Abstract
Staphylococcus aureus strains (n = 50) causing complicated skin and skin structure infections produced various levels of phenol-soluble modulin alpha-type (PSMα) peptides; some produced more than twice that produced by the control strain (LAC USA300). TR-700 (oxazolidinone) and clindamycin strongly inhibited PSM production at one-half the MIC but exhibited weak to modest induction at one-fourth and one-eighth the MICs, primarily in low producers. Adequate dosing of these agents is emphasized to minimize the potential for paradoxical induction of virulence.
TEXT
Secreted exotoxins such as toxic shock syndrome toxin 1 (TSST-1), α-hemolysin (Hla), and Panton-Valentine leukocidin (PVL) have been shown to contribute in part to the virulence of Staphylococcus aureus (1, 2, 14). The phenol-soluble modulin alpha-type (PSMα) peptides (1–4) are one of the most recently discovered peptides that have been implicated in the pathogenesis of community-associated methicillin-resistant S. aureus (CA-MRSA) complicated skin and skin structure infections (cSSSIs), bacteremia, and pneumonia (2, 16). Like the PVL toxin, the PSM peptides target primarily neutrophils, leading to pore formation and inflammatory mediator release. PSMα3 is the most cytolytic, with 3 to 10 μg/ml shown to cause 25 to 60% lysis of human neutrophils (5). PSMα peptides are secreted as both nonformylated and formylated forms, with the latter at a significantly higher quantity and greater cytotoxicity (16). Previous studies have shown that protein synthesis inhibitors at sub-MICs inhibit the production of Hla, PVL, TSST-1, and other virulence factors in the laboratory and a few clinical S. aureus strains (3, 4, 8, 11). We sought to investigate the effects of subinhibitory concentrations of the protein synthesis-inhibiting antibiotics, clindamycin and a second-generation oxazolidinone, TR-700, on PSM production.
(This study was presented at the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [abstract B-709], Boston, MA, 12 to 15 September 2010.)
We examined baseline production of PSMα1 to -4 in 50 PVL-positive methicillin-susceptible S. aureus (MSSA) and MRSA clinical isolates causing cSSSIs. Quantitation of formyl-PSMα peptides after 24 h of incubation was performed in duplicate by liquid chromatography-tandem mass spectrometry (LC-MS-MS). To assess the effect of antibiotics on PSM production in selected clinical isolates and the LAC (USA300) control strain, we used a modified CLSI broth macrodilution to determine the MIC, in which tryptic soy broth was used as the media and samples were shaken at 250 rpm for 24 h at 37°C. MIC50/90 values for TR-700 and clindamycin were 0.25/0.375 and 0.125/0.188 μg/ml, respectively. Supernatants were harvested after incubation with study drugs at one-half, one-fourth, and one-eighth the MICs for PSM quantitation and global regulator (agrA and RNAIII) expression analysis by reverse transcription-PCR (RT-PCR) (normalized to expression of gyrB).
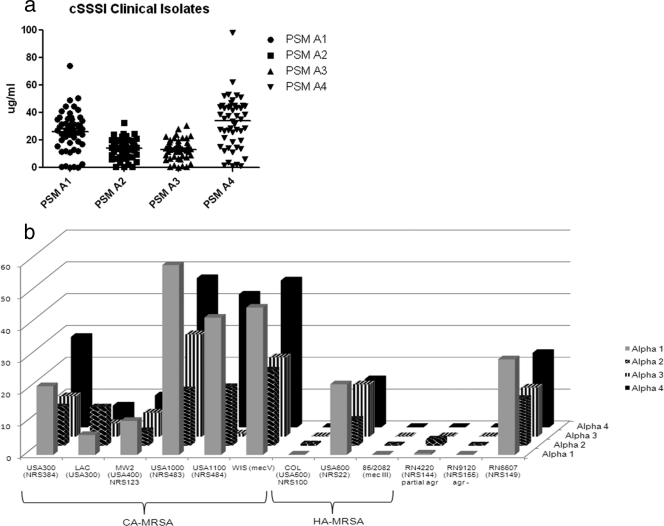
Baseline PSMα production among 50 clinical isolates varied from 0.22 to 98.24 μg/ml (Fig. 1). Some (14%) isolates produced more than twice the amount of PSMα1 to -4 peptides produced by the LAC strain. PSMα production did not differ by methicillin resistance, type of cSSSIs (cellulitis with or without abscess), or size of the abscess (>5 or ≤5 cm) caused by these strains.
Fig. 1.
