Abstract
The transcription factor SCL/tal-1 is essential for blood cell development. Though it is also expressed in vascular endothelium, SCL has been considered dispensable for vessel formation. Through transgenic rescue of hematopoietic defects of SCL−/− embryos and analysis of chimeras generated with SCL−/− ES cells tagged with a transgene expressed in vascular endothelial cells, we show that SCL is essential for angiogenic remodeling of the yolk sac capillary network into complex vitelline vessels. These findings establish a role for SCL in embryonic angiogenesis and argue for critical functions in both embryonic blood and vascular cells, the descendents of the presumptive hemangioblast.
Keywords: SCL/tal-1 gene, hematopoiesis, vascular development, tie-2 gene
Blood cell formation and vasculogenesis initiate at ∼E7.5 of mouse development in the yolk sac blood islands. The close temporal and spatial relationship of hematopoiesis and vascular development is consistent with the existence of a postulated common progenitor, the hemangioblast (Pardanoud et al. 1989). The SCL/tal-1 gene [hereafter termed SCL (stem cell leukemia)], which was originally identified through its translocation in acute T-cell lymphoblastic leukemia (Begley et al. 1989; Finger et al. 1989; Chen et al. 1990), encodes a basic helix–loop–helix transcription factor that is expressed specifically in hematopoietic cells (Green et al. 1991; Visvader et al. 1991), vascular endothelium (Hwang et al. 1993; Kallianpur et al. 1994), and the developing brain (Green et al. 1992). Embryos lacking SCL fail to develop past E9.5 because of the absence of yolk sac erythropoiesis (Robb et al. 1995; Shivdasani et al. 1995) and SCL−/− embryonic stem (ES) cells do not contribute to any hematopoietic lineages in chimeric mice (Porcher et al. 1996; Robb et al. 1996). Hence, SCL is believed to function either in programming commitment of ventral mesoderm to a hematopoietic fate or in the generation of hematopoietic pluripotential stem cells. From the presence of vascular endothelial cells in SCL−/− embryos it has been inferred that SCL is dispensable for vascular cell specification (Robb et al. 1995; Shivdasani et al. 1995).
To explore the potential involvement of SCL in nonhematopoietic pathways, we sought to rescue the hematopoietic defect in SCL−/− embryos by introducing an SCL cDNA–transgene specifically expressed in the hematopoietic compartment. By this approach, we uncovered an unsuspected but essential role for SCL in embryonic vessel development. By specifically tagging SCL−/− ES cells with an endothelial-expressed lacZ gene, the fate of SCL−/− endothelial cells was tracked in chimeras. This strategy provides evidence for a primary role for SCL in angiogenesis, a process entailing the proliferation and remodeling of pre-existing endothelial cells into a mature vascular network.
Results
Transgenic rescue of hematopoiesis
To rescue the hematopoietic component of the SCL/tal-1 knockout (Robb et al. 1995; Shivdasani et al. 1995), we first generated transgenic mice harboring SCL cDNA sequences under the control of regulatory sequences of the GATA-1 gene (McDevitt et al. 1997) (Fig. 1), as its hematopoietic expression profile mimics that of SCL (Visvader et al. 1991; Green et al. 1992). Of four founders, two independent lines of transgenic mice (GATA-1/SCL-12 and GATA-1/SCL-52) expressed transgene-derived RNA at a level closely approximating that of the endogenous SCL mRNA in yolk sac (Fig. 2). SCL−/− embryos carrying the GATA-1/SCL transgene were generated by interbreeding SCL−/+ Tg(GATA-1/SCL-12 or GATA-1/SCL-52) mice. Whereas SCL−/− yolk sacs are devoid of any visible blood (Fig. 1c), hematopoiesis is substantially rescued in SCL−/− Tg yolk sacs, evident as diffuse red color (Fig. 1b). SCL−/− Tg embryos, however, remained pale and growth retarded. Like SCL−/− embryos (Robb et al. 1995; Shivdasani et al. 1995), they failed to survive beyond E9.5 because of the vascular defects described below.
Figure 1.
