1N1 vaccination compared to those not primed with the seasonal vaccine (8). A similar result was observed in the setting with a prior seasonal influenza virus infection of ferrets (9). These studies implied that there is a priming effect of precedent exposure to seasonal influenza virus by vaccination or infection on the efficacy of a subsequent 2009 H1N1 vaccine.
In contrast, based on the phenomenon of “original antigenic sin,” it is also possible that a seasonal influenza virus vaccination could reduce the efficacy of a subsequent 2009 H1N1 vaccination. According to this intriguing phenomenon, antibody (Ab) or T cells specific to previously encountered virus may dominate the immune response to a new viral variant, and induction of protective immunity upon the vaccination or infection of the variant may be hampered (5, 6, 13). Recently, evidence of original antigenic sin was demonstrated in a murine model of sequential vaccinations with influenza virus A/PR/8/1934 (H1N1) and A/FM/1/1947 (H1N1) (12). In both immunization with DNA vaccines encoding hemagglutinin and infection with live virus, the Ab response following the secondary vaccination was exclusively directed to the original antigen rather than to the variant antigen. Therefore, the immune response to the initial antigen attenuated the immune response to the secondary antigen, resulting in diminished vaccine efficacy.
In the present study, the impact of a recent vaccination against seasonal influenza virus on the immune responses to subsequent 2009 H1N1 vaccination was assessed in a human vaccination program. We evaluated and compared the immune responses to the 2009 H1N1 vaccine in subjects enrolled in the nationwide vaccination program in the Republic of Korea with or without a history of the seasonal influenza virus vaccination given within the prior 3 months. We report here that individuals with a previous seasonal influenza virus vaccination displayed significantly lower Ab responses to the 2009 H1N1 vaccination than individuals who received the 2009 H1N1 vaccination alone.
MATERIALS AND METHODS
Study subjects and vaccination.
After receipt of informed consent, 71 high school students, who were enrolled in the nationwide vaccination program for 2009 H1N1, were recruited. All subjects were female and either 16 or 17 years old. There was no known clinical history of 2009 H1N1 infection in any subject, and all participants were devoid of any symptoms indicative of acute respiratory infection during this study. Two to three months before 2009 H1N1 vaccination, 34 out of 71 subjects had vaccination with a seasonal influenza virus vaccine composed of A/Brisbane/59/2007 (H1N1)-like virus, A/Brisbane/10/2007 (H3N2)-like virus, and B/Brisbane/60/2008-like antigen. All subjects were vaccinated with one dose of monovalent, inactivated, split-virus H1N1 vaccine (GREEN FLU-S; Green Cross, Yongin, Republic of Korea) containing 15 μg of hemagglutinin antigen of the virus prepared from A/California/7/2009 NYMC X-179A (H1N1). Whole-blood samples were collected before and 14 days after the 2009 H1N1 vaccination. Sera and peripheral blood mononuclear cells (PBMCs) were isolated from whole blood immediately after sampling. This research protocol was reviewed and approved by the Institutional Review Board of KAIST.
During data analysis, subjects with high preexisting Ab responses to 2009 H1N1 (hemagglutination inhibition [HI] titer of ≥1:320) were excluded due to a ceiling effect of Ab response (see Fig. S1 in the supplemental material). Figure S1 in the supplemental material demonstrates that Ab titers to 2009 H1N1 prior to the 2009 H1N1 vaccination inversely correlated with the Ab responses after the vaccination and that the subjects with prevaccination titers of ≥1:320 had severely impaired Ab responses. Accordingly, 62 subjects were included in the analyses by HI assay. In the multivariable logistic regression analysis, one subject was excluded since T cell response data were not available.
Serologic analyses by HI assays.
The HI assay was performed as described previously (15, 19). Briefly, obtained sera were treated with receptor-destroying enzyme (RDE) to inactivate nonspecific inhibitors with a final serum dilution of 1:10. RDE-treated sera were serially diluted 2-fold, and equal volumes of virus (8 hemagglutinating units/50 μl) were added to each well. The microplates were incubated at room temperature for 30 min, followed by the addition of 0.5% turkey red blood cells (RBCs). The plates were gently mixed and incubated at 37°C for 30 min. The HI titer was determined by the reciprocal of the last dilution that contained turkey RBCs with no agglutination. The limit of detection for the HI assays done was set to ≤20 HI units. Geometric mean titers (GMTs) were calculated for each group of serum samples.
