Abstract
We demonstrate improved ethanol yield and productivity through cofermentation of cellobiose and galactose by an engineered Saccharomyces cerevisiae strain expressing genes coding for cellodextrin transporter (cdt-1) and intracellular β-glucosidase (gh1-1) from Neurospora crassa. Simultaneous fermentation of cellobiose and galactose can be applied to producing biofuels from hydrolysates of marine plant biomass.
TEXT
In addition to lignocellulosic biomass, marine plant biomass is considered a potential feedstock for producing biofuels. Marine biomass lacks the recalcitrant cell wall structures that are found in lignocellulosic biomass. Therefore, it is relatively easier to release fermentable sugars from marine biomass than from terrestrial biomass. Moreover, a recent study predicted that the use of croplands for corn or energy crops could increase greenhouse gases because of changes in land use (13), which suggests that biofuel production from marine biomass is an alternative option for reducing greenhouse gases through carbon sequestration. In particular, macroalgae are attractive because of their wide geographical distribution and high growth rate. A red seaweed (Gelidium amansii) abundant on the coastlines of Southeast Asia contains about 20% cellulose and 60% agar (galactan), while cellulosic biomass (switchgrass) consists of 31% cellulose, 20% hemicellulose, and 18% lignin (8, 14). A combined treatment of weak acid and enzyme (cellulase) of red seaweed will produce a mixture of cellobiose and galactose. Because Saccharomyces cerevisiae cannot ferment cellobiose, treatment with β-glucosidase is required to generate fermentable hydrolysates containing glucose and galactose.
While Saccharomyces cerevisiae can ferment both glucose and galactose, prevalent in hydrolysates of marine biomass, this yeast ferments glucose and galactose sequentially with a diauxic lag period, which results in the reduction of overall ethanol productivity (6, 11). Moreover, the ethanol yield from galactose is lower than the yield from glucose (1, 10). At least three different approaches to enhance galactose fermentation by S. cerevisiae have been undertaken. First, overexpression of a positive regulator (GAL4) and deletion of negative regulators (GAL6, GAL90, and MIG1) were shown to be effective in improving galactose fermentation (10, 12). Second, overexpression of a pivotal enzyme (encoded by PGM2) resulted in a 70% increase in galactose uptake rates (1). Third, overexpression of a truncated transcriptional activator (TUP1) mediating glucose repression resulted in higher ethanol productivity from a mixture of glucose and galactose through shortening the lag period between glucose and galactose fermentations (7). However, these approaches failed to achieve simultaneous fermentation of glucose and galactose because of the tight regulation of galactose metabolic enzymes by galactose (5, 6) and the strong transcriptional repression of galactose permease (GAL2) by glucose (9). In order to overcome these problems, we demonstrated simultaneous fermentation of cellobiose and galactose by an engineered S. cerevisiae strain expressing genes coding for a cellodextrin transporter (cdt-1) and an intracellular β-glucosidase (gh1-1) from Neurospora crassa (2, 3). This cofermentation strategy offers higher productivity and yield of ethanol than does a parental strain that consumes glucose first and then ferments galactose only after depletion of glucose.
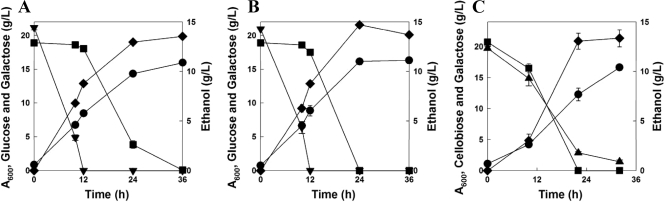
In order to investigate the degree of glucose repression on galactose fermentation, an S. cerevisiae D452-2 (MATα leu2 his3 ura3 can1) strain was cultured on medium containing either glucose or galactose and inoculated into 50 ml of yeast extract-peptone (YP) medium containing both glucose (20 g/liter) and galactose (20 g/liter) (4). All fermentation experiments were performed at 30°C with the same initial cell density (optical density at 600 nm [OD600], ∼1) under oxygen-limited conditions. As expected, strong preferential utilization of glucose was observed, regardless of preculture conditions. Both glucose- and galactose-grown cells consumed glucose rapidly, and galactose utilization started only after complete depletion of glucose (Fig. 1A and B). This is a typical fermentation characteristic of S. cerevisiae due to catabolic (glucose) repression (6, 11). While S. cerevisiae cells grown on galactose consumed galactose slightly faster than did the cells grown on glucose, severe catabolic repression was observed before galactose consumption. Ethanol yields from a sugar mixture of glucose and galactose were similar (0.34 versus 0.37 g ethanol/g sugar, respectively), regardless of the preculture conditions. However, galactose-grown cells showed higher volumetric productivity (0.61 g ethanol/liter·h) than glucose-grown cells (0.38 g/liter·h).
Fig. 1.