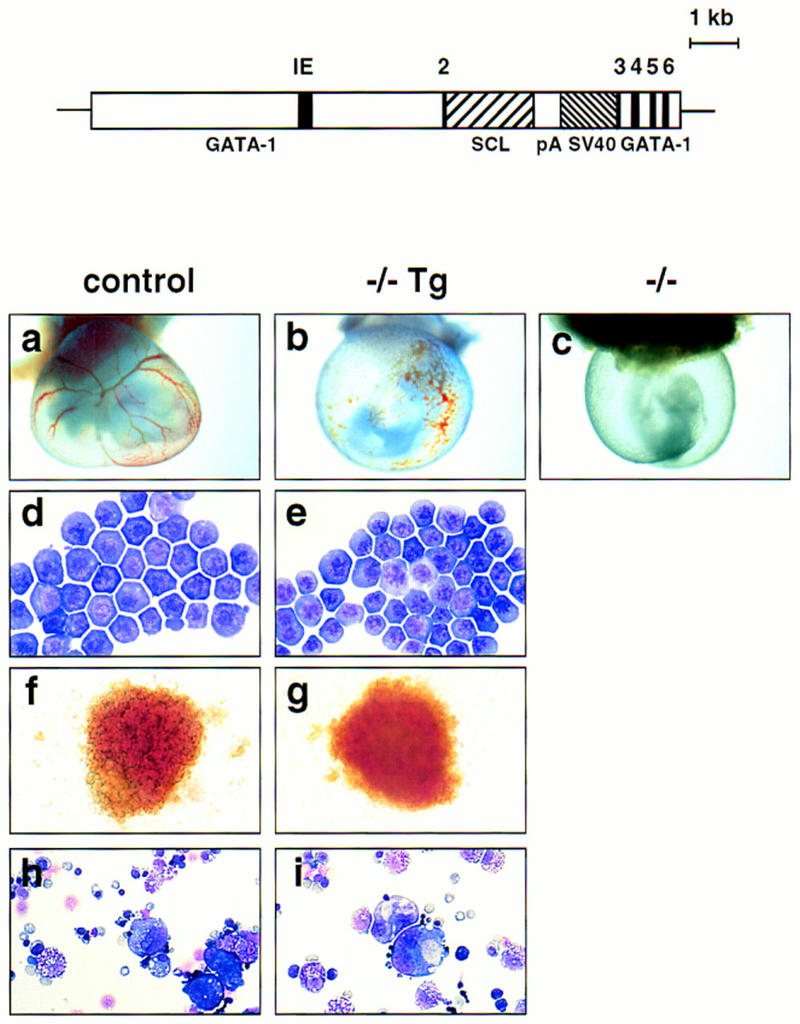
Rescue of hematopoiesis in SCL−/− yolk sacs by introduction of the GATA-1/SCL transgene. (Top) Structure of the GATA-1/SCL transgenic construct: 7 kb of GATA-1 upstream sequence and ∼1.5 kb of sequence downstream of exon 3 flank the SCL cDNA. (IE) The first erythroid promoter of the murine GATA-1 gene. The rabbit globin poly(A) addition region and SV40 sequence provide polyadenylation signals and allow transgene detection. (Bottom) Phenotypes of wild-type (either +/+ or +/−) (a) SCL−/− Tg (b), and SCL−/− (c) embryos with intact visceral yolk sacs (E9.5). Blood-filled vessels are visible in wild-type and −/− Tg yolk sacs. Note the lack of branching vitelline vessels in the −/− Tg yolk sac. Original magnification, 18.75×(d,e) May–Grunwald–Giemsa-stained blood cells from E9.5 wild-type (d) and SCL−/− (e) yolk sacs. Original magnification, 1000×. (f, g) Day 7 BFU-E colonies generated by in vitro differentiation of wild-type or SCL−/− Tg yolk sac cells. Original magnification, 400×. (h,i) May–Grunwald–Giemsa staining of mixed colonies at day 7 of differentiation from wild-type and −/− Tg yolk sacs. Mixed colonies were harvested and cytospun before staining. Original magnification, 320×.
Figure 2.
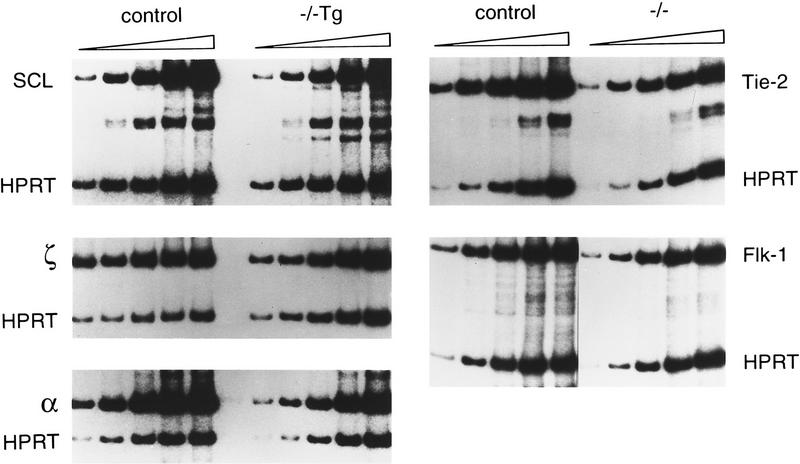
RNA expression of SCL and globins in SCL−/− Tg yolk sacs and of receptor tyrosine kinases in SCL−/− cells. RT–PCR analyses were performed with RNAs obtained from single yolk sacs at E9.5. Samples for SCL and the globins were amplified for six cycles in the presence of HPRT primers, before addition of SCL, or α or ζ globin primers. Aliquots were then removed after an additional 16, 18, 20, 22, and 24 cycles. Levels of globin transcripts were 1.3- to 3.1-fold lower in SCL−/− Tg yolk sacs compared to that in controls. PCR reactions for Tie-2 and Flk-1 were performed for four cycles prior to the addition of HPRT primers and amplified for a further 18, 20, 22, 24, and 26 cycles.
