Abstract
Finafloxacin is a new fluoroquinolone antibiotic with the unique property of increasing antibacterial activity at pH values lower than neutral. Whereas its antibacterial activity at neutral pH matches that of other quinolones in clinical use, it is expected to surpass this activity in tissues and body fluids acidified by the infection or inflammation processes. Pharmacokinetic parameters of oral single and multiple doses of up to 800 mg of finafloxacin and safety/tolerability observations were assessed in a phase I study including 95 healthy volunteers. Finafloxacin is well absorbed after oral administration, generating maximum concentrations (Cmaxs) in plasma at least comparable to those of other fluoroquinolones, with a half-life of around 10 h. About one-third of the dose is excreted unchanged in the urine. Renal elimination appears to be a saturable process leading to slight increases of the area under the concentration-time curve extrapolated to infinity and dose normalized (AUC∞,norm) at dosages of 400 mg and above. Safety and tolerability data characterize finafloxacin as a drug with a favorable safety profile. In particular, adverse reactions regarded as class-typical of fluoroquinolones, such as, e.g., electrocardiogram (ECG) changes, neurotoxic effects, or hypoglycemia, were not observed in the study population.
INTRODUCTION
Fluoroquinolones in clinical use constitute a class of antibiotics that are both effective and, in general, rather well tolerated (9). Nevertheless, improvements of the safety profile are as desirable as improved antibacterial activity in many clinical situations. Finafloxacin is a new fluoroquinolone that exhibited good antibacterial activity in preclinical studies in vitro and in animal infection models (5, 6, 10, 13) and was also selected as a development candidate because of its low toxicity and good tolerability in preclinical models (12).
Another property that provides finafloxacin with the potential to offer a therapeutic advantage is the pH dependence of its antibacterial activity which, in contrast to that of the older fluoroquinolones, is increased at lower pH, with a maximum around pH 5 to 6, whereas its activity is roughly similar to that of the older fluoroquinolones at neutral pH (10). However, in many infection sites the pH is below neutral, impairing the activity of other fluoroquinolones, whereas that of finafloxacin is expected to increase.
This paper presents pharmacokinetic and safety and tolerability data from the first human exposure study of finafloxacin.
(Part of the research reported in this paper was presented in a poster session at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy-Infectious Diseases Society of America 46th Annual Meeting, Washington, DC, 25 to 28 October 2008 [11]).
MATERIALS AND METHODS
Trial design.
This was a double-blind, placebo-controlled, randomized, single- and multiple-dose escalation study. Healthy adult male and female volunteers received oral single or multiple doses of finafloxacin. They stayed overnight in the phase I unit of Swiss Pharma Contract Ltd. (now Covance Basel Clinical Research Unit), Allschwil, Switzerland, for the duration of treatment. The investigation was approved by the cantonal ethics committee and was carried out with the agreement of the Swiss Medical Authority (SwissMedic; reference number 2007DR1190). It was carried out in accordance with good clinical practice (guideline CPMP/ICH/135/95), from 2 August 2007 to 27 March 2008.
Single-dose escalation (part A) was by oral dose from 25 mg to 50 mg, 100 mg, 200 mg, 400 mg, and 800 mg; multiple-oral-dose escalation (part B) was from 150 mg to 300 mg, 600 mg, and 800 mg, with each dose given once daily (q.d.) for 7 days. Each group (except for three volunteers in part A who received only finafloxacin at the 25-mg dose) consisted of eight volunteers, two of whom received placebo in a double-blinded manner. All volunteers in parts A and B of the study received only one treatment of finafloxacin or placebo. In part C of the study 20 volunteers were included who had tested positive for Helicobacter pylori by a urea breath test. They received 600 mg of finafloxacin orally q.d. for 7 days. Volunteers in parts A and B were not tested for H. pylori infection prior to or after treatment.
The tablet formulation contained 54.6 mg finafloxacin hydrochloride corresponding to 50 mg finafloxacin-free base (betaine). Dosages mentioned in this paper are expressed as the free base. Test drug was provided as tablets containing 50 mg of finafloxacin or a matching placebo. Administration was in the morning under fasting conditions, except for six subjects in part C, who were dosed after a high-fat breakfast. Details of the study parts are given in Table 1.
Table 1.