(a and b) Baseline PSMα1 to -4 production in clinical isolates and reference strains. (a) Clinical isolates that caused cSSSIs (n = 50). Horizontal lines represent median values for each PSMα peptide. The interquartile ranges (IQRs) for PSMα1 to -4 were 16.6 to 35.3, 7.8 to 19.4, 8.5 to 17.5, and 17.5 to 44.6 μg/ml, respectively. (b) Staphylococcus aureus control strains. Baseline PSM production of CA, hospital-associated (HA), and laboratory strains of S. aureus was measured after 24 h of incubation at 37 °C and shaking at 200 rpm. Laboratory strain NRS155 is the isogenic agr knockout of NRS149, and NRS144 has a partial agr defect.
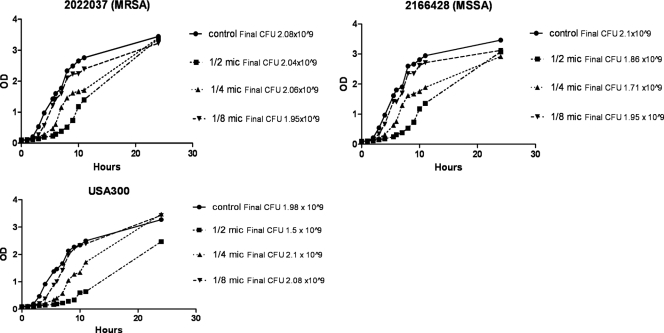
Clinical strains were grouped by baseline PSM production to select for representative high, medium, and low producers for studying the effect of subinhibitory TR-700 and clindamycin on PSM production. Experiments on growth kinetics with or without drugs were performed on the LAC strain and two clinical strains (MRSA and MSSA) (Fig. 2). At one-half the MICs of both drugs, growth was delayed, with a lower final cell count in LAC but to a lesser degree in the clinical isolates selected, whereas minimal to no effect on growth was observed at one-fourth and one-eighth the MICs for both agents. Measured PSMα concentrations were normalized to the number of CFU at the time of harvest to account for differences in growth and cell counts.
Fig. 2.
Growth curves of two clinical isolates (MRSA, MSSA) and the LAC (USA300) control strain at sub-MICs of TR-700. Growth curves with clindamycin were similar (data not shown). Optical densities (ODs) were read every hour for 10 hours, and a final OD reading was taken at 24 hours.
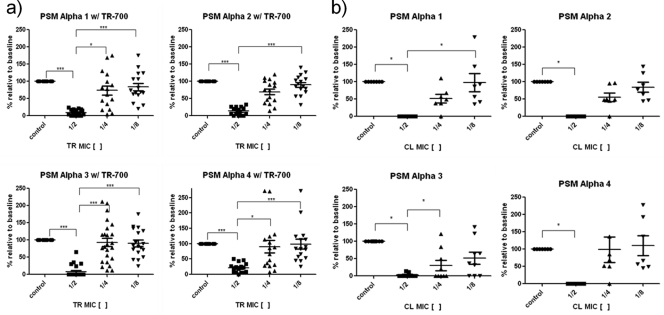
Overall, 21 clinical isolates and the LAC strain were tested. TR-700 at one-half the MIC had a pronounced inhibitory effect on PSM production in a dose-dependent manner, though the effect varied for all four alpha subtypes (Fig. 3a). PSMα3 was the most inhibited, in which nearly all isolates tested produced no measurable amounts, while PSMα4 was the least inhibited, with production decreasing to a median of 21% of the baseline value. Interestingly, paradoxical induction of PSMα was observed for TR-700 at one-fourth and one-eighth the MICs affecting primarily strains with low baseline production of PSMα3. The highest level of PSMα3 induced with TR-700 was 4.6 μg/ml from 2.5 μg/ml. Similarly, in the LAC control strain, another low baseline producer of PSMα3, TR-700 at one-half the MIC significantly inhibited PSM production to 30% of the baseline, while one-fourth and one-eighth the MICs increased PSM production by 40% and 45%, from 3.9 μg/ml to 5.43 and 5.63 μg/ml, respectively.
Fig. 3.
(a and b) Effect of subinhibitory concentrations of TR-700 (TR) (a) and clindamycin (CL) (b) on PSM production. Data represent the medians with IQRs. One-way analysis of variance (ANOVA) with Dunnett's posttest was used for statistical analysis. Note that an increase in PSM production from the baseline at one-fourth and one-eighth the MICs of TR-700 and clindamycin occurred primarily in low PSM producers. ***, P < 0.0001; *, P < 0.007.
Compared to TR-700, clindamycin had a stronger inhibitory effect on PSM production overall in a subset of the above-mentioned isolates (n = 7) (Fig. 3b). Complete inhibition of PSMα1 to -4 was observed at one-half the MIC in all but two strains. Against PSMα3, one-fourth the MIC completely inhibited production in 5 of 7 clinical isolates. Like TR-700, PSM production was weakly induced above the baseline level at one-fourth and one-eighth the MICs in two clinical isolates and the LAC strain. Others have documented in the LAC strain that both clindamycin (at 67% of the MIC) and linezolid (at 10% of the MIC) stimulated PSM production by 3.5 and 1.5 times above the baseline, respectively (6).