Blood cells present in the yolk sacs of SCL−/− Tg embryos appeared morphologically normal (Fig. 1d,e), indicating rescue of primitive erythropoiesis. Yolk sacs contained progenitors capable of generating multiple lineages, including erythroid, myeloid, mast, and megakaryocytic cells in hematopoietic colony assays (Shivdasani et al. 1995) (Fig. 1f–i). As colonies derived from yolk sacs at this stage are of the definitive (or adult) type, we conclude that definitive hematopoiesis is also restored by SCL expression. Rescue of multiple lineages by the GATA-1/SCL transgene suggests that the transcription factors GATA-1 and SCL genes act at a similarly early point within the hematopoietic regulatory hierarchy, an inference consistent with their expression during embryogenesis (Silver and Palis 1997). Rescue, however, was quantitatively incomplete. The mean colony number per SCL−/− Tg yolk sac (based on four experiments) was 3.8-fold less for erythroid colonies/BFU-E (37 ± 8 for −/−Tg vs. 144 ± 50 for control cells, +/+ or +/−), 2.9-fold less for myeloid colonies (101 ± 50 vs. 249 ± 110) and 4.5-fold less for mixed colonies (41 ± 7 vs. 186 ± 30). The reduced potential of the SCL−/− Tg yolk sac cells to form erythroid colonies was also reflected in their relative levels of ζ, α, and βh1 globin RNA (Fig. 2; data not shown). The inability to achieve full rescue may be attributable to improper timing or regulation of SCL transgene expression imposed by the GATA-1 control sequences.
SCL−/− and SCL−/− Tg yolk sacs lack vitelline vessels
The pattern of vasculature within the SCL−/− Tg yolk sacs was striking (Fig. 1b). Rather than the distinctive network of branching vitelline vessels visualized in normal embryos (Fig. 1a), a disorganized array of capillaries was observed, a finding corroborated by whole-mount staining with anti-PECAM antibodies, a specific marker of differentiated endothelial cells (Baldwin et al. 1994). The absence of vitelline vessels, but presence of an interconnecting network of smaller vessels, was also noted for SCL−/− yolk sacs (Fig. 3a–c). Individual endothelial cells of control and SCL−/− yolk sacs, however, stained similarly for PECAM (Fig. 3d–f) and CD34 (Fig. 3g,h), another marker of differentiated endothelial cells (Baumhueter et al. 1994). Both the endothelial and endodermal cell layers, as well as the smooth muscle/pericytes that constitute the lining of vessels, appeared intact by histological analysis (Fig. 3i,j) and electron microscopy (data not shown). Taken together, these data indicate that specification of vascular cells proceeds normally in the absence of SCL but that angiogenesis is defective, either as a primary or secondary consequence of the deficiency. As to be expected, this defect persists in the SCL−/− Tg embryos, as regulatory sequences of the GATA-1-based-transgene are inactive in vascular cells. Histological analysis of the SCL−/− and SCL−/− Tg embryos proper suggested that the vasculature may be more disorganized than in wild-type littermates, but severe growth retardation and necrosis by E9.5 precluded a detailed analysis. We speculate that the SCL−/−Tg embryos die by E9.5 due to the absence of connecting blood vessels between the yolk sac and embryo.
Figure 3.