Demographic data
| Subject group, dose regimen, and dose (mg) | Total no. of subjects | No. of male subjects | No. of female subjects | Mean body weight (kg) | Mean BMI (kg/m2) | Mean age (yr) |
|---|---|---|---|---|---|---|
| Part A, single dose | ||||||
| 25 | 3 | 2 | 1 | 76.4 | 23.8 | 41.7 |
| 50 | 6 | 6 | 0 | 72.7 | 23.5 | 39.3 |
| 100 | 6 | 5 | 1 | 76.4 | 24.2 | 38.3 |
| 200 | 6 | 5 | 1 | 74.1 | 24.4 | 36.7 |
| 400 | 6 | 5 | 1 | 76.3 | 25.1 | 37.8 |
| 800 | 6 | 5 | 1 | 73.3 | 23.7 | 37.0 |
| Placebo, single dose | 10 | 7 | 3 | 72.1 | 23.9 | 44.4 |
| Part B, 7 days q.d. | ||||||
| 150 | 6 | 5 | 1 | 70.0 | 23.5 | 44.0 |
| 300 | 6 | 5 | 1 | 78.4 | 24.4 | 46.8 |
| 600 | 6 | 6 | 0 | 79.1 | 24.3 | 43.7 |
| 800 | 6 | 6 | 0 | 81.2 | 24.8 | 44.8 |
| Placebo, multiple dose | 8 | 7 | 1 | 79.6 | 25.7 | 42.3 |
| Part C, 7 days q.d. | ||||||
| 600 | 20 | 20 | 20 | 75.7 | 24.3 | 41.6 |
A series of blood samples was collected for the measurement of finafloxacin concentrations in plasma and for the determination of blood chemistry and hematology safety parameters. Samples for pharmacokinetics were obtained for part A before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 36, and 48 h after dosing. For part B, samples for pharmacokinetics were obtained as follows: on day 1 before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12 h after dosing; before dosing on days 2, 4, and 6; and on day 7 before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 36, and 48 h after dosing. In part C, plasma samples for pharmacokinetics were obtained before dosing on days 1 and 7 and at 1, 2, 3, 4, 6, and 24 h after dosing on day 7. Due to the use of this abbreviated sampling schedule for part C subjects, only maximum plasma concentrations (Cmaxs) are reported (data not shown). Blood laboratory data were measured at screening, prior to first dosing, and 48 h after completion of dosing in all volunteers. An additional blood laboratory sample was taken before dosing on day 4 for study parts B and C.
Urine was collected in the periods 0 to 2, 2 to 4, 4 to 8, 8 to 12, 12 to 24, and 24 to 48 h after dosing from all volunteers in part A for determination of finafloxacin concentration. Analyses were performed by a validated liquid chromatography-tandem mass spectrometry (LC/MS-MS) method; the lower limit of quantification was 5 μg/liter in plasma and 100 μg/liter in urine. Pharmacokinetic parameters were calculated using noncompartmental analysis. Safety-relevant results of each dosing step cohort were assessed before the next higher dose was used.
Female volunteers were included on the basis of being menopausal for at least 2 years (confirmed by plasma 17β-estradiol and follicle-stimulating hormone [FSH] concentrations) or with documentation of being surgically sterilized.
In study part C only a limited number of blood samples were drawn and analyzed to demonstrate exposure since the primary objective of this part was to gain preliminary information on H. pylori eradication. Consequently, this part of the study is considered in this publication for supporting the phase I safety database only.
Pharmacokinetic analysis.
Pharmacokinetic parameters of finafloxacin were calculated using standard noncompartmental methods using WinNonlin, version 4.1 (Pharsight, Mountain View, CA).
Samples found to be below the limit of quantification were not accounted for during evaluation; i.e., they were treated as if there were no value at this time.
In part A and part B, pharmacokinetic parameters were calculated as follows. Maximum observed plasma concentration (Cmax) and time to reach maximum plasma concentration (tmax) were directly derived from the experimental data. Areas under the plasma concentration-time curve to 24 h after dosing (AUC24h) and to the last quantifiable point (AUC0-tlast) were calculated by the linear trapezoidal rule until tmax and by the log linear trapezoidal rule thereafter. The area under the plasma concentration-versus-time curve extrapolated to infinity (AUC∞) was also calculated. The AUC∞ and Cmaxs were also expressed in dose-normalized terms (AUC∞,norm and Cmax,norm). Time-concentration curves were generated using WinNonlin, version 4.1.
In the subjects in whom a single dose was administered (part A), urine was collected for 48 h after dosing, and the cumulative recovered amounts of finafloxacin (total and as a percentage of dose) were calculated. The average renal clearance (CLR) of finafloxacin was calculated by dividing the absolute amount of finafloxacin recovered in the urine by the plasma AUC0-tlast.
For the subjects treated in part C, a simplified sampling schedule was used, and only Cmax is reported.
