Abstract
The adherent-invasive Escherichia coli (AIEC) pathotype, which has been associated with Crohn's disease, shows similar traits to human and animal extraintestinal pathogenic E. coli (ExPEC) with respect to their phylogenetic origin and virulence gene profiles. Here, we demonstrate that animal ExPEC strains generally do not share the AIEC phenotype. In contrast, this phenotype is very frequent among animal intestinal pathogenic E. coli (InPEC) strains, particularly of feline and canine origin, that genetically resemble ExPEC. These results strengthen the particular identity and disease specificity of the AIEC pathotype and the putative role animals might play in the transmission of AIEC-like strains to humans.
TEXT
Adherent-invasive Escherichia coli (AIEC) is a recently described pathotype that has been repeatedly associated with Crohn's disease (2, 4, 8, 9, 13). Nowadays, this pathotype can be identified only by phenotypical traits, because no specific virulence genes have been discovered to date. The identification of AIEC strains is based on their ability to adhere to and to invade intestinal epithelial cells, as well as their capacity to survive and replicate within macrophages (4). The AIEC strains isolated to date are clonally diverse and belong to distinct serotypes, and even though they primarily fall into the B2 phylogroup, AIEC strains belonging to the A, B1, and D phylogroups have also been isolated (2, 8, 9, 11, 13). Moreover, AIEC strains carry different sets of virulence-associated gene characteristics of extraintestinal pathogenic E. coli (ExPEC) strains (2, 4, 9), and some strains, including the prototype strain LF82, have been found to be genetically similar to distinct avian pathogenic E. coli (APEC) (2, 3, 10, 14). Due to the similarities observed between these pathotypes, we compared several genotypic and phenotypic characteristics of AIEC and human ExPEC strains in a previous study (10). We observed that although both pathotypes were genetically similar (in terms of virulence gene profiles, phylogenetic backgrounds, and pulsed-field gel electrophoresis profiles), the majority of human ExPEC strains did not share the AIEC phenotype, which gives to the pathotype a particular identity.
It is against this background that we sought to examine the AIEC phenotype among animal strains, either from extraintestinal or intestinal infections, commonly showing an ExPEC-typical genotype in order to (i) evaluate the impact of the similar phylogenetic background and virulence profile among animal strains on their AIEC-like phenotype properties, (ii) study host and disease specificity of the AIEC pathotype, and (iii) seek out a putative zoonotic risk of AIEC strains between humans and animals.
We analyzed the AIEC phenotype of 79 ExPEC strains causing extraintestinal disease (Table 1) and 45 intestinal pathogenic E. coli (InPEC) strains causing enteritis to animals (Table 2). Together, these strains were adopted from a larger collection of animal strains with available sequence types (C. Ewers and L. H. Wieler, unpublished data; strains have been deposited in the MLST database [http://mlst.ucc.ie/mlst/mlst/dbs/Ecoli/]). Virulence-associated genes linked with the ExPEC group were determined as described recently (5). Strains were selected with respect to sequence types (STs) that had already been found in AIEC strains from patients with Crohn's disease, including ST131, ST73, ST10, and ST101. A broad range of serotypes was included, and we preferentially chose motile strains with malX and kpsMTII virulence genes, which are common traits in AIEC strains (10, 15). This strain set is, of course, biased toward strains with characteristics frequently found in AIEC. However, this was intended, as we aimed to increase the chance to identify the AIEC phenotype among strains from different animal species rather than to provide prevalence data.
Table 1.