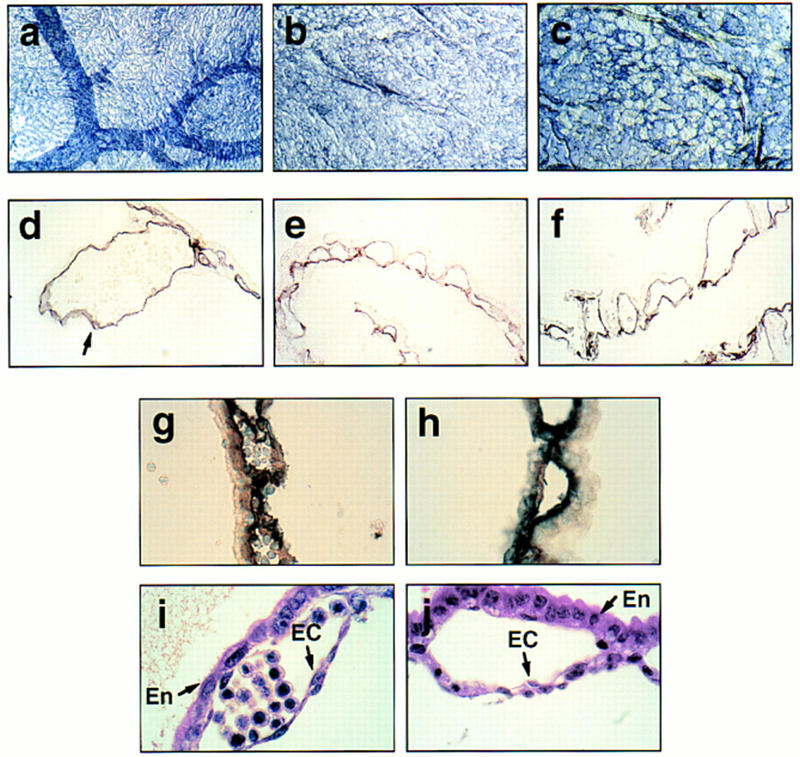
Defective angiogenesis in SCL−/− and SCL−/− Tg yolk sacs. (a–c) Whole-mount staining with anti-PECAM antibody of E9.5 yolk sacs derived from wild-type, SCL−/− and −/−Tg embryos shows the presence of capillaries and absence of vitelline vessels in both SCL−/− and SCL−/− Tg yolk sacs. Original magnification, 18.75×. (d–f) Sections of anti-PECAM whole-mount-stained yolk sacs from a, b, and c, revealing specific staining of endothelial cells. The arrow designates a vitelline vessel, only observed in control yolk sacs. Original magnification, 400×. (g,h) Sections of wild-type and mutant (−/−) yolk sac stained with anti-CD34, a marker of endothelium and early hematopoietic cells. Original magnification, 1000×. (i,j) Hematoxylin and eosin staining of wild-type and SCL−/− yolk sac sections. (En) extraembryonic endoderm; (EC) endothelial cell. Original magnification, 1000×.
Endothelial receptor tyrosine kinases are expressed in SCL−/− yolk sacs
Several genes are essential for the development and integrity of the yolk sac vascular system (Risau 1997). These include receptor tyrosine kinases and their ligands. The transcriptional status of several of these genes was evaluated by RT–PCR to search for potential SCL target genes in endothelial cells (Fig. 2; data not shown). The expression of the endothelial RTKs, Flk-1, Tie-1, and Flt-4, in SCL−/− yolk sacs was comparable to that observed in normal yolk sacs, although Tie-2 and Flt-1 RNA levels appeared to be diminished slightly (about two fold). These findings suggest that the number of endothelial cells in mutant yolk sacs is approximately the same as in wild type. The levels of TEL (Wang et al. 1997) and Gα13 (Offermanns et al. 1997) transcripts also appeared normal. Hence, none of the above genes are likely to be essential early targets of the SCL transcription factor in vascular endothelium.
Aberrant vasculature in SCL−/−/ROSA chimeras
Although it remained formally plausible that the angiogenic defect was merely secondary to the absence of blood in SCL−/− embryos, it seemed improbable for at least two reasons. (1) The defect was evident in yolk sacs displaying substantial hematopoietic rescue; and (2) other hematopoiesis-defective embryos, such as those lacking GATA-1 or GATA-2 (Shivdasani and Orkin 1996), have normal vasculature, despite a marked decrease in blood volume. To distinguish between primary or secondary effects of SCL loss on angiogenesis, we generated two different types of chimeras. Because ES cells often display a marked bias in tissue distribution following blastocyst injection, we produced chimeras with either marked ES cells or blastocysts to follow the fate of donor SCL−/− ES cells in developing yolk sacs.
SCL−/− ES cells were first injected into blastocysts of β-galactosidase (lacZ)-expressing ROSA26 mice (Zambrowicz et al. 1997). The degree of chimerism was estimated by lacZ staining of sections through E9.5 chimeric yolk sacs (data not shown). Of particular note, we observed that the level of contribution by the SCL−/− cells correlated with the vascular, as well as the hematopoietic, phenotypes of the yolk sacs. Typical phenotypes of SCL−/−/ROSA chimeras are shown in Figure 4a–c. Yolk sacs estimated to be ∼80% chimeric (Fig. 4a) were pale and exhibited a patchy, disorganized network of capillaries lacking vitelline vessels, thereby recapitulating the SCL−/− Tg vascular phenotype. Although a more well-developed vascular network was evident in yolk sacs exhibiting ∼20% chimerism (Fig. 4b), the vessels were uniformly dilated with no clear distinction between large and small vessels. Conversely, vitelline vessels were readily visualized in low chimeric (<5% ) yolk sacs (Fig. 4c). The analysis of SCL−/−/ROSA chimeras, therefore, suggests that the presence of SCL−/− cells in the developing yolk sac leads to abnormal vessel formation.