Fermentation profiles of a mixture of glucose (20 g/liter) and galactose (20 g/liter) (A and B) and a mixture of cellobiose (20 g/liter) and galactose (20 g/liter) (C) by an engineered S. cerevisiae strain (D452-2BT). Glucose severely repressed galactose fermentation, regardless of preculture conditions (cells grown on glucose [A] or on galactose [B]). However, cellobiose and galactose were fermented simultaneously (C). All values are the means of the results for two independent fermentations, and error bars represent the standard deviations of the results between two fermentations. Symbols: •, OD; ▾, glucose; ■, galactose; ▴, cellobiose; ⧫, ethanol.
To bypass the problems caused by glucose repression, we attempted cofermentation of cellobiose and galactose using an engineered S. cerevisiae (D452-2BT) strain. The D452-2BT strain contained two plasmids expressing a cellodextrin transporter (cdt-1) and an intracellular β-glucosidase (gh1-1) (3). D452-2BT cells grown on medium containing cellobiose as a sole carbon source were inoculated into YP medium containing both 20 g/liter of cellobiose and 20 g/liter of galactose. The D452-2BT cells consumed the cellobiose and galactose simultaneously and produced 13 g/liter of ethanol within 22 h (Fig. 1C). Although cells were grown on cellobiose, a dimer of glucose, the repression of galactose utilization was not observed (Fig. 1C). Coconsumption of galactose and cellobiose suggests that glucose generated from cellobiose by β-glucosidase intracellularly might not cause glucose repression, as is the case when glucose is added extracellularly. We have also observed similar levels of glucose derepression when cellobiose and xylose were cofermented by an engineered yeast strain containing both cellobiose and xylose fermentation pathways (3).
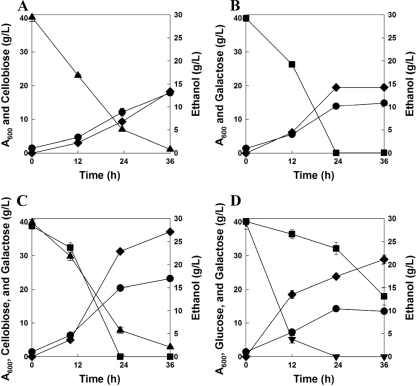
In order to demonstrate the beneficial effects of the cofermentation on ethanol yield and productivity, we performed four fermentation experiments using different sugar concentrations. Cellobiose (40 g/liter), galactose (40 g/liter), cellobiose and galactose (40 g/liter of each), and glucose and galactose (40 g/liter of each) were used as carbon sources. The D452-2BT strain was able to ferment cellobiose and galactose simultaneously; a total of 80 g/liter of sugars (cellobiose and galactose) were consumed within 34 h (Fig. 2C). Although double the amount of sugars was consumed by the D452-2BT strain, cellobiose and galactose consumption rates were almost identical to those in single-sugar fermentation experiments using either galactose or cellobiose alone (Fig. 2A and B). As a result, ethanol productivity during the cofermentation improved drastically over that of single-sugar fermentations (Table 1). When a mixture of glucose and galactose was used, the D452-2BT strain consumed glucose rapidly, but galactose fermentation began only after glucose depletion (Fig. 2C and D). While the specific ethanol productivity (0.35 g ethanol/g cell·h) from the mixture of glucose and galactose was similar to that of the cofermentation (0.33 g ethanol/g cell·h) of cellobiose and galactose during the glucose consumption period from 0 to 24 h, the galactose fermentation rate after glucose depletion was much lower than the galactose fermentation rate during the cofermentation. Therefore, overall volumetric ethanol productivity from diauxic fermentation of glucose and galactose was much lower than that of cofermentation of cellobiose and galactose (0.58 versus 0.75 g ethanol/liter·h, respectively). In summary, simultaneous fermentation of cellobiose and galactose exhibited improved cell growth (64%), ethanol titer (29%), ethanol yield (6%), and ethanol productivity (29%) compared to that of sequential fermentation of glucose and galactose. Moreover, cofermentation of cellobiose and galactose resulted in yields and productivities comparable to or better than those of single-sugar fermentation using the same amount of an individual sugar (Table 1).
Fig. 2.
Synergistic effects of cofermentation of cellobiose and galactose on ethanol yield and productivity compared to ethanol yield and productivity of single-sugar fermentations and glucose-galactose mixture fermentation. (A) 40 g/liter of cellobiose; (B) 40 g/liter of galactose; (C) a mixture of 40 g/liter of cellobiose and 40 g/liter of galactose; (D) a mixture of 40 g/liter of glucose and 40 g/liter of galactose. All values are the means of the results for two independent fermentations, and error bars represent the standard deviations of the results between two fermentations. Symbols: •, OD; ▾, glucose; ■, galactose; ▴, cellobiose; ⧫, ethanol.
Table 1.