Origin and characteristics of the 79 animal ExPEC strains included in this studya
| Strain | Host species | Pathotype | Disease | Serotype | ST | ST complex | Ancestral group | ADH_I |
INV_I |
REPL_I |
AIEC phenotype | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||||||
| IMT2312 | Chicken | APEC | Colibacillosis | O131:NM | 10 | STC10 | A | 0.2 | 0.1 | ND | ND | − | ||
| IMT2488 | Chicken | APEC | Colibacillosis | Ont:H4 | 10 | STC10 | A | 0.4 | 0.2 | 0.048 | 0.030 | ND | − | |
| IMT9238 | Chicken | APEC | Colibacillosis | Ont:H6 | 10 | STC10 | A | 0.4 | 0.1 | 0.022 | 0.011 | ND | − | |
| IMT9706 | Chicken | APEC | Colibacillosis | O119:Hnt | 10 | STC10 | A | 0.3 | 0.1 | 0.030 | 0.014 | ND | − | |
| IMT5110 | Chicken | APEC | Colibacillosis | O149:Hnt | 100 | STC165 | A | 0.1 | 0.1 | 0.001 | 0.001 | ND | − | |
| IMT9712 | Chicken | APEC | Colibacillosis | O22:H31 | 373 | STC168 | A | 3.7 | 3.6 | 0.031 | 0.039 | ND | − | |
| IMT2358 | Chicken | APEC | Colibacillosis | O78:H4 | 915 | None | ABD | 0.1 | 0.1 | 0.008 | 0.000 | ND | − | |
| IMT5215 | Chicken | APEC | Colibacillosis | O5:H10 | 93 | STC168 | AxB1 | 0.3 | 0.2 | 0.019 | 0.003 | ND | − | |
| IMT1939 | Duck | APEC | Colibacillosis | O78:H51 | 155 | STC155 | AxB1 | 0.3 | 0.1 | 0.050 | 0.049 | ND | − | |
| IMT2095 | Chicken | APEC | Colibacillosis | O8:H19 | 162 | STC469 | AxB1 | 2.7 | 2.7 | 0.038 | 0.010 | ND | − | |
| IMT5475 | Duck | APEC | Colibacillosis | O8:H4 | 359 | STC101 | AxB1 | 0.2 | 0.1 | 0.004 | 0.004 | ND | − | |
| IMT5494 | Duck | APEC | Colibacillosis | O29:H51 | 359 | STC101 | AxB1 | 0.5 | 0.2 | ND | ND | − | ||
| IMT2087 | Chicken | APEC | Colibacillosis | O78:H9 | 23 | STC23 | B1 | 0.2 | 0.2 | 0.088 | 0.061 | ND | − | |
| IMT2125 | Chicken | APEC | Colibacillosis | O78:H9 | 23 | STC23 | B1 | 0.1 | 0.1 | 0.007 | 0.005 | ND | − | |
| IMT2113 | Chicken | APEC | Colibacillosis | Ont:NM | 101 | STC101 | B1 | 0.2 | 0.2 | 0.001 | 0.001 | ND | − | |
| IMT5124 | Chicken | APEC | Colibacillosis | O78:Hnt | 369 | STC23 | B1 | 0.1 | 0.1 | 0.001 | 0.001 | ND | − | |
| IMT2097 | Chicken | APEC | Colibacillosis | O1:H7 | 95 | STC95 | B2 | 0.3 | 0.3 | 0.052 | 0.015 | ND | − | |
| IMT2272 | Chicken | APEC | Colibacillosis | O2:Hnt | 95 | STC95 | B2 | 0.2 | 0.0 | 0.018 | 0.007 | ND | − | |
| IMT2297 | Chicken | APEC | Colibacillosis | O2:H5 | 95 | STC95 | B2 | 0.2 | 0.0 | 0.047 | 0.016 | ND | − | |
| IMT2532 | Chicken | APEC | Colibacillosis | O18:H7 | 95 | STC95 | B2 | 0.3 | 0.2 | 0.014 | 0.010 | ND | − | |
| IMT2537 | Chicken | APEC | Colibacillosis | O18:H7 | 95 | STC95 | B2 | 0.2 | 0.1 | 0.015 | 0.009 | ND | − | |
| IMT2545 | Chicken | APEC | Colibacillosis | O1:H5 | 95 | STC95 | B2 | 0.1 | 0.1 | 0.001 | 0.001 | ND | − | |
| IMT4517 | Poultry | APEC | Colibacillosis | O2:H7 | 95 | STC95 | B2 | 0.2 | 0.2 | 0.023 | 0.011 | ND | − | |
| IMT4533 | Chicken | APEC | Colibacillosis | O18:Hnt | 95 | STC95 | B2 | 0.4 | 0.4 | 0.038 | 0.008 | ND | − | |
| IMT5211 | Chicken | APEC | Colibacillosis | O18:H7 | 95 | STC95 | B2 | 0.2 | 0.1 | 0.021 | 0.020 | ND | − | |