Dose proportionality was explored by means of plots of dose-normalized Cmax and AUC∞ versus dose, by comparison using analysis of variance (ANOVA) of dose-normalized Cmax and of CL/F (the inverse of dose-normalized AUC∞ used as a parameter in itself), considered after natural logarithm transformation.
RESULTS
Volunteers.
A total of 95 male and female adult healthy volunteers were included in the study, 18 of whom received placebo tablets. The body weight (BW) of the volunteers, aged 19 to 55 years, was between 59.6 and 97.7 kg, and the body mass index (BMI) ranged from 20.1 to 27.9 kg/m2 (Table 1).
Plasma pharmacokinetics.
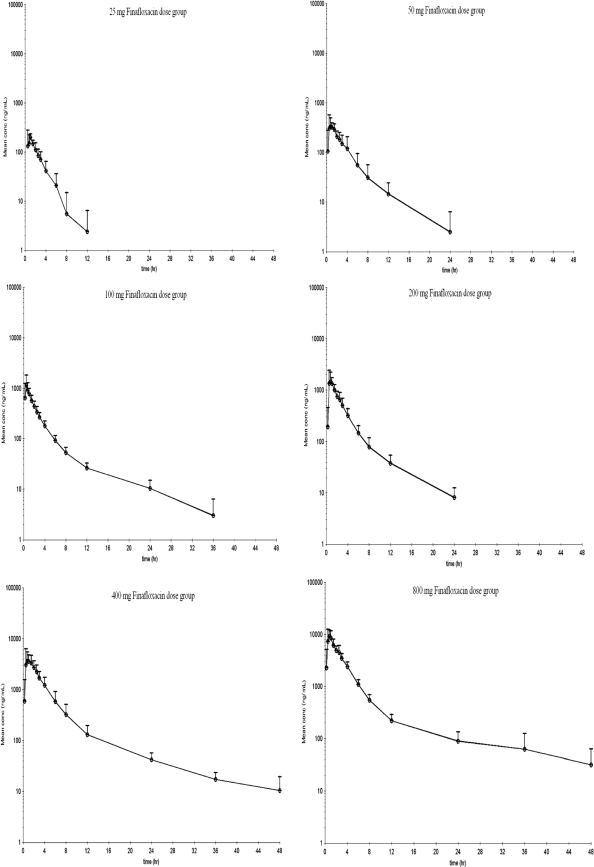
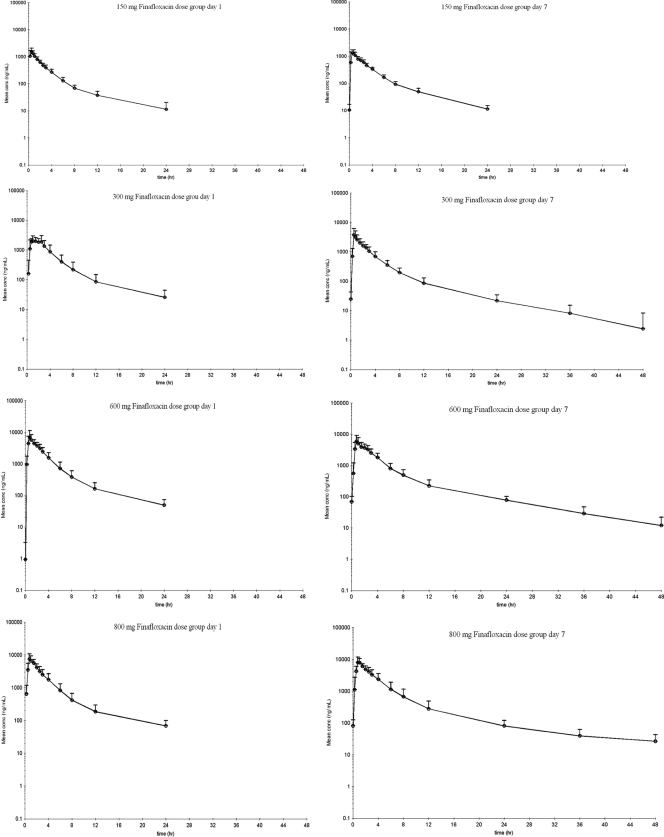
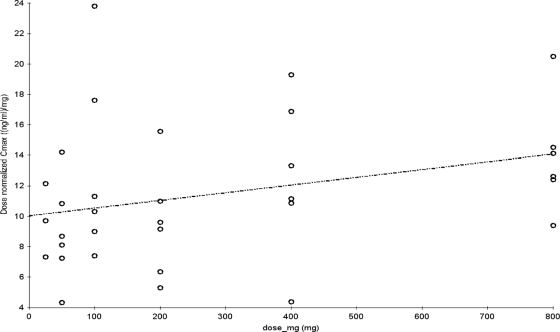
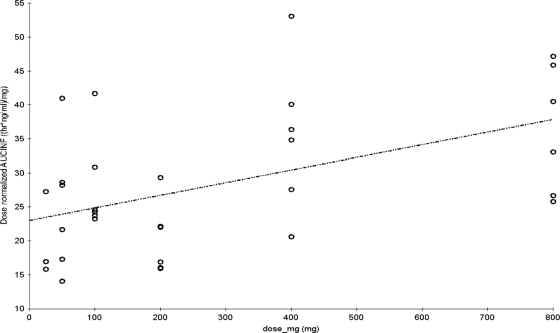
Pharmacokinetic parameters for finafloxacin in plasma are shown in Tables 2 and 3. Concentration-versus-time curves, plotted on a logarithmic concentration scale for each single-dose group (part A), are shown in Fig. 3. Figure 4 shows the concentration-versus-time curves on a logarithmic concentration scale for each of the multiple-dose groups (part B) on day 1 and day 7, after dosing for 7 days q.d. Graphical examination of Cmaxs tended to deviate slightly from dose proportionality over the tested dose range (Fig. 1). However, the dose-normalized Cmaxs would be compatible to a linear increase of Cmax with dose (Tables 2 and 3), and an exploratory (due to the small sample size) comparison by means of ANOVA of the logarithmic transformed dose-normalized Cmaxs among dose levels did not show statistically significant differences for part A (P = 0.2821) or for part B (P = 0.8361). Regarding AUC∞, graphical examination also suggested a deviation from dose