Abstract
Antibiotic lock therapy (ALT) is an adjunctive procedure to prevent or treat central venous catheter infections, ensuing catheter-related bacteremia, and catheter-related metastatic infections. Daptomycin is a cyclic lipopeptide that is rapidly bactericidal against methicillin-susceptible and -resistant Staphylococcus aureus. The efficacies of daptomycin against central venous catheter biofilms, catheter-related bacteremia, and catheter-related metastatic infections were evaluated by adapting a previously reported central venous catheter biofilm model in rats. Combined daptomycin ALT and systemic dosing resulted in the clearance of an established in vivo S. aureus central venous catheter biofilm after just two daily ALT treatments (30 min with daptomycin at 5 mg/ml) with concurrent systemic daptomycin dosing (40 mg/kg of body weight/day subcutaneously [s.c.]; equivalent exposure of 6 mg/kg/day in people). Daptomycin ALT solutions formulated in either saline or lactated Ringer's solution were equally fast in eradicating established in vivo methicillin-resistant Staphylococcus epidermidis (MRSE) central venous catheter biofilms. However, the lactated Ringer's formulation was superior to that of saline in sustaining the bacterial clearance of treated central venous catheters (83% versus 50%). In MRSE-infected central venous catheter studies, 3 days of daptomycin or vancomycin ALT (18 h at 5 mg/ml) with systemic s.c. dosing (40 mg/kg/day daptomycin or 100 mg/kg/day vancomycin) was equally effective 1 week posttherapy in maintaining cleared central venous catheters (90% [n = 10] versus 100% [n = 8]). These results suggest that daptomycin ALT, along with systemic dosing, could be an effective treatment option for the prevention or eradication of staphylococcal central venous catheter biofilm infections, thereby reducing the occurrence of catheter-related bacteremia or catheter-related metastatic infections.
INTRODUCTION
Central venous catheters are essential for patients receiving hemodialysis, chemotherapy, or parenteral nutrition. More than 5 million central venous catheters are placed annually in the United States alone (30). Data from 599 U.S. facilities representing 16,225,498 patient days in an intensive care unit (ICU) showed that 33,587 catheter-related bacteremias occurred during 8,628,109 catheter days from 1997 to 2007, demonstrating 1 catheter-related bacteremia every 257 catheter days (5). Among critically ill patients developing catheter-related bacteremia, mortality rates are as high as 35%, while survivors incur increased hospital costs of at least $40,000 and increased time spent in the ICU of 8 to 20 days (13, 36).
Cases of nosocomial catheter-related bacteremia are caused most frequently by catheters infected with persistent biofilm populations of Staphylococcus epidermidis or Staphylococcus aureus (17, 39). Bacterial central venous catheter biofilms occur when planktonic bacteria irreversibly adhere to the submerged surface of the catheter and create an external polysaccharide matrix (“slime layer”) structure (21, 33). Antibiotic MICs for stationary-phase bacteria embedded in such biofilms may be 100- to 1,000-fold greater than those for planktonic cells (30). The management of a central venous catheter infection usually includes catheter removal and the initiation of appropriate systemic antibiotic coverage.
Antibiotic lock therapy (ALT) is an alternative approach that has been investigated (1, 2), particularly for patients with central venous catheters colonized by coagulase-negative staphylococci (14, 43, 47). With the primary goal of the prevention or resolution of catheter-related bacteremia, ALT involves instilling an antibiotic solution (often combined with heparin) into the hub of the central venous catheter when it is not in use to achieve a concentration of the antibiotic that is manyfold higher than the MIC for the bacteria. The antibiotic solution is allowed to remain for a specified length of time, generally 18 to 24 h (3, 30). ALT is also intended to minimize the need for systemic antibiotics by preventing the colonization of the catheter or eradicating organisms from an infected catheter as well as to prolong the life of the catheter in critically ill patients, for whom reintroducing a catheter may be difficult and for whom catheters are often the only means available for administering nutrients and/or therapeutics (2, 22). ALT has been used successfully for patients receiving long-term parenteral nutrition or chemotherapy as well as for patients requiring dialysis or those infected with human immunodeficiency virus (11, 16, 23, 25, 26, 31). A systematic review and meta-analysis of 11 randomized, controlled ALT clinical trials showed a significant reduction in the incidence of catheter-related bacteremia as well as the rate at which central venous catheters were removed because of infection (49). However, ALT is associated with antibiotic leakage into the systemic circulation and the development of resistance as well as systemic side effects and compatibility issues (3).