IFN-γ ELISpot assay.
Gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays were performed using cryopreserved PBMCs. In brief, duplicate cultures of 300,000 PBMCs per well were stimulated by inactivated seasonal influenza A/Brisbane/59/2007 (H1N1)-like virus at a final concentration of 0.1 μg/ml. Also, for negative and positive controls, PBMCs were incubated with phosphate-buffered saline (PBS) and with phytohemagglutinin. After 30 h, plates were developed and IFN-γ spot-forming units (SFUs) were determined with an ELISpot analyzer (Cellular Technology Ltd., Shaker Heights, OH). Antigen-specific IFN-γ SFUs were quantified by subtracting the mean SFUs in negative-control wells from the mean SFUs in antigen-stimulated wells. A response was considered positive when antigen-specific IFN-γ SFUs were greater than 10 SFUs per 300,000 PBMCs.
Statistical analysis.
We calculated the significance of differences in response rates indicating vaccine effectiveness by means of Fischer's exact test. In analyzing differences in GMTs of HI, log-transformed values were compared using the Mann-Whitney U test. The correlation between two variables was represented by Spearman's rank coefficient. We used logistic regression to model the individual effect of dichotomized variables on the Ab response to H1N1 vaccination after adjustment for covariates. All analyses were performed with PASW Statistics 18. A P value of <0.05 was considered to indicate statistical significance.
RESULTS
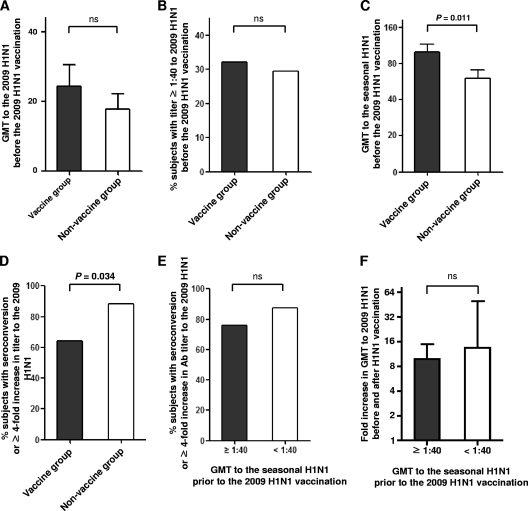
In the present study, Ab responses to influenza virus were evaluated by HI assays. For the HI assay, 62 subjects were evaluated (28 subjects with a history of the seasonal influenza vaccination within 3 months [vaccine group] and 34 subjects without prior vaccination [nonvaccine group]). Table S1 in the supplemental material presents the raw data of HI assays of the 62 subjects. Between the vaccine and nonvaccine groups, there was no significant difference in Ab responses to 2009 H1N1 prior to 2009 H1N1 vaccination, presented by GMT (Fig. 1A), and the proportion of subjects with Ab titers of ≥1:40 (Fig. 1B). As expected, the vaccine group presented significantly higher GMTs than the nonvaccine group to the seasonal H1N1 contained in the seasonal influenza virus vaccine (P = 0.011) (Fig. 1C). We next investigated the Ab response to the 2009 H1N1 vaccine in the subjects with or without a recent history of the seasonal influenza vaccination. Importantly, the vaccine group experienced decreased Ab response to the 2009 H1N1 vaccine compared to that of the nonvaccine group, as determined by the proportion of subjects with seroconversion or a ≥4-fold increase in 2009 H1N1-specific Ab titer (P = 0.034) (Fig. 1D).
Fig. 1.