proportionality (Fig. 2), and AUC∞,norm tended to increase with increasing doses, while its reciprocal total body clearance (CL/F) tended to decrease with increasing doses. Statistical testing using ANOVA showed significant differences among these parameters between dose levels in subjects who received single doses in part A (P = 0.0075) but not in the subjects who received multiple doses in part B (P = 0.6928).
Table 2.
Plasma pharmacokinetics of finafloxacin in healthy volunteers after single-dose administration (part A)a
| Dose (mg) | No. of subjects | Cmax (mg/liter) | Cmax,norm [(mg/liter)/mg] | AUC∞ (h · mg/liter) | AUC∞,norm [(h · mg/liter)/mg] | tmax (h) | t1/2 (h) | CL/F (liters/h) | Vz/F (liters) |
|---|---|---|---|---|---|---|---|---|---|
| 25 | 3 | 0.24 | 0.0096 | 0.42 | 0.017 | 1.00 | 1.28 | 59.0 | 109 |
| 50 | 6 | 0.44 ± 0.16 | 0.0088 | 1.26 ± 0.48 | 0.025 | 0.88 | 3.8 ± 2.7 | 45.0 ± 17.1 | 220 ± 127 |
| 100 | 6 | 1.32 ± 0.62 | 0.013 | 2.80 ± 0.72 | 0.028 | 0.50 | 7.2 ± 3.2 | 37.2 ± 7.5 | 389 ± 202 |
| 200 | 6 | 1.90 ± 0.73 | 0.0095 | 4.08 ± 1.05 | 0.020 | 0.75 | 4.6 ± 1.9 | 51.5 ± 11.7 | 348 ± 194 |
| 400 | 6 | 5.06 ± 2.09 | 0.013 | 14.2 ± 4.44 | 0.036 | 1.00 | 10.0 ± 4.4 | 30.8 ± 10.4 | 487 ± 363 |
| 800 | 6 | 11.1 ± 2.96 | 0.014 | 29.2 ± 7.50 | 0.036 | 0.88 | 10.5 ± 2.2 | 29.0 ± 7.7 | 435 ± 130 |
Where indicated, values are means ± standard deviations. Cmax, peak plasma concentration; AUC, area under the concentration-time curve; tmax, time to reach Cmax (for this parameter median values are reported); t1/2 terminal half life; CL/F, total body clearance; Vz/F, distribution volume based on the terminal phase; norm, values normalized by the administered dose per kg of body weight; AUC∞, AUC extrapolated to infinite time.
Table 3.