| IMT5214 | Chicken | APEC | Colibacillosis | O1:NM | 95 | STC95 | B2 | 0.2 | 0.1 | 0.001 | 0.001 | ND | − | |
| IMT8989 | Chicken | APEC | Colibacillosis | O1:H7 | 95 | STC95 | B2 | 0.2 | 0.0 | 0.030 | 0.043 | ND | − | |
| IMT9232 | Chicken | APEC | Colibacillosis | O1:H7 | 95 | STC95 | B2 | 0.7 | 0.1 | 0.022 | 0.016 | ND | − | |
| IMT9241 | Chicken | APEC | Colibacillosis | O2:H4 | 95 | STC95 | B2 | 0.6 | 0.2 | 0.018 | 0.014 | ND | − | |
| MT78 | Chicken | APEC | Colibacillosis | O2:Hnt | 95 | STC95 | B2 | 6.1 | 7.0 | 0.997 | 0.859 | 34.6 | 25 | − |
| IMT5112 | Chicken | APEC | Colibacillosis | O6:NM | 127 | STC127 | B2 | 0.1 | 0.1 | 0.000 | 0.000 | ND | − | |
| 2363 | Chicken | APEC | Colibacillosis | Ont:Hnt | 135 | None | B2 | 0.62 | 0.16 | 0.038 | 0.000 | ND | − | |
| IMT21073 | Chicken | APEC | Colibacillosis | O2:H1 | 135 | None | B2 | 1.83 | 0.46 | 0.205 | 0.092 | 58 | 28 | − |
| IMT2295 | Chicken | APEC | Colibacillosis | O2:Hnt | 135 | None | B2 | 0.04 | 0.05 | 0.014 | 0.003 | ND | − | |
| IMT2288 | Chicken | APEC | Colibacillosis | O2:H5 | 140 | STC95 | B2 | 0.4 | 0.4 | 0.018 | 0.006 | ND | − | |
| IMT4534 | Chicken | APEC | Colibacillosis | O2:Hnt | 140 | STC95 | B2 | 0.2 | 0.0 | 0.026 | 0.005 | ND | − | |
| IMT5128 | Chicken | APEC | Colibacillosis | O2:H5 | 140 | STC95 | B2 | 0.3 | 0.3 | 0.028 | 0.004 | ND | − | |
| IMT2290 | Chicken | APEC | Colibacillosis | O2:H6 | 141 | None | B2 | 1.7 | 0.2 | 0.045 | 0.032 | ND | − | |
| IMT2477 | Chicken | APEC | Colibacillosis | O2:H6 | 141 | None | B2 | 0.2 | 0.2 | 0.043 | 0.005 | ND | − | |
| IMT5160 | Chicken | APEC | Colibacillosis | Orough:H11 | 141 | None | B2 | 0.3 | 0.1 | 0.028 | 0.024 | ND | − | |
| IMT2112 | Poultry | APEC | Colibacillosis | O2:H5 | 355 | STC73 | B2 | 0.3 | 0.1 | 0.032 | 0.003 | ND | − | |
| IMT9713 | Chicken | APEC | Colibacillosis | O125:H10 | 372 | None | B2 | 0.1 | 0.1 | 0.002 | 0.001 | ND | − | |
| IMT4542 | Chicken | APEC | Colibacillosis | O2:Hnt | 1168 | STC95 | B2 | 0.3 | 0.0 | 0.044 | 0.016 | ND | − | |
| IMT2111 | Chicken | APEC | Colibacillosis | O1:H15 | 38 | STC38 | D | 1.2 | 0.4 | 0.030 | 0.005 | ND | − | |
| IMT2487 | Chicken | APEC | Colibacillosis | O77:H18 | 69 | STC69 | D | 0.2 | 0.0 | 0.019 | 0.007 | ND | − | |
| IMT2282 | Chicken | APEC | Colibacillosis | O23:H15 | 70 | None | D | 0.2 | 0.0 | 0.025 | 0.007 | ND | − | |
| K416/97-2 | Chicken | APEC | Colibacillosis | O2:Hnt | 115 | None | D | 1.9 | 2.6 | 0.043 | 0.024 | ND | − | |
| IMT2294 | Chicken | APEC | Colibacillosis | O2:H9 | 115 | None | D | 0.0 | 0.0 | 0.011 | 0.006 | ND | − | |
| IMT2261 | Chicken | APEC | Colibacillosis | O166:H15 | 349 | STC349 | D | 8.3 | 3.1 | 0.066 | 0.055 | ND | − | |
| IMT14972 | Dog | UPEC | UTI | Ont:NM | 10 | STC10 | A | 0.0 | 0.0 | 0.000 | 0.000 | ND | − | |
| VB 973164 | Pig | UPEC | UTI | O128:NM | 10 | STC10 | A | 0.0 | 0.0 | ND | ND | − | ||
| VB 985583 | Dog | UPEC | UTI | Ont:NM | 10 | STC10 | A | 0.0 | 0.0 | 0.000 | 0.000 | ND | − | |
| VB 991926 | Cat | UPEC | UTI | O49:H32 | 10 | STC10 | A | 0.0 | 0.0 | 0.004 | 0.004 | ND | − | |