In this study, we characterize an optimized rat model of central venous catheter infection, originally described by Ulphani and Rupp (45), caused by methicillin-resistant S. epidermidis (MRSE) and methicillin-susceptible S. aureus (MSSA). Daptomycin is equally active against methicillin-susceptible and methicillin-resistant S. aureus strains both in vitro (35) and in vivo (32). We used the model to examine the efficacy of daptomycin administered by ALT and parenterally against catheter infections and related secondary infections of the blood and organs. Daptomycin was chosen because it has broad, rapid in vitro and in vivo bactericidal activities against staphylococci (24, 32, 35), including biofilm-producing strains; retains bactericidal activity against nondividing bacteria (29); and has been shown to rapidly penetrate S. epidermidis biofilms (44).
MATERIALS AND METHODS
Bacterial test strains.
Strains of S. epidermidis (ATCC 35984, ATCC 49461, and ATCC 700565 [all MRSE]; American Type Culture Collection) and S. aureus (ATCC 25923 [MSSA]) were evaluated. S. epidermidis strain ATCC 35984 is a biofilm producer reference strain (7, 27). In a preliminary biofilm production evaluation, the three additional staphylococcal strains produced biofilms equivalent to that of ATCC 35984 (data not shown).
Antibiotic susceptibility testing.
Susceptibilities to daptomycin and vancomycin were evaluated by a standard broth microdilution method according to Clinical and Laboratory Standards Institute guidelines (8). In accordance with those guidelines, daptomycin susceptibility was determined by using Mueller-Hinton broth adjusted to contain physiological levels of calcium (50 mg/liter).
Culture media and growth conditions.
All cultures used to inoculate central venous catheters were started from glycerol stocks. Planktonic populations were prepared by the subculturing of cultures grown overnight in fresh media and incubation for 2.5 h at 37°C before central venous catheters were inoculated. MSSA cultures were grown in tryptic soy broth (Sigma, St. Louis, MO), and MRSE cultures were grown in Mueller-Hinton broth (Becton Dickinson, Sparks, MD).
Antibacterial agents and vehicles.
Daptomycin powder was provided by Cubist Pharmaceuticals, Inc. (Lexington, MA). Vancomycin hydrochloride and heparin sodium salt (cell culture suitable) for in vivo studies were purchased from Sigma-Aldrich, Inc. (Atlanta, GA). Sterile 0.9% saline and lactated Ringer's solution were obtained from Sigma-Aldrich, Inc. For the in vitro daptomycin stability study, USP-grade heparin was obtained from Hospira (10 units/ml) and Baxter (10,000 units/ml).
Animals.
Male Sprague-Dawley rats with preimplanted jugular vein central venous catheters (polyurethane, 0.025-in. internal diameter, and 40-μl volume) were obtained from Charles River Labs (Wilmington, MA). Initial weights were 175 to 200 g per rat. Rats received water and Agway rodent chow ad libitum throughout the experiments. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Cubist Pharmaceuticals, Inc., before the studies were initiated.
Rat central venous catheter biofilm model.
The rat central venous catheter biofilm model described previously by Ulphani and Rupp (45) was employed, with modifications. Jugular vein central venous catheters were inoculated by the instillation of 0.2 ml of saline containing either 1 × 108 CFU MRSE or 1 × 106 CFU MSSA. One hour later, catheters were flushed with heparin saline (0.3 ml; 400 U/ml). Central venous catheter infections were monitored by daily blood sampling through the catheter. Blood samples (0.3 ml) were processed for CFU/ml values by standard methods (serial dilution, plating, incubation, and CFU counting). To maintain central venous catheter patency, catheters were flushed with heparin saline immediately following each blood draw.
Model optimization.