Ab responses to the 2009 H1N1 influenza virus vaccine in groups with (vaccine group) or without (nonvaccine group) a recent history of the seasonal influenza virus vaccination. (A and B) Baseline GMTs to 2009 H1N1 (A) and proportions of subjects with Ab titers of ≥1:40 (B) were determined prior to the 2009 H1N1 vaccination by HI assay and compared between vaccine and nonvaccine groups. (C) Baseline GMTs to seasonal H1N1 were determined prior to the 2009 H1N1 vaccination by HI assay and compared between vaccine and nonvaccine groups. (D) Proportions of subjects with seroconversion or a ≥4-fold increase in Ab titer to 2009 H1N1 were compared between vaccine and nonvaccine groups. (E and F) Proportions of subjects with seroconversion or a ≥4-fold increase in Ab titer to 2009 H1N1 (E) and a fold increase in Ab titer to 2009 H1N1 (F) were compared between groups with high (≥1:40) and low (<1:40) Ab titers to seasonal H1N1. Error bars represent 95% confidence intervals. A P value of <0.05 was considered statistically significant. ns, not significant.
As many subjects presented high GMTs to the seasonal H1N1 even without a recent seasonal influenza virus vaccination (Fig. 1C), we analyzed the relationship between preexisting Ab titer to the seasonal H1N1 virus and Ab response to the 2009 H1N1 vaccine. However, we found no difference in vaccine response to 2009 H1N1 between groups with high (≥1:40) and low (<1:40) Ab titers to the seasonal H1N1, as analyzed by the proportion of subjects with seroconversion or a ≥4-fold increase in Ab titer (Fig. 1E). Furthermore, no correlation was found during an analysis of preexisting Ab titer to the seasonal H1N1 virus versus the fold increase in the GMT to 2009 H1N1 (Fig. 1F). In summary, a recent vaccination history against the seasonal influenza virus reduced the vaccine response to the 2009 H1N1 vaccine, though a preexisting Ab response to seasonal H1N1 virus did not influence the 2009 H1N1 vaccine response.
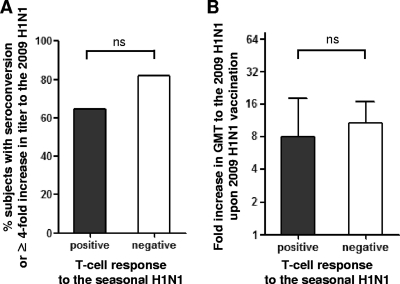
Next, we explored if preexisting T cell immunity to the seasonal H1N1 virus could influence the immune response to the 2009 H1N1 vaccination. To assess preexisting T cell responses, PBMCs were stimulated with inactivated seasonal H1N1 particles, and an IFN-γ enzyme-linked immunospot (ELISpot) assay was performed. We found that the presence of preexisting T cell responses to seasonal H1N1 virus did not affect vaccine response to 2009 H1N1, as evaluated by the proportion of subjects with seroconversion or a ≥4-fold increase in Ab titer (Fig. 2A) and fold increases in GMT to 2009 H1N1 (Fig. 2B).
Fig. 2.
Effect of preexisting T cell responses to seasonal H1N1 virus on the Ab responses to the 2009 H1N1 vaccine. (A and B) Proportions of subjects with seroconversion or a ≥4-fold increase in Ab titer to 2009 H1N1 (A) and a fold increase in Ab titer to 2009 H1N1 (B) were compared between groups with or without preexisting T cell responses to seasonal H1N1 particles, as measured by IFN-γ ELISpot assay. Error bars represent 95% confidence intervals. A P value of <0.05 was considered statistically significant. ns, not significant.
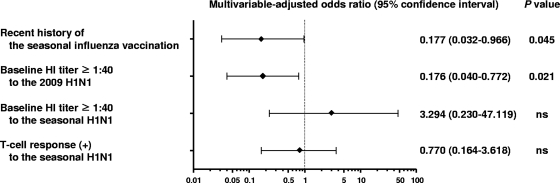
Finally, we performed multivariable logistic regression analysis to identify contributing factors affecting the vaccine response to 2009 H1N1. Our results showed that a recent history of seasonal influenza virus vaccination was an independent factor to make a significant impact on Ab response to the 2009 H1N1 vaccine (P = 0.045) (Fig. 3). As expected by the ceiling effect of Ab response (see Fig. S1 in the supplemental material), a baseline titer of ≥1:40 to 2009 H1N1 was also an independent factor to reduce Ab response to 2009 H1N1 (P = 0.021). A similar result was obtained in the analysis without exclusion of the subjects with a prevaccination titer of ≥1:320 to 2009 H1N1 (see Fig. S2 in the supplemental material).