Figure 4.
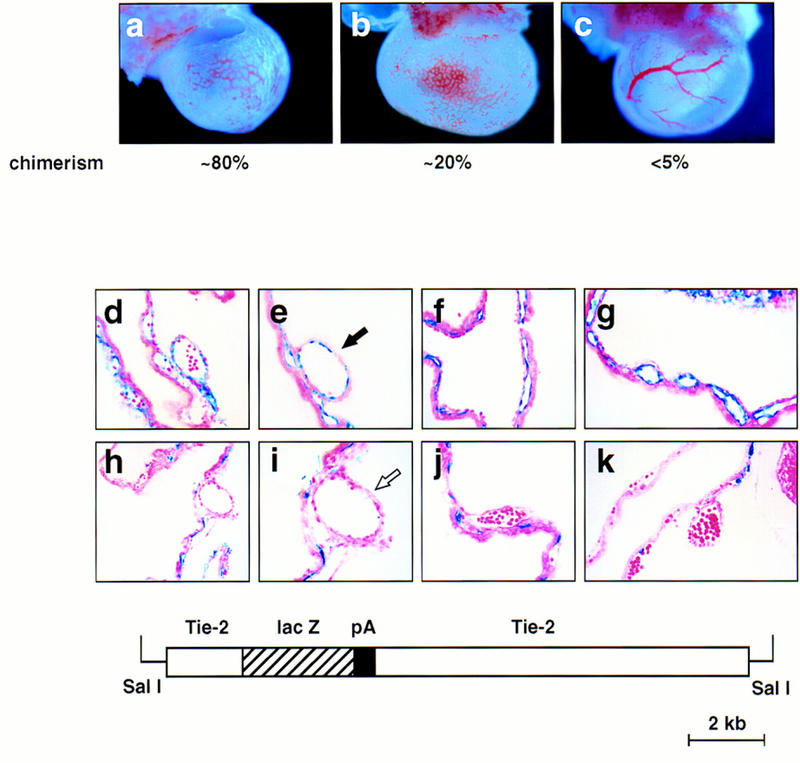
SCL−/− cells contribute to the endothelium of small but not large vessels in chimeric yolk sacs. The contribution of SCL-deficient cells to the endothelium of E9.5 chimeras was analyzed by β-galactosidase whole-mount staining. (a–c) β-Galactosidase-stained yolk sacs derived from SCL−/−/ROSA chimeras. The approximate degree of chimerism is shown below each. Note the disorganized vascular architecture in the higher percentage chimeras, a and b. Original magnification, 22.5×. (f–k) Sections of stained yolk sacs of chimeric embryos derived from Tie-2–lacZ-marked SCL+/− or SCL−/− ES cells. (d,e) SCL+/− cells contribute uniformly to capillaries and vitelline vessels in chimeric yolk sacs. Note the contribution of lacZ-positive cells to large vessels, as indicated by the solid arrow in e. (f–k) In contrast, SCL−/−-derived endothelial cells were not detectable lining the lumens of vitelline vessels, as highlighted by the open arrow in i. Note that the SCL−/− ES cells contribute efficiently to capillaries. Original magnification, 400×, except for a and f, which were 200×. The structure of the Tie2–lacZ transgene (kindly provided by T. Sato, University of Texas Southwestern Medical School, Dallas) is shown in the bottom panel.
Tagged SCL−/− endothelial cells do not contribute to large vessels in chimeras
To investigate a primary role for SCL in angiogenesis, we generated a second type of chimera using wild-type blastocysts and SCL−/− ES cells harboring a Tie-2–lacZ reporter gene (Schlaeger et al. 1997) (Fig. 4, bottom). The Tie-2–lacZ transgene directs uniform and high-level expression to all endothelial cells. In control experiments we demonstrated that SCL−/+/Tie-2–lacZ cells readily contributed to both small and large vessels of the developing yolk sac vasculature (Fig. 4d,e; note solid arrow in e). In contrast, SCL−/−/Tie-2–lacZ cells contributed efficiently to small capillaries but were not observed in vitelline vessels of multiple chimeras of varying extents generated from three different marked SCL−/− ES cell clones (Fig. 4f–k; note open arrow in i). In addition, the aberrant vascular architecture observed in whole yolk sacs containing a high proportion of SCL−/−-derived endothelial cells paralleled the phenotypes observed for SCL−/− and SCL−/− Tg embryos (Fig. 4f,g), in which only small capillaries were detectable. These data provide direct evidence for an intrinsic defect in the capacity of SCL−/− endothelial cells to participate in the formation of large vessels during the process of angiogenesis.