Plasma pharmacokinetics of finafloxacin in six healthy volunteers per dose after multiple-dose administration (part B)a
| Dose (mg) | Day no. | Cmax (mg/liter) | Cmax,norm [(mg/liter)/mg] | AUC24h (h · mg/liter) | AUC24h,norm [(h · mg/liter)/mg] | tmax (h) | t1/2 (h) | CL/F (liters/h) | Vz/F (liters) |
|---|---|---|---|---|---|---|---|---|---|
| 150 | 1 | 1.69 ± 0.38 | 0.011 | 3.76 ± 0.82 | 0.025 | 0.50 | |||
| 150 | 7 | 1.50 ± 0.52 | 0.010 | 4.01 ± 0.64 | 0.027 | 0.50 | 5.3 ± 0.6 | 37.4 ± 5.8 | 282 ± 25 |
| 300 | 1 | 2.96 ± 0.88 | 0.0099 | 8.75 ± 3.50 | 0.029 | 1.50 | |||
| 300 | 7 | 4.15 ± 2.11 | 0.014 | 9.09 ± 3.83 | 0.030 | 0.63 | 6.5 ± 2.5 | 37.4 ± 17.5 | 337 ± 155 |
| 600 | 1 | 7.67 ± 3.42 | 0.013 | 18.7 ± 7.63 | 0.031 | 0.75 | |||
| 600 | 7 | 6.76 ± 2.20 | 0.011 | 19.2 ± 6.40 | 0.032 | 0.88 | 8.8 ± 3.1 | 32.5 ± 10.2 | 409 ± 175 |
| 800 | 1 | 8.68 ± 1.96 | 0.011 | 20.8 ± 7.42 | 0.026 | 0.75 | |||
| 800 | 7 | 8.95 ± 3.11 | 0.011 | 26.1 ± 8.60 | 0.033 | 0.88 | 14.0 ± 5.5 | 31.6 ± 10.6 | 659 ± 467 |
Where indicated, values are means ± standard deviations. AUC24h, area under the plasma concentration-time curve to 24 h after dosing. For the explanation of other symbols, see the footnote to Table 2.
Fig. 3.
Arithmetic mean plus standard deviation of plasma finafloxacin concentration-versus-time profile on a logarithmic concentration scale in part A subjects.
Fig. 4.
Arithmetic mean plus standard deviation of serum finafloxacin concentration-versus-time profile on a logarithmic concentration scale in part B subjects.
Fig. 1.
Dose proportionality of Cmax after single-dose administration of finafloxacin.
Fig. 2.
Dose proportionality of AUC∞ after single-dose administration of finafloxacin.
The absorption of finafloxacin was rapid, with tmax values of 0.25 to 2.50 h and median values clustering in the 0.50- to 1.50-h range among the different dose groups in parts A and B of the study. Geometric mean trough concentrations on day 7 of part B for the 300-, 600-, and 800-mg dose groups were 0.025, 0.063, and 0.073 mg/liter, respectively.
Urine pharmacokinetics.
Finafloxacin concentrations in urine were determined after all single-dose administrations. Data of four highest dose groups are shown in Table 4. Approximately 30% of each dose was recovered in urine as unchanged finafloxacin (Table 5).
Table 4.
Finafloxacin concentrations in urine (part A)
| Collection period (h) | Median finafloxacin concn (mg/liter [range]) at a dose of: |
|||
|---|---|---|---|---|
| 100 mg | 200 mg | 400 mg | 800 mg | |
| 0–2 | 36.4 (16.5–101) | 52.2 (30.6–159) | 85.5 (50.8–134.0) | 89.8 (38.0–242.0) |
| 2–4 | 43.8 (19.3–61.9) | 48.4 (34.9–166) | 83.2 (44.7–328) | 112.5 (62.6–193.0) |
| 4–8 | 14.2 (6.3–36.5) | 22.4 (11.9–91.7) | 80.4 (29.4–207) | 137.6 (43.9–257.0) |
| 8–12 | 4.9 (1.1–6.9) | 10.5 (2.0–31.5) | 26.6 (9.3–63.9) | 22.2 (12.1–87.1) |
| 12–24 | 2.1 (0.7–4.8) | 2.3 (0.9–13.0) | 8.6 (5.0–11.3) | 11.4 (7.5–54.0) |
| 24–48 | 0.8 (0.1–1.1) | 0.7 (0.5–1.6) | 2.1 (1.4–7.5) | 1.9 (0.7–73.8) |
Table 5.
Urine pharmacokinetic parameters of finafloxacin after single-dose administration (part A)
| Dose (mg) | No. of subjects | Recovery in urine (% of dose [range]) | CLR (liters/h)a |
|---|---|---|---|
| 25 | 3 | 27.8 (27.0–29.2) | 17.0 (10.5–19.2) |
| 50 | 6 | 31.9 (25.8–38.1) | 14.2 (10.8–17.5) |
| 100 | 6 | 35.5 (26.8–44.1) | 13.3 (10.1–16.5) |
| 200 | 6 | 32.1 (19.3–44.9) | 15.8 (12.8–18.8) |
| 400 | 6 | 28.5 (20.0–37.0) | 8.6 (4.9–12.2) |
| 800 | 6 | 33.5 (26.1–40.8) | 9.6 (7.5–11.6) |
Median (minimum-maximum) values are reported for the 25-mg dose; mean values (95% confidence limits) are reported for the other dose levels. CLR, renal clearance.