Fig. 3.
Multivariable logistic regression analysis. Multivariable logistic analysis was performed to identify contributing factors which affect the Ab response to the 2009 H1N1 vaccine. One subject was excluded from this analysis since T cell response data were not available (n = 61 subjects) (see Table S1 in the supplemental material). A recent history of the seasonal influenza vaccination and preexisiting Ab response to 2009 H1N1 negatively influenced the efficacy of the 2009 H1N1 vaccine with statistical significance. Shown are the adjusted odds ratios, with bars indicating the 95% confidence intervals. A P value of <0.05 was considered statistically significant. ns, not significant.
Taken together, our findings demonstrate that a recent history of the seasonal influenza virus vaccination reduced the Ab response to the 2009 H1N1 vaccine. These data suggest that an original antigenic sin effect of the seasonal influenza virus vaccination may be diminishing the Ab responses to the 2009 H1N1 vaccine.
DISCUSSION
In planning a mass vaccination program against a new pandemic influenza virus, it is very important to determine how a previous vaccination against seasonal influenza virus may affect the response to a subsequently administered pandemic vaccine. In the present study, we showed that individuals previously given the seasonal influenza virus vaccination exhibited a significantly lower response to the 2009 H1N1 vaccine than those without a recent prior seasonal influenza vaccination. Interestingly, a previous study demonstrated that prior vaccination against seasonal influenza virus was associated with increased risk of medically attended 2009 H1N1 infection (18). This might be explained by reduced immune responses to 2009 H1N1 after the seasonal influenza virus vaccination. Our findings are in contrast to a recent ferret study that showed that administration of a seasonal influenza virus vaccine had a positive immunological priming effect on subsequent 2009 H1N1 vaccination (8). The difference in results between these two studies might be explained by the vaccine formula. In the current study, the 2009 H1N1 vaccine did not contain any adjuvant, as 2009 H1N1 vaccines were shown to induce protective Ab responses without adjuvant (10, 14, 17, 21). In the ferret study, however, the priming effect of the previous seasonal influenza virus vaccination was strong only when MF59-adjuvanted 2009 H1N1 vaccine was administered to animals that had received prior vaccination with MF59-adjuvanted seasonal influenza virus vaccine. In a future study, the effect of adjuvant needs to be evaluated in the setting of subsequent vaccination for different influenza strains.
Our finding that a recent history of seasonal influenza virus vaccination reduces the Ab responses to the 2009 H1N1 vaccine supports the previously described phenomenon of original antigenic sin. In original antigenic sin, preexisting influenza virus immunity hampers the induction of immunity against a new variant. In the present study, however, preexisting immunity to the seasonal H1N1 virus, as measured by Ab and T cell responses, did not appear to influence the vaccine response to 2009 H1N1. Although we measured the preexisting immunity specific to the seasonal H1N1 virus [A/Brisbane/59/2007 (H1N1)], this may, in fact, have been induced by other strains cross-reactive to the seasonal H1N1 virus. It is possible that a past infection or vaccination with cross-reactive strains might distort the original antigenic sin effect of the seasonal H1N1 immunity on Ab response to the 2009 H1N1 vaccine. Actually, six different H1N1 strains have been included in seasonal influenza vaccines over the past 15 years. In the future, a larger study needs to be performed to evaluate the effect of preexisting immunity to seasonal influenza virus on Ab response to 2009 H1N1.
In the present study, we recruited study subjects ages 16 and 17 from a single suburban city. It is possible that the vaccine response might be affected by various factors, such as age of subjects and regional viral variants (6). Therefore, the relatively young and uniform age of our subjects from a single suburban city allowed us to reduce confounding effects caused by age and regional viral variants. Our study in a homogeneous population should be extended to a larger heterogeneous population to generalize our results. The future study will verify the original antigenic sin effect between seasonal H1N1 and 2009 H1N1. In the future study, the kinetics of antibody responses need to be investigated by sampling of sera at multiple time points, since our current study examined HI titer at an early time point, day 14.