Comparison of cellobiose-galactose cofermentation result with sole-carbon-source fermentation by engineered S. cerevisiae (D452-2BT)a
| Added sugar (concn) for fermentation expt | OD (A600) | EtOH concn (g/liter) | EtOH yield (g/g) | Vol PEtOH (g ethanol/liter·h) | Sp. PEtOH (g ethanol/g cell·h) |
|---|---|---|---|---|---|
| Cellobiose (40 g/liter) | 18 ± 0.59 | 13 ± 0.30 | 0.34 ± 0.01 | 0.37 ± 0.01 | 0.17 ± 0.02 |
| Galactose (40 g/liter) | 14 ± 0.06 | 14 ± 0.12 | 0.36 ± 0.01 | 0.61 ± 0.01 | 0.26 ± 0.02 |
| Cellobiose (20 g/liter) + galactose (20 g/liter) | 12 ± 1.02 | 13 ± 0.80 | 0.35 ± 0.02 | 0.59 ± 0.04 | 0.32 ± 0.01 |
| Cellobiose (40 g/liter) + galactose (40 g/liter) | 23 ± 0.20 | 27 ± 0.04 | 0.36 ± 0.01 | 0.75 ± 0.02 | 0.33 ± 0.02 |
| Glucose (40 g/liter) + galactose (40 g/liter) | 14 ± 0.04 | 21 ± 0.93 | 0.34 ± 0.01 | 0.58 ± 0.01 | 0.35 ± 0.02 |
Vol PEtOH and Sp. PEtOH denote volumetric ethanol (EtOH) productivity and specific EtOH productivity, respectively. All values are the means of results of two independent fermentations; errors represent the standard deviations of results between two fermentations. Sp. PEtOH was calculated during the period from 0 to 24 h of each fermentation.
Through cofermentation of cellobiose and galactose, we were able to remove glucose repression, which delays the utilization of nonglucose sugars. This cofermentation strategy has advantages over the current sequential fermentation of glucose and galactose from the hydrolysates of marine biomass. First, the addition of β-glucosidase is not required, as the engineered strain is capable of fermenting cellobiose, so the enzyme cost is lower. Second, the overall fermentation period can be reduced because the engineered strain consumes cellobiose and galactose simultaneously and volumetric productivity is increased. These benefits will contribute to economic biofuel production from marine biomass.
Acknowledgments
This work was supported by funding from Energy Biosciences Institute to Yong-Su Jin.
Footnotes
Published ahead of print on 24 June 2011.
REFERENCES
- 1. Bro C., Knudsen S., Regenberg B., Olsson L., Nielsen J. 2005. Improvement of galactose uptake in Saccharomyces cerevisiae through overexpression of phosphoglucomutase: example of transcript analysis as a tool in inverse metabolic engineering. Appl. Environ. Microbiol. 71:6465–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galazka J. M., et al. 2010. Cellodextrin transport in yeast for improved biofuel production. Science 330:84–86 [DOI] [PubMed] [Google Scholar]
- 3. Ha S. J., et al. 2011. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc. Natl. Acad. Sci. U.S.A. 108:504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hosaka K., Nikawa J., Kodaki T., Yamashita S. 1992. A dominant mutation that alters the regulation of INO1 expression in Saccharomyces cerevisiae. J. Biochem. 111:352–358 [DOI] [PubMed] [Google Scholar]
- 5. Ideker T., et al. 2001. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292:929–934 [DOI] [PubMed] [Google Scholar]
- 6. Johnston M., Flick J. S., Pexton T. 1994. Multiple mechanisms provide rapid and stringent glucose repression of GAL gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:3834–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee K. S., et al. 2011. Improved galactose fermentation of Saccharomyces cerevisiae through inverse metabolic engineering. Biotechnol. Bioeng. 108:621–631 [DOI] [PubMed] [Google Scholar]
- 8. Mosier N., et al. 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96:673–686 [DOI] [PubMed] [Google Scholar]
- 9. Nehlin J. O., Carlberg M., Ronne H. 1991. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 10:3373–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostergaard S., Olsson L., Johnston M., Nielsen J. 2000. Increasing galactose consumption by Saccharomyces cerevisiae through metabolic engineering of the GAL gene regulatory network. Nat. Biotechnol. 18:1283–1286 [DOI] [PubMed] [Google Scholar]
- 11. Ostergaard S., Walloe K. O., Gomes S. G., Olsson L., Nielsen J. 2001. The impact of GAL6, GAL80, and MIG1 on glucose control of the GAL system in Saccharomyces cerevisiae. FEMS Yeast Res. 1:47–55 [DOI] [PubMed] [Google Scholar]
- 12. Ronnow B., Olsson L., Nielsen J., Mikkelsen J. D. 1999. Derepression of galactose metabolism in melibiase producing bakers' and distillers' yeast. J. Biotechnol. 72:213–228 [DOI] [PubMed] [Google Scholar]
- 13. Searchinger T., et al. 2008. Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319:1238–1240 [DOI] [PubMed] [Google Scholar]
- 14. Wi S. G., Kim H. J., Mahadevan S. A., Yang D. J., Bae H. J. 2009. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. 100:6658–6660 [DOI] [PubMed] [Google Scholar]