| VB 905336 | Cat | UPEC | UTI | Ont:NM | 10 | STC10 | A | 0.8 | 1.0 | 0.000 | 0.000 | ND | − | |
| VB 993928 | Cat | UPEC | UTI | Ont:H35 | 10 | STC10 | A | 0.1 | 0.0 | 0.030 | 0.013 | ND | − | |
| IMT15499 | Pig | UPEC | UTI | Unknown | 880 | STC10 | A | 0.5 | 0.6 | 0.011 | 0.004 | ND | − | |
| IMT15532 | Pig | UPEC | UTI | Unknown | 891 | STC10 | A | 0.5 | 0.6 | 0.050 | 0.000 | ND | − | |
| VB 960678.1 | Dog | UPEC | UTI | O19:NM | 1254 | STC101 | AxB1 | 0.0 | 0.0 | 0.000 | 0.000 | ND | − | |
| IMT15513 | Pig | UPEC | UTI | O86:Hnt | 101 | STC101 | B1 | 0.4 | 0.4 | 0.046 | 0.016 | ND | − | |
| VB 996714.1 | Dog | UPEC | UTI | O15:H10 | 101 | STC101 | B1 | 0.1 | 0.0 | 0.047 | 0.042 | ND | − | |
| IMT14958 | Cat | UPEC | UTI | O22:H1 | 73 | STC73 | B2 | 0.2 | 0.3 | 0.047 | 0.038 | ND | − | |
| IMT14966 | Dog | UPEC | UTI | O6:H1 | 73 | STC73 | B2 | 0.4 | 0.5 | 0.023 | 0.024 | ND | − | |
| IMT14980 | Cat | UPEC | UTI | O6:H1 | 73 | STC73 | B2 | 0.0 | 0.0 | 0.004 | 0.003 | ND | − | |
| IMT14995 | Cat | UPEC | UTI | O6:H1 | 73 | STC73 | B2 | 0.0 | 0.0 | 0.015 | 0.023 | ND | − | |
| IMT15033 | Cat | UPEC | UTI | O25:H1 | 73 | STC73 | B2 | 0.1 | 0.1 | 0.052 | 0.003 | ND | − | |
| IMT9096 | Cat | UPEC | UTI | O2:H1 | 73 | STC73 | B2 | 0.8 | 0.9 | 0.035 | 0.007 | ND | − | |
| IMT12556 | Dog | UPEC | UTI | O25b:H4 | 131 | None | B2 | 0.6 | 0.7 | 0.041 | 0.008 | ND | − | |
| VB 977549 | Dog | UPEC | UTI | O25b:H4 | 131 | None | B2 | 0.1 | 0.1 | ND | ND | − | ||
| VB 999294 | Dog | UPEC | UTI | O25b:H4 | 131 | None | B2 | 0.3 | 0.2 | ND | ND | − | ||
| VB 973707 | Dog | UPEC | UTI | O25b:H4 | 131 | None | B2 | 4.3 | 0.6 | ND | ND | − | ||
| VB 984674 | Dog | UPEC | UTI | O25b:H4 | 131 | None | B2 | 0.4 | 0.3 | ND | ND | − | ||
| BF 187735 | Dog | UPEC | UTI | O25b:H4 | 131 | None | B2 | 0.3 | 0.2 | ND | ND | − | ||
| VB 991463 | Dog | UPEC | UTI | O2:Hnt | 135 | None | B2 | 1.85 | 0.49 | 0.103 | 0.004 | 976 | 210 | + |
| IMT15525 | Pig | ExPEC | GTI | Ont:H21 | 101 | STC101 | B1 | 0.4 | 0.5 | 0.048 | 0.018 | ND | − | |
| IMT15459 | Pig | ExPEC | GTI | O114:Hnt | 101 | STC101 | B1 | 0.9 | 1.2 | 0.045 | 0.002 | ND | − | |
| IMT15466 | Pig | ExPEC | GTI | Ont:H21 | 101 | STC101 | B1 | 0.2 | 0.3 | 0.063 | 0.039 | ND | − | |
| IMT15469 | Pig | ExPEC | GTI | Orough:NM | 101 | STC101 | B1 | 0.5 | 0.6 | 0.007 | 0.006 | ND | − | |
| IMT15472 | Pig | ExPEC | GTI | Ont:H21 | 101 | STC101 | B1 | 0.3 | 0.4 | 0.034 | 0.015 | ND | − | |
| IMT15497 | Pig | ExPEC | GTI | Ont:NM | 101 | STC101 | B1 | 0.1 | 0.1 | 0.001 | 0.001 | ND | − | |
Adhesion index (ADH_I), calculated as the mean number of bacteria per cell, and invasion index (INV_I), calculated as the percentage of intracellular bacteria of total bacteria inoculated, are detailed. Survival/replication index (REPL_I), calculated as the percentage of intracellular bacteria at 24 h postinfection, has been investigated only for those invasive strains. AIEC phenotype is positive (+) if ADH_I ≥ 1, INV_I ≥ 0.1, and REPL_I ≥ 100. Abbreviations: ND, not determined; UTI, urinary tract infection; GTI, genital tract infection.