Pandemic spread of 2009 H1N1 and the nationwide vaccination program in the Republic of Korea enabled us to study the effect of recent seasonal influenza virus vaccination on the Ab response to subsequent 2009 H1N1 vaccination. As a result, we demonstrate here that a recent history of the seasonal influenza virus vaccination led to a reduction in Ab responses to the 2009 H1N1 vaccine, in a manner consistent with the original antigenic sin phenomenon.
Supplementary Material
ACKNOWLEDGMENTS
We thank the volunteers who participated in the present study and the staff of Pyeongtaek Girls' High School.
This study was supported by the Korean Research Foundation Grant funded by the government of the Republic of Korea (KRF-2008-313-E00245) and by the Research Program for New Drug Target Discovery through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0020471).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 3 August 2011.
REFERENCES
- 1. CDC 2009. Announcement: national influenza vaccination week—January 12 to 16, 2010. MMWR Morb. Mortal. Wkly. Rep. 58:1444 [Google Scholar]
- 2. CDC 2009. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400–402 [PubMed] [Google Scholar]
- 3. CDC 2009. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm. Rep. 58:1–8 [PubMed] [Google Scholar]
- 4. Clark T. W., et al. 2009. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N. Engl. J. Med. 361:2424–2435 [DOI] [PubMed] [Google Scholar]
- 5. Davenport F. M., Hennessy A. V. 1957. Predetermination by infection and by vaccination of antibody response to influenza virus vaccines. J. Exp. Med. 106:835–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davenport F. M., Hennessy A. V. 1956. A serologic recapitulation of past experiences with influenza A; antibody response to monovalent vaccine. J. Exp. Med. 104:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawood F. S., et al. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605–2615 [DOI] [PubMed] [Google Scholar]
- 8. Del Giudice G., et al. 2009. Seasonal influenza vaccine provides priming for A/H1N1 immunization. Sci. Transl. Med. 1:12re1. [DOI] [PubMed] [Google Scholar]
- 9. Ellebedy A. H., et al. 2010. Contemporary seasonal influenza A (H1N1) virus infection primes for a more robust response to split inactivated pandemic influenza A (H1N1) virus vaccination in ferrets. Clin. Vaccine Immunol. 17:1998–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greenberg M. E., et al. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361:2405–2413 [DOI] [PubMed] [Google Scholar]
- 11. Hancock K., et al. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 [DOI] [PubMed] [Google Scholar]
- 12. Kim J. H., Skountzou I., Compans R., Jacob J. 2009. Original antigenic sin responses to influenza viruses. J. Immunol. 183:3294–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klenerman P., Zinkernagel R. M. 1998. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394:482–485 [DOI] [PubMed] [Google Scholar]
- 14. Nolan T., et al. 2010. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 303:37–46 [DOI] [PubMed] [Google Scholar]
- 15. Palmer D. F., Coman M. T., Dowdle W. R., Schild G. C. 1975. Advanced laboratory techniques for influenza diagnosis. Immunol. Ser. 6:51–52 [Google Scholar]
- 16. Pascua P. N., et al. 2009. Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection. PLoS One 4:e8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plennevaux E., Sheldon E., Blatter M., Reeves-Hoche M. K., Denis M. 2010. Immune response after a single vaccination against 2009 influenza A H1N1 in U.S.A.: a preliminary report of two randomised controlled phase 2 trials. Lancet 375:41–48 [DOI] [PubMed] [Google Scholar]
- 18. Skowronski D. M., et al. 2010. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med. 7:e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song M. S., et al. 2009. Investigation of the biological indicator for vaccine efficacy against highly pathogenic avian influenza (HPAI) H5N1 virus challenge in mice and ferrets. Vaccine 27:3145–3152 [DOI] [PubMed] [Google Scholar]
- 20. WHO 2009. New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly. Epidemiol. Rec. 84:249–257 [PubMed] [Google Scholar]
- 21. Zhu F. C., et al. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361:2414–2423 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.