Abstract
Micafungin alone and combined with liposomal amphotericin B was evaluated against two strains of Histoplasma capsulatum. Micafungin was active in vitro against the mold but not the yeast form but was ineffective in vivo. Micafungin appears to be ineffective in treatment of histoplasmosis.
TEXT
Histoplasmosis is a life-threatening infection in patients with weakened immunity. While lipid formulations of amphotericin B and itraconazole are the preferred treatments, occasionally other agents are needed. In vitro and animal studies showed conflicting results using echinocandins in the treatment of histoplasmosis (4, 8, 11, 17). Caspofungin demonstrated limited efficacy in a pulmonary model (11) but was effective in an intravenous murine model of histoplasmosis (8). The reasons for the conflicting findings are unknown but may include the use of different Histoplasma isolates, routes of infection, or mouse strains (12). The objective of this study was to examine the efficacy of micafungin (MFG) compared to that of lipo-somal amphotericin B (L-AMB) against the two strains of Histoplasma capsulatum used in the conflicting studies.
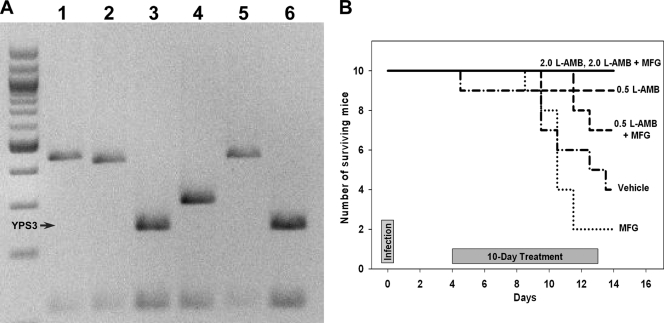
The H. capsulatum isolates were IU-CT (3, 11) and 94-255 (8). Genotyping using YPS3 (1, 9) showed that the 94-255 isolate did not express YPS3 and, thus, was a class 1 strain, whereas the IU-CT isolate expressed YPS3, which is characteristic for class 2 (Fig. 1A), the predominant type in North America (20).
Fig. 1.
(A) Genotyping of H. capsulatum isolates based on HaeIII-generated restriction fragment length polymorphism (RFLP) of the YPS3 gene. The first lane is a 100-bp ladder; lane 1, class 1 isolate (Downs); lane 2, class 1 isolate (UCLA531S); lane 3, class 2 isolate (G217B); lane 4, class 3 isolate (G186AS); lane 5, 94-255 isolate; lane 6, IU-CT isolate. (B) Survival of mice infected with H. capsulatum class 2 at 1 × 107 yeasts intranasally. 2.0, 0.5, dose in mg/kg.
The MICs or minimal effective concentrations (MECs) of the two strains were compared. The MIC for the yeast form was determined as the dilution at which there was no visible growth (6, 11, 16, 19), and the MEC for the mold was the lowest concentration of drug causing growth of small, rounded, compact hyphal forms, compared to the results in control wells (5). The fractional inhibitory concentration index (FICI) was determined using the checkerboard method to classify drug interaction between MFG and L-AMB, as follows: synergy (FICI ≤ 0.5), antagonism (FICI > 4.0), and no interaction (FICI > 0.5 to 4.0) (14).
L-AMB was highly effective in vitro against the yeast and mold forms of both strains, whereas MFG was effective only against the mold forms. L-AMB and MFG displayed no interaction with either the mold or yeast forms (Table 1), consistent with earlier reports (11, 13).
Table 1.
In vitro susceptibilities of yeast and mold forms of both strains of H. capsulatum to liposomal amphotericin B and micafungin
| H. capsulatum form and class | MIC of L-AMB (μg/ml) | MEC or MIC of MFG (μg/ml) | FIC index for L-AMB + MFG |
|---|---|---|---|
| Mold | |||
| 1 | 0.25 | 0.06 | 0.75 |
| 2 | 0.25 | 0.06 | 0.75 |
| Yeast | |||
| 1 | 0.25 | >64 | 0.62 |
| 2 | 0.125 | >64 | 0.62 |
Six-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were infected intranasally with 1 × 107 yeasts, based on preliminary experiments using the IU-CT class 2 isolate (1 × 107 to 2 × 107 yeasts caused 80 to 100% mortality). All experiments were performed twice to confirm reproducibility, and treatment groups contained 9 or 10 mice. Treatment was started on day 4 of infection and continued for 10 days. The groups were as follows: group 1, L-AMB at 0.5 mg/kg of body weight intraperitoneally (i.p.) every other day (q.o.d.); group 2, L-AMB at 2.0 mg/kg i.p. q.o.d.; group 3, MFG at 10 mg/kg i.p. once daily (q.d.); group 4, L-AMB at 0.5 mg/kg i.p. q.o.d. and MFG at 10 mg/kg i.p. q.d.; group 5, L-AMB at 2.0 mg/kg i.p. q.o.d. and MFG at 10 mg/kg i.p. q.d.; and group 6, vehicle control (5% dextrose-water solution) i.p. q.d. Twenty-four hours after the last antifungal injection, mice were sacrificed, and lungs and spleens were removed aseptically and weighed. Quantitative culture of organ homogenates to measure fungal burden was expressed as CFU per gram of tissue.