Table 2.
Origin and characteristics of 45 animal intestinal pathogenic strains included in this studya
| Strain | Host species | Serotype | ST | ST complex | Ancestral group | ADH_I |
INV_I |
REPL_I |
AIEC phenotype | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||||
| IMT15384 | Dog | O6:H1 | 10 | STC10 | A | 0.28 | 0.17 | 0.008 | 0.006 | ND | − | |
| IMT15419 | Dog | Ont:H32 | 10 | STC10 | A | 1.50 | 0.42 | 0.015 | 0.014 | ND | − | |
| VB 900719 | Dog | Ont:Hnt | 10 | STC10 | A | 15.25 | 4.60 | 0.445 | 0.049 | 449 | 410 | + |
| VB 903359 | Dog | Ont:NM | 10 | STC10 | A | 0.08 | 0.07 | 0.002 | 0.003 | ND | − | |
| BF 137999 | Dog | Ont:NM | 10 | STC10 | A | 0.06 | 0.05 | 0.001 | 0.001 | ND | − | |
| VB 904411 | Dog | Ont:Hnt | 10 | STC10 | A | 0.18 | 0.11 | 0.028 | 0.003 | ND | − | |
| VB 904860 | Dog | Ont:H37 | 10 | STC10 | A | 7.93 | 1.52 | 1.975 | 0.177 | 439 | 212 | + |
| VB 905637 | Dog | Ont:NM | 10 | STC10 | A | 0.28 | 0.05 | 0.002 | 0.002 | ND | − | |
| IMT 13347 | Swine | Unknown | 10 | STC10 | A | 20.25 | 11.67 | 0.178 | 0.018 | 259 | 95 | + |
| IMT 13359 | Swine | Unknown | 10 | STC10 | A | 1.48 | 0.11 | 0.678 | 0.315 | 942 | 138 | + |
| IMT 13398 | Swine | Unknown | 10 | STC10 | A | 3.55 | 1.39 | 0.063 | 0.036 | ND | − | |
| IMT 13425 | Swine | Unknown | 10 | STC10 | A | 0.01 | 0.01 | 0.001 | 0.001 | ND | − | |
| IMT 13497 | Swine | Unknown | 10 | STC10 | A | 0.00 | 0.00 | 0.001 | 0.001 | ND | − | |
| IMT 13473 | Swine | Unknown | 10 | STC10 | A | 0.00 | 0.00 | 0.000 | 0.000 | ND | − | |
| VB 934275 | Dog | Ont:NM | 617 | STC10 | A | 0.02 | 0.02 | 0.005 | 0.001 | ND | − | |
| IMT15422 | Dog | Ont:H37 | 1238 | STC10 | A | 9.08 | 2.67 | 0.235 | 0.115 | 609 | 97 | + |
| IMT15383 | Cat | O6:Hnt | 73 | STC73 | B2 | 0.34 | 0.22 | 0.310 | 0.014 | 477 | 85 | + |
| IMT15401 | Cat | O25:NM | 73 | STC73 | B2 | 4.93 | 0.39 | 0.423 | 0.180 | 1,153 | 159 | + |
| IMT15407 | Cat | O25:H1 | 73 | STC73 | B2 | 3.03 | 1.66 | 0.163 | 0.067 | 1,399 | 535 | + |
| IMT15412 | Cat | O6:H1 | 73 | STC73 | B2 | 1.43 | 0.67 | 0.370 | 0.021 | 658 | 169 | + |
| IMT15417 | Cat | O6:H1 | 73 | STC73 | B2 | 1.60 | 1.35 | 0.242 | 0.173 | 980 | 60 | + |
| IMT15418 | Cat | O25:H1 | 73 | STC73 | B2 | 0.16 | 0.21 | 0.040 | 0.001 | ND | − | |
| IMT15423 | Cat | O6:H1 | 73 | STC73 | B2 | 1.17 | 0.64 | 0.292 | 0.183 | 1,541 | 190 | + |
| IMT15430 | Cat | O25:H1 | 73 | STC73 | B2 | 6.39 | 1.05 | 0.155 | 0.000 | 2,074 | 1,081 | + |