Safety and tolerability.
Adverse events (AEs) observed during the study are summarized in Table 6. No distinction was made between drug-related, study-related, and unrelated AEs from any of the treatment groups. Nervous system (NS) events included mainly headache; gastrointestinal (GI) events included mainly diarrhea, loose stools, nausea, and flatulence; respiratory tract infection (RTI) events included mainly nasopharyngitis and rhinitis.
Table 6.
AEs observed in the study parts
| Subject group and dose (mg) | No. of subjects | No. (%) of subjects with AE | Total no. of AEs | No. of AEs per subject | No. of AEs by typea |
|||
|---|---|---|---|---|---|---|---|---|
| GI | NS | RTI | Other | |||||
| Part A, single dose | ||||||||
| 25 | 3 | 1 (33) | 3 | 0.33 | 1 | 1 | 0 | 1 |
| 50 | 6 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 6 | 2 (33) | 3 | 0.5 | 0 | 1 | 0 | 2 |
| 200 | 6 | 5 (83) | 7 | 1.17 | 1 | 4 | 0 | 2 |
| 400 | 6 | 1 (33) | 2 | 0.33 | 1 | 1 | 0 | 0 |
| 800 | 6 | 5 (83) | 11 | 1.83 | 2 | 1 | 0 | 8 |
| Placebo, single dose | 10 | 3 (33) | 5 | 0.5 | 0 | 3 | 0 | 2 |
| Part B, 7 days q.d. | ||||||||
| 150 | 6 | 4 (67) | 13 | 2.17 | 1 | 3 | 1 | 8 |
| 300 | 6 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| 600 | 6 | 3 (50) | 5 | 0.83 | 3 | 1 | 0 | 1 |
| 800 | 6 | 5 (83) | 10 | 1.67 | 1 | 1 | 1 | 7 |
| Placebo, multiple dose | 8 | 5 (63) | 12 | 1.5 | 1 | 3 | 2 | 6 |
| Part C | ||||||||
| Fasting subjects | 14 | 11 (79) | 16 | 1.14 | 8 | 2 | 1 | 5 |
| Fed subjects | 6 | 4 (67) | 10 | 1.67 | 5 | 2 | 0 | 3 |
| Total group, 7 days q.d., 600 mg | 20 | 15 (75) | 26 | 1.30 | 13 | 4 | 1 | 8 |
| Nonflatulence AE subjects | 11 (55) | 20 | 1.0 | 7 | 4 | 1 | 8 | |
GI, gastrointestinal; NS, nervous system; RTI, respiratory tract infections; Other, cardiovascular, musculoskeletal, eye, skin, local reaction at puncture site, and/or general.
The incidence of the AEs did not differ between the different dose levels or their corresponding placebo groups, with the one exception of GI effects (Table 6). This was mostly due to the higher incidence of gastrointestinal disturbances in the actively treated subjects. Flatulence had a higher incidence in part C than in the other parts of the study.
No AEs were seen in the laboratory data, apart from a few values which were slightly outside the normal range but without clinical relevance. These included a treatment-emergent increase of alanine aminotransferase (ALT) serum levels, which were detected in some subjects at the end-of-study (EOS) visit, which occurred 48 h after receipt of the last dose. The subjects in question included one subject each from the 200-mg and 400-mg dose groups who had received finafloxacin. Both exhibited a slightly increased ALT level, up to 1.33× the upper limit of normal (ULN) at EOS, from an already high value detected on day 1 before dosing. In part B, one subject who received 150 mg of finafloxacin had a treatment-emergent increase of ALT serum levels up to 1.57× ULN at EOS. Another, a subject in the placebo group, exhibited an ALT increase up to 1.71× ULN on day 4 and EOS (and up to 1.82× ULN in a retest on day 4), up from an already high value (1.07× ULN) detected at day 1 before dosing.
In part C, increases of ALT up to 1.5× ULN or up to 3× ULN were seen in two and five subjects, respectively. Two of the part C subjects had elevated initial ALT levels prior to the first dose (1.81× ULN and 3.14× ULN), which decreased toward the normal range during treatment. Of note, slight increases in ALT were not observed in three subsequent clinical trials in which a total of 98 subjects received finafloxacin at 400 mg or above (MerLion Pharmaceuticals GmbH, data on file).