Discussion
Our goal in the studies reported here was to identify nonhematopoietic roles of SCL in development. To this end, we used cell-specific transgenic rescue to reveal functions obscured by early embryonic lethality of a knockout embryo. The relatively efficient rescue of yolk sac hematopoiesis by a transgene driven by GATA-1 regulatory sequences suggests that GATA-1 and SCL act at a similarly early point within the hematopoietic regulatory hierarchy. Furthermore, GATA-1 gene-based constructs may be useful in the rescue of hematopoietic defects of other knockout embryos. Transgenic rescue provided initial evidence for a vascular defect in the absence of SCL, a finding corroborated and extended by chimera studies, particularly employing SCL−/− ES cells tagged with a Tie-2–lacZ reporter gene.
Blood vessels develop by two distinct processes, vasculogenesis and angiogenesis. Vasculogenesis involves the differentiation of endothelial cells from their mesodermal precursors and the subsequent assembly into a primary capillary plexus. During angiogenesis, these primary vessels are remodeled into a larger, more complex network. Although SCL appears to be dispensable for initial specification and proliferation of endothelial cells, the present work demonstrates that it is required for formation of branching vitelline vessels from a primary vascular plexus. Furthermore, the vascular defects associated with loss of SCL appear to be endothelial-specific, as shown by the Tie-2–lacZ transgene experiments and ultrastructure analysis indicating that the mesenchyme/smooth muscle layer is intact (data not shown).
Several genes encoding receptor tyrosine kinases (RTKs), such as Flk-1 (Shalaby et al. 1995, 1997), Flt-1 (Fong et al. 1995), Tie-1 (Puri et al. 1995; Sato et al. 1995), and Tie-2 (Dumont et al. 1994; Sato et al. 1995); and the ligands, such as vascular endothelial growth factor (VEGF) (Carmeliet et al. 1996a; Ferrara et al. 1996), angiopoietin-1 (Ang-1) (Suri et al. 1996), and tissue factor (TF) (Carmeliet et al. 1996b), are important for proper yolk sac vascular development (Risau 1997). Targeted disruption of these genes has provided insights into intracellular signaling mechanisms that govern blood vessel formation. However, none of these genes, nor the transcription factor TEL (Wang et al. 1997) or the G protein Gα13 (Offermanns et al. 1997), appear to be early targets of SCL, as shown by RT–PCR analysis.
The SCL−/− vascular defect most closely resembles that of embryos lacking Tie-2 (Dumont et al. 1994; Sato et al. 1995). Tie-2 has direct effects on the endothelium but has also been implicated in angiogenesis by virtue of an activating mutation that causes vascular dysmorphogenesis (Vikkula et al. 1996). Although targeted disruption of several other genes (Risau 1997), such as Ang-1, VEGF, TF, and aryl hydrocarbon receptor nuclear translocator (ARNT) (Maltepe et al. 1997), leads to similar yolk sac phenotypes, these seem to reflect aberrant interactions/signaling between the endothelium and surrounding mesenchymal cells. In contrast, SCL is required in a cell autonomous fashion for remodeling of endothelial cells into a mature vascular network.
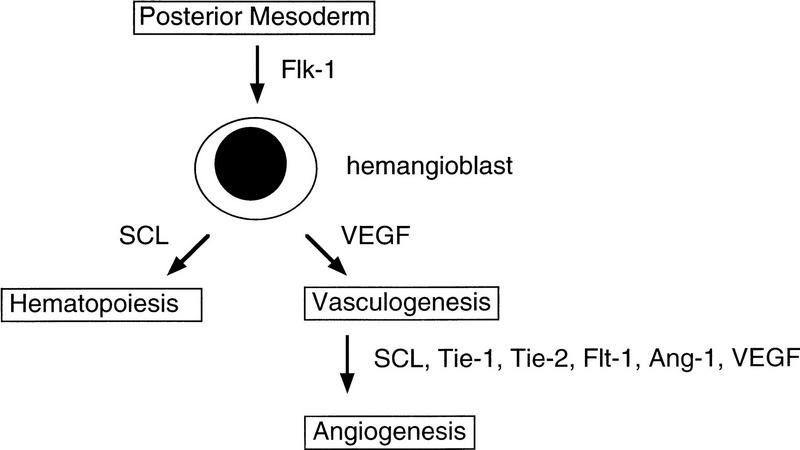
Taken together with earlier studies, our findings establish a dual function for SCL in hematopoiesis and vascular development (Fig. 5). The close relationship between hematopoiesis and vasculogenesis in the developing yolk sac blood islands is consistent with the existence of a common progenitor, the hemangioblast (Pardanoud et al. 1989). The requirement for the receptor Flk-1 in both hematopoiesis and vasculogenesis (Shalaby et al. 1995, 1997) is compatible with this model. Although our current data do not bear directly on the existence of the hemangioblast, they do establish SCL as having a unique regulatory function, positioned at the interface between blood and vascular development. Identifying critical target genes for SCL in vascular endothelial cells should elucidate novel angiogenic pathways and their possible relationship to those already defined.