| IMT15444 | Cat | O6:H1 | 73 | STC73 | B2 | 1.43 | 0.18 | 2.350 | 0.212 | 1,013 | 985 | + |
| IMT15445 | Cat | Unknown | 73 | STC73 | B2 | 3.28 | 0.81 | 0.238 | 0.138 | 741 | 494 | + |
| IMT15447 | Cat | O25:H1 | 73 | STC73 | B2 | 1.04 | 0.24 | 1.450 | 0.424 | 91 | 14 | − |
| IMT15448 | Cat | O6:H1 | 73 | STC73 | B2 | 1.08 | 0.55 | 0.580 | 0.312 | 777 | 456 | + |
| VB 900990 | Cat | O6:H1 | 73 | STC73 | B2 | 4.70 | 1.20 | 0.463 | 0.187 | 604 | 61 | + |
| VB 901947 | Cat | O22:Hnt | 73 | STC73 | B2 | 2.23 | 0.25 | 0.150 | 0.035 | 1,060 | 603 | + |
| VB 901857 | Cat | O6:H1 | 73 | STC73 | B2 | 0.16 | 0.22 | 0.390 | 0.156 | 762 | 408 | + |
| VB 902289 | Cat | O25:H1 | 73 | STC73 | B2 | 1.35 | 0.14 | 1.375 | 0.247 | 1,287 | 409 | + |
| VB 902302 | Cat | O6:H1 | 73 | STC73 | B2 | 5.20 | 3.32 | 0.248 | 0.032 | 83 | 23 | − |
| VB 902827 | Cat | O25:H1 | 73 | STC73 | B2 | 1.03 | 0.32 | 0.245 | 0.127 | 647 | 286 | + |
| VB 903648 | Cat | O25:H1 | 73 | STC73 | B2 | 4.45 | 3.61 | 1.378 | 0.735 | 345 | 138 | + |
| BF 138088 | Cat | O6:H1 | 73 | STC73 | B2 | 3.68 | 0.53 | 0.145 | 0.014 | 757 | 324 | + |
| VB 905481 | Cat | O25:H1 | 73 | STC73 | B2 | 1.93 | 0.53 | 1.268 | 0.848 | 522 | 349 | + |
| BF 138148 | Cat | O25:NM | 73 | STC73 | B2 | 0.12 | 0.06 | 0.018 | 0.004 | ND | − | |
| IMT15380 | Dog | O6:H1 | 73 | STC73 | B2 | 1.52 | 0.77 | 0.395 | 0.085 | 1,504 | 548 | + |
| IMT15424 | Dog | O22:H1 | 73 | STC73 | B2 | 1.04 | 0.09 | 0.234 | 0.164 | 639 | 17 | + |
| IMT15441 | Dog | O6:NM | 73 | STC73 | B2 | 0.12 | 0.09 | 0.004 | 0.002 | ND | − | |
| VB 900402 | Dog | Ont:H1 | 73 | STC73 | B2 | 2.18 | 1.10 | 0.039 | 0.024 | ND | − | |
| VB 900782 | Dog | O25:H1 | 73 | STC73 | B2 | 1.50 | 0.28 | 0.830 | 0.844 | 1,159 | 847 | + |
| VB 901616 | Dog | O25:H1 | 73 | STC73 | B2 | 0.37 | 0.39 | 0.079 | 0.075 | ND | − | |
| VB 903606 | Dog | O6:H1 | 73 | STC73 | B2 | 2.38 | 0.95 | 0.026 | 0.006 | ND | − | |
Adhesion index (ADH_I), calculated as the mean number of bacteria per cell, and invasion index (INV_I), calculated as the percentage of intracellular bacteria of total bacteria inoculated, are detailed. Survival/replication index (REPL_I), calculated as the percentage of intracellular bacteria at 24 h postinfection, has been investigated only for those invasive strains. AIEC phenotype is positive (+) if ADH_I ≥ 1, INV_I ≥ 0.1, and REPL_I ≥ 100. ND, not determined.