No concomitant increases in bilirubin, alanine aspartate transaminase (AST), or γ-glutamyl transferase (γGT) above the ULN were noted. There are no other data supporting a suspicion that H. pylori infection might influence ALT serum levels during treatment with finafloxacin.
There were no cardiovascular findings indicative of adverse drug reactions and, in particular, no corrected QT (QTc) prolongations in the electrocardiogram (ECG).
DISCUSSION
The value of a new fluoroquinolone is determined by efficacy parameters including (but not limited to) the MIC under the conditions at the site of infection, penetration to the infection site, the convenience of administration, and the safety and tolerability profile, including the adverse drug reactions. In this paper the first administration in humans of the new fluoroquinolone finafloxacin is described, and data on the resultant pharmacokinetics and adverse events are reported, including laboratory values and ECG measurements.
There was no relevant accumulation of finafloxacin following once daily (q.d.) administration of doses up to and including 800 mg (the highest dose tested) over 7 days. Maximal concentrations in plasma may increase slightly more than proportionally with dose, but these observed differences were not statistically significant. The small available sample size calls for caution in the interpretation of such tendencies in these data.
The observed changes of AUC∞,norm and half-life (t1/2) values among dose levels may at least partly be due to restrictions in the ability to accurately calculate these from subjects receiving lower doses. At the lower doses (Fig. 3 and 4) the plasma levels of finafloxacin fall below the limit of quantification at an earlier time point than at higher doses and are therefore no longer considered in the calculations. This can have two consequences: (i) that calculation of t1/2 is at least partially based on data belonging to the initial phases of the profile rather than to the actual terminal phase, deceptively suggesting faster elimination, and (ii) that a larger proportion of the AUC is disregarded. Combined, these factors may decrease the contribution of the extrapolated AUC to the total AUC∞value, leading to an artificially lower AUC∞,norm. There might also be a true effect leading to longer t1/2 at higher dosages as oral clearance, CL/F, tended to be slightly lower at single doses of 400 and 800 mg than at lower doses, suggesting that a saturable process might be involved that decreases clearance at increasing doses.
The urine recovery data in Table 5 suggest that renal clearance may be one of the processes responsible for the apparent reduced clearance and increased AUC∞value at doses of 300 mg and above. No statistical testing was performed, but the 95% confidence intervals do not overlap, which is the equivalent finding to a significant difference in confidence interval terms between the 200-mg and the two higher (400 mg and 800 mg) dose levels, and show limited overlap between the 50-mg and 100-mg and either the 400-mg and 800-mg dose levels (data not shown). Hence, dose-normalized AUC values after dosages below 300 mg q.d. tend to be lower than those after higher doses. This is apparently caused by the slight dose dependency of the renal elimination, which may include a saturable step.
The pharmacokinetic parameters of finafloxacin are grossly in line with those of newer fluoroquinolones in clinical practice (2, 3, 8, 14). A plasma elimination half-life of around 10 h would permit a once-daily dosing regimen.
Due to the nonavailability of a parenteral formulation at the time of the study, the absolute bioavailability of finafloxacin could not be determined. The time course of the plasma levels indicates fast absorption of the orally administered dose.
A comparison with other fluoroquinolones in terms of pharmacodynamic parameters should take into account that MIC values of finafloxacin decrease with lower than neutral pH values, which prevail at most infection sites and with the majority of infectious agents (except, e.g., urease-producing strains). The activity of finafloxacin in in vitro studies reaches its optimum at pH 5 to 6 in contrast to all other fluoroquinolones in clinical practice (9, 10). The Cmaxs of finafloxacin obtained after a single dose of 400 mg and above were in excess of the MICs reported for a number of clinically relevant bacteria including Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus at both neutral and acidic pH (4). The pharmacodynamic parameters that best describe the activity of fluoroquinolones, AUC/MIC and Cmax/MIC, will later be investigated both in vitro and in vivo to further characterize the pharmacokinetic/pharmacodynamic relationship for finafloxacin.
The urinary recovery data shown here indicate that absorption is at least one-third of the oral dose. Since urinary excretion is not uniform throughout the class of fluoroquinolones, no further conclusions can be drawn on the basis of analogy.
Finafloxacin concentrations in urine exceeded MIC values, determined in cation-adjusted Mueller-Hinton broth (pH 7.2 and pH 5.8) and synthetic urine medium (pH 5.8) for many of the causative bacteria of urinary tract infections, including E. coli, P. aeruginosa, and S. aureus, for 24 h after oral doses of 400 to 800 mg (4). Taking into consideration the higher MICs against pathogens in urine than in conventional in vitro test media, Wagenlehner et al. tested finafloxacin in urine and found bactericidal titers for most of the pathogens for at least 24 h after an 800-mg dose of finafloxacin (15).