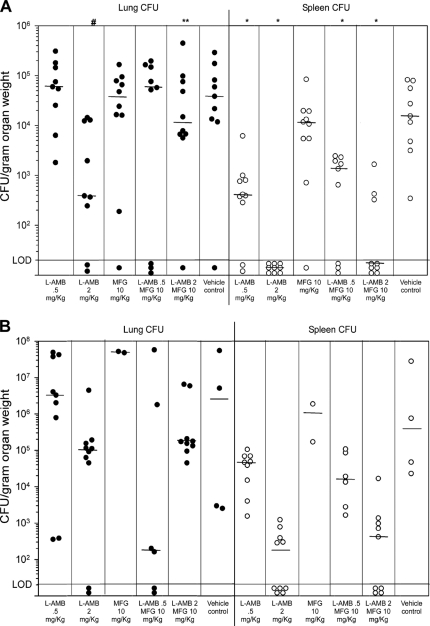
All mice infected with the 94-255 class 1 isolate survived to day 15, including those treated with the vehicle control. In preliminary experiments, mice survived infection with up to 1 × 108 class 1 yeasts, prohibiting analysis of mortality. Compared to that in vehicle-treated mice, the lung fungal burden was lower only in mice treated with L-AMB at 2.0 mg/kg alone (P = 0.013). Mice treated with 2 mg/kg L-AMB had lower lung fungal burdens than those receiving the combination of L-AMB at 2.0 mg/kg and MFG at 10 mg/kg (P = 0.045), suggesting in vivo antagonism (Fig. 2A). The spleen fungal burden was lower in all groups (P < 0.05) except for MFG alone (P = 0.54).
Fig. 2.
Quantitative culture results for lungs (filled circles) and spleens (open circles) from mice that were sacrificed 14 days after intranasal infection with 107 IU-CT (A) and 94-255 (B) yeasts. Each data point represents the result for one animal. Horizontal bars represent the medians of the corresponding data columns. Lower limit of detection (LOD) is 20 CFU/g of organ tissue. P values using paired comparisons (Mann-Whitney test), after adjustment by step-down Bonferroni multiple-comparison procedure, are listed above the columns as follows: #, P = 0.013 compared to vehicle-treated mice; **, P = 0.045 compared to mice treated with L-AMB at 2.0 mg/kg; *, P < 0.005 compared to vehicle-treated mice.
Following infection with the IU-CT class 2 isolate, only 2 mice (20%) receiving MFG and 4 (40%) vehicle control mice survived, compared to all 10 receiving L-AMB at 2 mg/kg, 9 (90%) receiving L-AMB, 9 (90%) receiving L-AMB at 2.0 mg/kg with MFG, 9 (90%) receiving L-AMB at 0.5 mg/kg alone, and 7 (70%) receiving L-AMB at 0.5 mg/kg with MFG (Fig. 1B). Cox proportional hazards regression analysis showed treatment with MFG to be associated with reduced survival (P < 0.0001). L-AMB at 2.0 mg/kg alone or combined with MFG appeared to reduce the fungal burden in the lung and spleen, but too few vehicle control mice survived for statistical analysis (Fig. 2B). YPS3 was expressed in the IU-CT class 2 strain, perhaps contributing to the higher mortality, as YPS3 is a virulence factor associated with extrapulmonary dissemination (2, 10).
Since echinocandins exert their antifungal activity by inhibiting 1,3 beta-d-glucan (BG) synthase (7), in vitro activity against the mold but not the yeast might be related to the difference in the glucan composition between the two growth forms. The cell walls of the mold contain mainly beta-1,3 glucan, but the yeast form contains predominantly alpha-glucan (13, 15, 18). The BG assay was performed by Associates of Cape Cod, Inc. (East Falmouth, MA), on pooled mouse serum samples obtained on day 15 after infection and on culture supernatant from the yeast of both isolates using the same methods as for the in vitro susceptibility testing. BG was not detected in pooled serum from mice infected with either strain and treated with either L-AMB or MFG or from uninfected control mice. Similarly, in vitro, BG was not detected in the yeast culture supernatant that contained either L-AMB or MFG but was detected in control wells (Table 2).
Table 2.