Figure 5.
Dual function for SCL in the developing embryo. SCL has distinct roles in regulating angiogenesis (sprouting and remodeling of the vasculature) and early hematopoietic events (prior to commitment of progenitors to different lineages). The sites of action of relevant genes in blood vessel development, as determined by targeted disruption, are shown. (Ang-1) Angiopoietin-1. The existence of the bipotential hemangioblast is hypothetical.
Materials and methods
Constructs and transgenic mice production
A 1.4-kb murine SCL cDNA fragment was linked to the rabbit β-globin polyadenylation region and a 900-bp fragment spanning the SV40 early intronic region and polyadenylation signal (Lindeman et al. 1995), which also allow transgene detection, before insertion into a vector containing regulatory sequences derived from the murine GATA-1 gene (McDevitt et al. 1997). The final insert, released by SalI digestion, was introduced by pronuclear injection into fertilized eggs derived from a CD1 strain (Charles River). Southern blot analysis with the 900-bp SV40 fragment was used to identify transgenics; progeny tail DNA was subsequently typed by PCR using SV40 primers. Two independent transgenic lines were established and were crossed with SCL heterozygous mice (129 × C57BL/6N) (Shivdasani et al. 1995) to produce SCL+/− Tg strains, which were then mated to produce SCL−/− Tg embryos.
A 15-kb Tie-2–lacZ insert was isolated from pT2HlacZpA1I.7 (Schlaeger et al. 1997) by SalI digestion and ligated to a fivefold molar excess of XhoI-digested PGK-puromycin cassette (2.1 kb) for 2 hr at 16°C before final purification. The DNA was electroporated into either SCL−/− or SCL+/− J1 ES cells; the SCL+/− had also undergone two rounds of G418 selection to provide a control line (Porcher et al. 1996). Puromycin-resistant clones were typed by Southern blot analysis and PCR for detection of the lacZ insert.
Yolk sac progenitor assay
These were performed essentially as described (Shivdasani et al. 1995). The yield for each collagenase-treated yolk sac (E9.5) was 1 × 104 to 5 × 104/ml. The disaggregated cells were plated in α-minimal essential medium supplemented with α methylcellulose, 30% FCS, 1% BSA, and 2 U/ml of erythropoietin, 50 ng/ml of recombinant c-kit ligand, 5 ng/ml of IL-11, and 10 ng/ml of IL-3.
Semiquantitative RT–PCR
RNAs were isolated from individual E9.5 yolk sacs and genomic DNA from the embryos used to confirm their genotype. RT–PCR was performed as described (Weiss et al. 1994), using two sets of primers, one representing the specific gene and the other HPRT. In the case of SCL, 10% dimethylsulfoxide was included in the reactions. The sequence of the oligonucleotide primers has been described elsewhere; SCL (Porcher et al. 1996), HPRT and globins (Weiss et al. 1994), and Tel, Flk-1, Tie-2, and other RTKs (Carmeliet et al. 1996). Relative quantitations versus HPRT were performed using a PhosphorImager (Molecular Dynamics). Control experiments without reverse transcriptase in the cDNA synthesis reactions did not show these specific PCR products.
Immunohistochemistry and histology
Whole-mount immunohistochemistry using anti-PECAM monoclonal antibody MEC13.3 (Pharmingen) was performed essentially as described (Schlaeger et al. 1995). The stained yolk sacs were postfixed in 4% paraformaldehyde, embedded in paraffin wax, and sectioned at 8 μm. Affinity purified polyclonal anti-CD34 antibody (Baumhueter et al. 1994) was used to stain 10-μm cryosections of dissected E9.5 embryos and yolk sacs that had been previously fixed with 4% paraformaldehyde. Ten-micrometer sections were used for immunohistochemical staining; alkaline phosphatase staining was used for antibody detection.