Finafloxacin has also been shown to exhibit good in vitro activity against resting and slowly growing bacteria, which is relevant to the physiological status of bacteria during infection (7).
These properties suggest that finafloxacin could offer therapeutic benefits in infections of acidic body compartments or when there are indications involving inflammatory processes which create a slightly acidic environment, e.g., in skin infections, intra-abdominal infections, bronchial and lung infections, or urinary tract infections. Activity under low-pH conditions might also be particularly valuable for the use of finafloxacin in regimens to eradicate infections with H. pylori (1).
Safety and tolerability data confirm expectations on the basis of the preclinical results. No relevant differences between treatment and placebo or individual dose groups were seen with regard to the nature, the incidence, or the severity of the adverse events, other than for some gastrointestinal adverse effects, notably flatulence. Flatulence was, in addition, more frequent in the part C subjects, raising the possibility of an association to the H. pylori infection carried by the part C subjects. These observations characterize finafloxacin as a drug with a very favorable safety profile within the fluoroquinolone class.
ACKNOWLEDGMENTS
This work was supported by an unrestricted grant from MerLion Pharmaceuticals GmbH to Covance Clinical Research Unit AG, Basel, Switzerland.
A. Andresen, H.-D. Heilmann, H. Labischinski, H. Patel, A. Vente, and W. Stubbings were employed by or currently work for MerLion Pharmaceuticals GmbH, Berlin, Germany.
Footnotes
This publication is dedicated to Harald Labischinski, who sadly died unexpectedly in August 2010.
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Buissonnière A., et al. 2008. Antimicrobial activity of finafloxacin (FIN) against Helicobacter pylori in vitro and in vivo, poster F1-2038. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA), 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 2. Chien S.-C., et al. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 41:2256–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalhoff A., Schmitz F.-J. 2003. In vitro antibacterial activity and pharmacodynamics of new quinolones. Eur. J. Clin. Microbiol. Infect. Dis. 22:203–221 [DOI] [PubMed] [Google Scholar]
- 4. Dalhoff A., Stubbings W., Schubert S. 2011. Comparative in vitro activities of the novel antibacterial finafloxacin against selected gram-positive and gram-negative bacteria tested in Mueller-Hinton broth and synthetic urine. Antimicrob. Agents Chemother. 55:1814–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endermann R., Ladel C., Stubbings W., Labischinski H. 2008. Pharmacokinetics (PK) and in vivo efficacy of oral finafloxacin (FIN) and comparators in rodent models of systemic infection, poster F1-2044. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 6. Endermann R., Ladel C., Stubbings W., Labischinski H. 2008. In vivo efficacy of finafloxacin in difficult to treat animal models of infection, poster F1-2045. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA), 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 7. Goh C. Y., Goh F., Stubbings W., Kroll H.-P., Labischinski H. 2008. Bactericidal activity of finafloxacin against difficult to kill growth forms of Escherichia coli, poster F1-2042. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA), 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 8. Gonzalez M. A., et al. 1984. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob. Agents Chemother. 26:741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hooper D. C., Rubinstein E. 2003. Quinolone antimicrobial agents, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 10. Kresken M., Körber-Irrgang B., Labischinski H., Stubbings W. 2008. Effect of pH on the in vitro activity of finafloxacin against gram-negative and gram-positive bacteria, poster F1-2037. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA), 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 11. Patel H., et al. 2008. A phase I study to determine safety, tolerability and pharmacokinetics (PK) of finafloxacin (FIN) in healthy subjects, poster F1-2048. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 12. Schmuck G., Wasinska-Kempka G., Vente A., Labischinski H. 2008. In vitro toxicological profiling of finafloxacin, poster F1-2047. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA), 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 13. Schubert S., Stubbings W., Dalhoff A. 2008. Comparative inhibitory and bactericidal activities of finafloxacin and ciprofloxacin against gram-negative and gram-positive UTI-pathogens under physiological conditions and at varying pH-values. Poster F1-2041. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA), 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 14. Stass H., Dalhoff A., Kubitza D., Schühly U. 1998. Pharmacokinetics, safety and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 42:2060–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagenlehner F. M., et al. 2011. Urinary pharmacokinetics and bactericidal activity of finafloxacin (200 and 800 mg) in healthy volunteers receiving a single oral dose. Chemotherapy 57:97–107 [DOI] [PubMed] [Google Scholar]