As for the ExPEC strain collection, the present study includes 49 APEC strains isolated from colibacillosis in poultry; 24 uropathogenic E. coli strains (UPEC) from cystitis and pyelonephritis in cats, dogs, and pigs; and six strains causing upper genital tract infections in pigs (Table 1) (7). As determined by STRUCTURE analysis of concatenated sequences of the seven housekeeping genes included in MLST analyses (6), the distribution of ancestral groups was 50.6% B2, 17.7% A, 15.2% B1, and 7.6% D. Seven other strains were categorized under hybrid groups AxB1 (7.6%) and ABD (1.3%), which resemble highly recombining strains supposed to have acquired genetic material from all four major phylogenetic groups (16). With regard to the InPEC strain collection, we included 22 strains causing enteritis in cats, 17 in dogs, and six in swine. These strains belonged to phylogroups B2 (64.4%) and A (35.6%) (Table 2).
To identify the AIEC phenotype, we determined the ability of strains to adhere to and to invade intestinal epithelial cells, as well as their capacity to survive and replicate within macrophages by performing gentamicin protection assays over intestine-407 epithelial cells (ATCC CCL-6) and murine J774A1.1 macrophages (ATCC TIB-67), respectively (9). Assays for strains resistant to gentamicin were carried out with kanamycin at a final concentration of 100 μg·ml−1. All assays were performed on 24-well plates in triplicate. AIEC prototype strain LF82 and nonpathogenic E. coli strain C600 were used as controls.
In parallel, we performed correspondence analysis to determine whether or not a particular distribution of virulence-associated genes correlated with each pathotype (Canoco software version 4.5 for Windows; biplot scaling). We compared the 124 animal strains included in the present study with 22 human intestinal AIEC and 37 human mucosa-associated E. coli (non-AIEC) strains from a previous study (10). Fifty-two virulence genes were included in the analysis after removing genes that were present in all the strains or in a single strain.
Only one out of the 79 animal ExPEC strains analyzed shared the AIEC phenotype despite the mindful selection of strains. Approximately 16% of APEC strains were adherent to intestine-407 cells, but only two were invasive (IMT21073 and BEN2332, formerly named MT78). These results are in agreement with those of a previous study that described the adhesion and invasion ability of the MT78 strain to avian heterophils and macrophages (12). Among the UPEC and genital tract infection-associated strains, 10.2% were classified as adherent, but only UPEC strain VB 991463, which belonged to the same phylogroup (B2) and sequence type (ST135) as the LF82 strain, presented the complete AIEC phenotype. These results provided evidence for a particular identity of the AIEC pathotype, since animal ExPEC strains, although being genetically similar to AIEC strains, did not share the AIEC phenotype.
Contrary to animal ExPEC, up to 57.7% of preselected animal InPEC strains phenotypically resembled the AIEC pathotype, and a higher proportion (72.4%) was observed among B2 isolates. These InPEC strains caused enteritis to animals but genetically resembled ExPEC strains rather than classical intestinal pathogenic E. coli. Similarly, the AIEC pathotype is genetically similar to ExPEC but different from diarrheagenic E. coli pathovars and has been particularly related to ileal Crohn's disease and granulomatous colitis in boxer dogs, a disease which highly resembles ulcerative colitis and Crohn's disease in humans (2, 4, 9, 15). To our knowledge, this is the first study demonstrating the occurrence of AIEC in cats, even though these animals also suffer from inflammatory bowel disease (1). Although the nonarbitrary strain selection might have accounted for the exceptional high frequency of AIEC among intestinal strains from cats (81.8%), it stays a matter of concern that warrants future investigations.
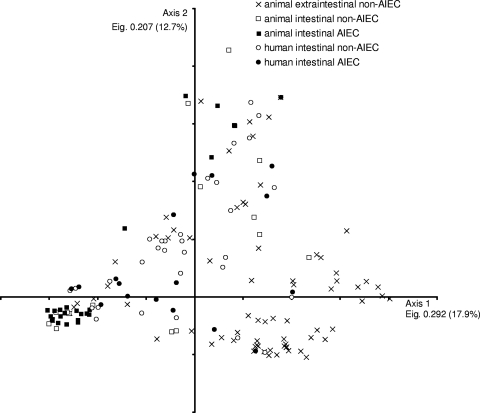
Correspondence analysis of virulence gene (VG) profiles showed that no particular VG distribution was associated to a certain group of strains (see Fig. 1). Strains clustered with regard to their phylogenetic affiliation (data not shown) but not by their extraintestinal/intestinal origin, human/animal host source, or AIEC phenotype. Human and animal AIEC strains appeared scattered in the plot, indicating their genetic diversity and the lack of a genetic marker for the identification of that pathotype. However, we found malX and kpsMTII more prevalent in animal and human strains with the AIEC phenotype (71.4% of AIEC-like versus 47% of non-AIEC [P = 0.003] and 71.4% versus 52% [P = 0.013], respectively, taking into account a total of 173 strains used for correspondence analysis). These virulence genes have already been detected in the LF82 strain and four other AIEC strains from human intestine, two human UPEC and two sepsis-causing E. coli strains also with the AIEC phenotype, and three AIEC strains from boxer dogs (10, 15).