Generation and analysis of chimeras
Chimeras were generated by blastocyst injection as described (Hogan et al. 1994). Mice carrying the ROSA26 gene trap integration (Zambrowicz et al. 1997) were obtained from Jackson Laboratories [TgR(ROSA26)RSor strain]. For the ROSA chimera experiments, blastocysts were collected from matings between homozygous ROSA26 and C57BL/6NTacfBR (Taconic) mice. A maximum of 16–18 ES cells were injected into the blastocyst cavity. Chimeric percentages were estimated by β-galactosidase staining of yolk sacs. For Tie-2–lacZ transgenic ES cell experiments, blastocysts were collected from matings between C57BL/6NTacfBRs (Taconic). To produce relatively low chimeric embryos, the number of ES cells injected into the blastocyst cavity was, on average, 8–10. Following transfer into pseudopregnant foster females, the embryos were recovered at day 9.5 or 10.5 of development. Embryos were fixed and processed for β-galactosidase as described previously (Hogan et al. 1994). After staining, embryos and their yolk sacs were postfixed with 4% paraformaldehyde and embedded in paraffin wax. Samples were sectioned at 8 μm and counterstained with neutral red.
Acknowledgments
We are grateful to Drs. M. McDevitt and T. Sato for providing vectors containing the GATA-1 regulatory sequences and Tie-2/lacZ cassette, respectively, and Dr. L. Laskey for providing CD34 antibody. We thank Carol Browne and Kerrianne Cunniff for technical assistance. J.V. was the recipient of a Union Internationale Centre de Cancer (UICC)/American Cancer Society Senior Fellowship. S.H.O. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL orkin@rascal.med.harvard.edu; FAX (617) 355-7262.
References
- Baldwin H, Shen H, Yan H, DeLisser H, Chung A, Mickanin C, Trask T, Kirschbaum N, Newman P, Albelda S, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Baumhueter S, Dybdal N, Kyle C, Laskey L. Globular vascular expression of murine CD34, a sialo mucin-like endothelial lingand for L-selectin. Blood. 1994;84:2554–2565. [PubMed] [Google Scholar]
- Begley C, Aplan P, Denning S, Haynes B, Waldmann T, Kirsch I. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci. 1989;86:10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996a;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Müller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996b;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- Chen Q, Cheng J-T, Tsai L-H, Schneider N, Buchanan G, Carroll A, Crist W, Ozanne B, Siciliano MJ, Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990;9:415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont D, Gradwohl JG, Fong G-H, Puri M, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore M. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Finger LR, Kagan J, Christopher G, Kurtzberg J, Hershfield MS, Nowell PC, Croce CM. Involvement of the TCL5 gene on human chromosome 1 in T cell leukemia and melanoma. Proc Natl Acad Sci. 1989;86:5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong G-H, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Green AR, Salvaris E, Begley CG. Erythroid expression of the helix-loop-helix gene SCL. Oncogene. 1991;6:475–479. [PubMed] [Google Scholar]
- Green AR, Lints T, Visvader J, Harvey R, Begley CG. SCL is coexpressed with GATA-1 in haemopoietic cells but is also expressed in developing brain. Oncogene. 1992;7:653–660. [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Hwang L-Y, Siegelman M, Davis L, Oppenheimer-Marks N, Baer R. Expression of the TAL1 proto-oncogene in cultured endothelial cells and blood vessels of the spleen. Oncogene. 1993;8:3043–3046. [PubMed] [Google Scholar]
- Kallianpur AR, Jordan JE, Brandt SJ. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood. 1994;83:1200–1208. [PubMed] [Google Scholar]
- Lindeman GJ, Harris AW, Bath ML, Eisenman RN, Adams JM. Overexpressed max is not oncogenic and attenuates myc-induced lymphoproliferation and lymphomagenesis in transgenic mice. Oncogene. 1995;10:1013–1017. [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc Natl Acad Sci. 1997;94:7976–7981. doi: 10.1073/pnas.94.15.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel J-P, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science. 1997;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- Pardanoud L, Yassine F, Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis, and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Robb L, Lyons I, Li R, Hartley L, Köntgen F, Harvey RP, Metcalf D, Begley CG. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Elwood N, Elefanty AG, Köntgen F, Li R, Barnett D, Begley CG. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 1996;15:4123–4129. [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embyronic and adult transgenic mice. Proc Natl Acad Sci. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger TM, Qin Y, Fujiwara Y, Magram J, Sato TN. Vascular endothelial cell lineage-specific promoter in transgenic mice. Development. 1995;121:1089–1098. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi T, Gertsenstein M, Wu X-F, Breitman M, Schuch AC. Failure of blood-island formation and vasculogenesis in Flk-1 deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer K-D, Schuch AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T cell leukaemia protein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- Silver L, Palis J. Initiation of murine embryonic erythropoiesis: A spatial analysis. Blood. 1997;89:1154–1164. [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Vikkula M, Boon LM, Carraway III KL, Calvert JT, Kiamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- Visvader J, Begley CG, Adams JM. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene. 1991;6:187–194. [PubMed] [Google Scholar]
- Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the ETs-related factor TEL. EMBO J. 1997;16:4374–4383. doi: 10.1093/emboj/16.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MJ, Keller G, Orkin SH. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1− embryonic stem cells. Genes & Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA βgeo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]