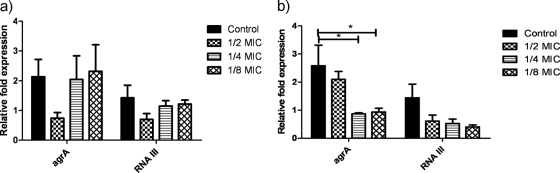
Like PVL and Hla, PSMs are under strict control of agrA and RNAIII of the agr system (9, 10, 16). Others have examined the effect of antibiotics on RNAIII expression after 8 h during postexponential growth phase when the agr system would be maximally expressed (6). We extended the previous investigation to determine whether the effects persist after overnight incubation. Expression of agrA and RNAIII appears to follow the direction of PSM inhibition at sub-MICs of TR-700, even after 24 h (Fig. 4). While clindamycin inhibited both agrA and RNAIII, inhibition was less at one-half the MIC than one-eighth the MIC, suggesting differential response of these regulators, or perhaps the inhibitory effect of clindamycin on agrA is less sustained over time than that of TR-700.
Fig. 4.
(a and b) Effect of subinhibitory concentrations of TR-700 (a) and clindamycin (b) on expression of agrA and RNAIII. n = 5 isolates. Expression is normalized to that of gyrB. *, P = 0.017.
Our study had limitations. First, we chose subinhibitory concentrations of antibiotics that would likely be encountered in the clinical setting due to improper dosing or other parameters impacting drug levels at the site of infection. However, growth was delayed in some strains at one-half the MIC. We normalized measured PSMα levels to the number of CFU to account for possible cell count differences, recognizing that this may not completely account for alteration in growth patterns and, in turn, PSM production. Second, we did not obtain samples at earlier time points during growth, which could better characterize responses of global regulators. Finally, the in vitro effects of subinhibitory TR-700 and clindamycin will need in vivo confirmation to clarify their clinical relevance (13).
Taken together, our findings indicated that S. aureus strains causing cSSSIs produced various amounts of PSMα1 to -4. Our study results support the antivirulence potential of protein synthesis-inhibiting antibiotics in decreasing PSM production, especially at one-half the MIC, consistent with the published literature on other exotoxins (3, 4, 12, 15). However, it is notable that these antibiotics can cause paradoxical effects on PSM production, albeit a weak to moderate induction predominantly in low PSM producers. Our results underscore the importance of adequate dosing of these agents in order to minimize the potential for paradoxical induction of virulence.
Acknowledgments
This study was supported by a research grant from Trius Therapeutics.
We thank Bixin Xi for his technical assistance in the development and optimization of the mass spectrometry assay for PSM measurement. We also thank the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for providing the control strains used.
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Bubeck Wardenburg J., Bae T., Otto M., Deleo F. R., Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405–1406 [DOI] [PubMed] [Google Scholar]
- 2. Diep B. A., et al. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U. S. A. 107:5587–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dumitrescu O., et al. 2008. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clin. Microbiol. Infect. 14:384–388 [DOI] [PubMed] [Google Scholar]
- 4. Herbert S., Barry P., Novick R. P. 2001. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect. Immun. 69:2996–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hongo I., et al. 2009. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J. Infect. Dis. 200:715–723 [DOI] [PubMed] [Google Scholar]
- 6. Joo H. S., Chan J. L., Cheung G. Y., Otto M. 2010. Subinhibitory concentrations of protein synthesis-inhibiting antibiotics promote increased expression of the agr virulence regulator and production of phenol-soluble modulin cytolysins in community-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:4942–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. Koszczol C., Bernardo K., Kronke M., Krut O. 2006. Subinhibitory quinupristin/dalfopristin attenuates virulence of Staphylococcus aureus. J. Antimicrob. Chemother. 58:564–574 [DOI] [PubMed] [Google Scholar]
- 9. Li M., et al. 2007. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66:1136–1147 [DOI] [PubMed] [Google Scholar]
- 10. Li M., et al. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novick R. P., Jiang D. R. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709–2717 [DOI] [PubMed] [Google Scholar]
- 12. Ohlsen K., et al. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 42:2817–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pragman A. A., Schlievert P. M. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42:147–154 [DOI] [PubMed] [Google Scholar]
- 14. Sloane R., et al. 1991. A toxic shock syndrome toxin mutant of Staphylococcus aureus isolated by allelic replacement lacks virulence in a rabbit uterine model. FEMS Microbiol. Lett. 78:239–244 [DOI] [PubMed] [Google Scholar]
- 15. Stevens D. L., et al. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 195:202–211 [DOI] [PubMed] [Google Scholar]
- 16. Wang R., et al. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 [DOI] [PubMed] [Google Scholar]