Fig. 1.
Correspondence analysis of the distribution of 52 virulence-associated genes in 79 animal ExPEC, 45 animal InPEC (this study), 22 human AIEC, and 37 human mucosa-associated non-AIEC strains (data obtained from reference 10). Eigenvalues (Eig.) and percentages of variance are provided for each axis.
The detection of AIEC strains in the intestinal tracts of cats, dogs, and swine provides further support for the absence of host specificity of this pathotype, which has already been observed by Simpson et al. in boxer dogs (15). They show that the AIEC pathotype is disease specific rather than host specific. We hold up this hypothesis because the InPEC strains included in the present study were isolated from animals with enteritis. Unfortunately, detailed data on the clinical history of the strains were not available, and thus it was not possible to correlate the presence of AIEC with the type or severity of intestinal disease. Nevertheless, our data support the association of AIEC with an altered intestinal state possibly linked to inflammatory bowel disease. Finally, the presence of AIEC in the intestine of several animal species suggests a putative zoonotic risk. Further studies analyzing the real prevalence of AIEC in well-characterized diseased animals are needed in order to detect putative reservoirs of AIEC strains, even in the intestine of asymptomatic carriers, and to evaluate the dimension of the risk with respect to the implementation of prevention and control measures and thus to public health.
Acknowledgments
We are grateful to Maryvonne Dho-Moulin for kindly providing APEC strain MT78. We also thank Ivonne Stamm and Peter A. Kopp from the IDEXX VetMedLab (Ludwigsburg, Germany) for providing E. coli strains from animal sources.
This work was funded by the Spanish Ministry of Education and Science (SAF2010-15896) and supported by the ANR in the frame of ERA-NET Pathogenomics and from the Deutsche Forschungsgemeinschaft (German Research Foundation; WI 1436/5-3).
Footnotes
Published ahead of print on 24 June 2011.
REFERENCES
- 1. Allenspach K. 2011. Clinical immunology and immunopathology of the canine and feline intestine. Vet. Clin. North Am. Small Anim. Pract. 41:345–360 [DOI] [PubMed] [Google Scholar]
- 2. Baumgart M., et al. 2007. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 1:403–418 [DOI] [PubMed] [Google Scholar]
- 3. Boudeau J., Barnich N., Darfeuille-Michaud A. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272–1284 [DOI] [PubMed] [Google Scholar]
- 4. Darfeuille-Michaud A., et al. 2004. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127:412–421 [DOI] [PubMed] [Google Scholar]
- 5. Ewers C., et al. 2010. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-β-lactamase-producing Escherichia coli among companion animals. J. Antimicrob. Chemother. 65:651–660 [DOI] [PubMed] [Google Scholar]
- 6. Falush D., Stephens M., Pritchard J. K. 2003. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grobbel M., et al. 2007. Antimicrobial susceptibility of Escherichia coli from swine, horses, dogs and cats as determined in the BfT-GermVet monitoring program 2004-2006. Berl. Munch. Tierarztl. Wochenschr. 120:391–401 [PubMed] [Google Scholar]
- 8. Martin H. M., et al. 2004. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 127:80–93 [DOI] [PubMed] [Google Scholar]
- 9. Martinez-Medina M., et al. 2009. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm. Bowel Dis. 15:872–882 [DOI] [PubMed] [Google Scholar]
- 10. Martinez-Medina M., et al. 2009. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J. Clin. Microbiol. 47:3968–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masseret E., et al. 2001. Genetically related Escherichia coli strains associated with Crohn's disease. Gut 48:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mellata M., et al. 2003. Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infect. Immun. 71:494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sasaki M., et al. 2007. Invasive Escherichia coli are a feature of Crohn's disease. Lab. Invest. 87:1042–1054 [DOI] [PubMed] [Google Scholar]
- 14. Sepehri S., Kotlowski R., Bernstein C. N., Krause D. O. 2009. Phylogenetic analysis of inflammatory bowel disease associated Escherichia coli and the fimH virulence determinant. Inflamm. Bowel Dis. 15:1737–1745 [DOI] [PubMed] [Google Scholar]
- 15. Simpson K. W., et al. 2006. Adherent and invasive Escherichia coli is associated with granulomatous colitis in Boxer dogs. Infect. Immun. 74:4778–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wirth T., et al. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]