Beta glucan concentrations in infected mouse serum and in vitro yeast culture supernatants
| Specimen and treatment | Concn of beta glucan (pg/ml) |
||
|---|---|---|---|
| Class 1 H. capsulatum | Class 2 H. capsulatum | Uninfected or uninoculated control | |
| Infected mouse serum, day 15 of infection | <31 | ||
| L-AMB at 2.0 mg/kg q.o.d. | <31 | <31 | |
| MFG at 10 mg/kg q.d. | <31 | <31 | |
| No-drug controls | 125 | Interference, no result | |
| In vitro culture supernatant (yeast form) | <31 | ||
| L-AMB at 2.0 mg/kg q.o.d. | <31 | <31 | |
| MFG at 10 mg/kg q.d. | <31 | <31 | |
| No-drug controls | 104 | >500 | |
In conclusion, while L-AMB was highly effective in vitro in both yeast and mold forms, MFG was effective only against the mold in vitro and was not effective in vivo with either strain of H. capsulatum. No in vitro interaction was detected between L-AMB and MFG. The echinocandins appear to be ineffective in the treatment of histoplasmosis.
Acknowledgments
This work was partly supported by a VA Career Development Award (CDA-2) to C.A.H. and an unrestricted grant by Astellas Pharma Global Development, Inc., to L.J.W. Part of this work was supported by a University of Texas Pan-American Faculty Research Council Award (FRC Molecular Strategy 135BIOL07 to R.Z.).
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Bohse M. L., Woods J. P. 2007. Expression and interstrain variability of the YPS3 gene of Histoplasma capsulatum. Eukaryot. Cell 6:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohse M. L., Woods J. P. 2007. RNA interference-mediated silencing of the YPS3 gene of Histoplasma capsulatum reveals virulence defects. Infect. Immun. 75:2811–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connolly P., et al. 2000. Comparison of a new triazole, posaconazole, with itraconazole and amphotericin B for treatment of histoplasmosis following pulmonary challenge in immunocompromised mice. Antimicrob. Agents Chemother. 44:2604–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Espinel-Ingroff A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Espinel-Ingroff A., et al. 2005. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M38-A document) for amphotericin B, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 43:5243–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fothergill A. W. 2007. Update: antifungal susceptibility testing, p. 13 Presented at Focus on Fungal Infections 17, San Diego, CA, March 7 to 9, 2007 [Google Scholar]
- 7. Georgopapadakou N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269–280 [DOI] [PubMed] [Google Scholar]
- 8. Graybill J. R., et al. 1998. Treatment of histoplasmosis with MK-991 (L-743,872). Antimicrob. Agents Chemother. 42:151–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutierrez M. E., Canton A., Connolly P., Zarnowski R., Wheat L. J. 2008. Detection of Histoplasma capsulatum antigen in Panamanian patients with disseminated histoplasmosis and AIDS. Clin. Vaccine Immunol. 15:681–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holbrook E. D., Rappleye C. A. 2008. Histoplasma capsulatum pathogenesis: making a lifestyle switch. Curr. Opin. Microbiol. 11:318–324 [DOI] [PubMed] [Google Scholar]
- 11. Kohler S., et al. 2000. Comparison of the echinocandin caspofungin with amphotericin B for treatment of histoplasmosis following pulmonary challenge in a murine model. Antimicrob. Agents Chemother. 44:1850–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayfield J. A., Rine J. 2007. The genetic basis of variation in susceptibility to infection with Histoplasma capsulatum in the mouse. Genes Immun. 8:468–474 [DOI] [PubMed] [Google Scholar]
- 13. Nakai T., et al. 2003. In vitro antifungal activity of micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odds F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 15. Rappleye C. A., Engle J. T., Goldman W. E. 2004. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol. Microbiol. 53:153–165 [DOI] [PubMed] [Google Scholar]
- 16. Rex J. H., et al. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 17. Rodriguez-Arellanes G., et al. 2009. Combined therapy with amphotericin B and caspofungin in an experimental model of disseminated histoplasmosis. Rev. Invest. Clin. 61:4–10 [PubMed] [Google Scholar]
- 18. Rodriguez-Brito S., Nino-Vega G., San-Blas G. 2010. Caspofungin affects growth of Paracoccidioides brasiliensis in both morphological phases. Antimicrob. Agents Chemother. 54:5391–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waitz J. A., et al. 1997. Reference method of broth dilution antifungal susceptibility testing of yeasts; approved standard. M27-A. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 20. Zarnowski R., Connolly P. A., Wheat L. J., Woods J. P. 2007. Production of extracellular proteolytic activity by Histoplasma capsulatum grown in Histoplasma-macrophage medium is limited to restriction fragment length polymorphism class 1 isolates. Diagn. Microbiol. Infect. Dis. 59:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]