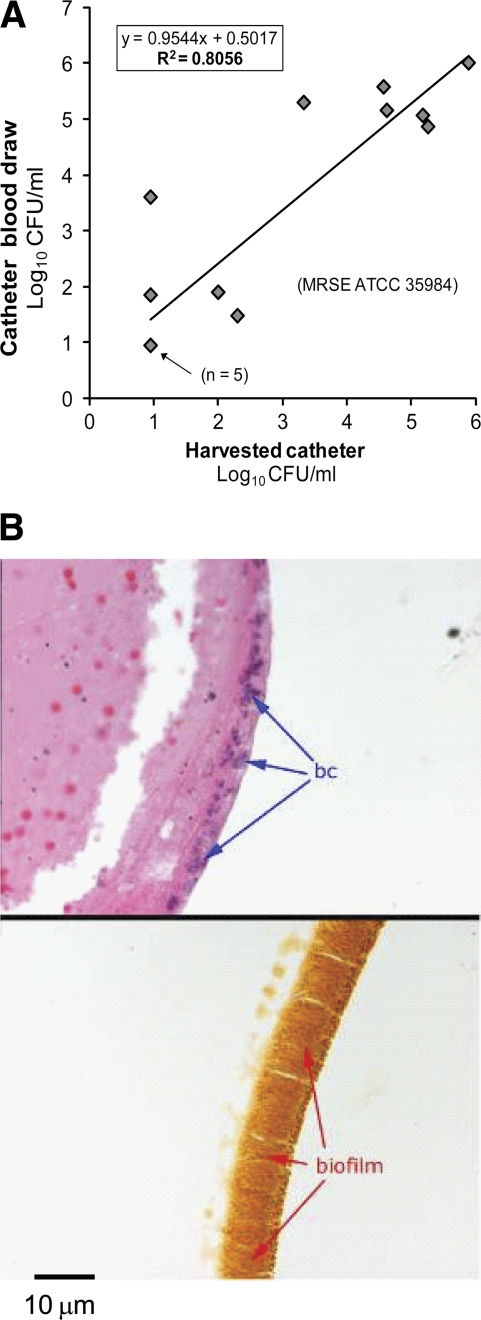
In preliminary experiments, the bacterial CFU/ml of catheter-drawn blood samples was positively correlated (R2 = 0.8056) with the CFU/ml of the corresponding harvested central venous catheters (Fig. 1A). Histological analysis of catheters at 4 to 8 days postinoculation showed that those with biofilm layers approximately 10 μm thick were associated with elevated bacterial counts in the blood (Fig. 1B) and with the development of disseminated infection. Thus, ALT was evaluated only in animals with demonstrated mature catheter infections, on the basis of having consistently elevated bacterial counts (≥104 CFU/ml) in their catheter blood samples from 1 to 4 days postinoculation.
Fig. 1.
Characterization of the rat central venous catheter biofilm infection model. (A) Correlation of daily central venous catheter blood draws and harvested catheter CFU. Rat central venous catheters were infected with Staphylococcus epidermidis ATCC 35984. At various time points, rats were sacrificed immediately following central venous catheter blood draws, and catheters were harvested and processed for the quantification of CFU/ml. Each data point (from a single animal, except where noted) represents the log10 CFU/ml from a central venous catheter blood draw and the corresponding log10 CFU/ml value from the harvested catheter. Central venous catheter blood draws are a good measure of the level of central venous catheter infection based on the high correlation seen in these data. (B) Harvested catheters, infected 4 days previously, were submerged in 10% formalin for 72 h before processing for routine sectioning and staining. The top photomicrograph, a hematoxylin- and eosin-stained section, shows remnant blood cells in the interior of the catheter (left side) and darkly stained bacterial cells (labeled “bc”) along the internal catheter edge. The bottom photomicrograph is a Gram-stained section showing a biofilm nearly 10 μm wide.
Close to 100% of animals with infections caused by S. epidermidis survived the entire experimental period. However, when S. aureus was used as the infecting organism, significant mortality occurred even with the lower inoculum of 106 CFU/0.2 ml (data not shown). Therefore, in experiments with MSSA as the biofilm agent, it was necessary to treat animals with systemic daptomycin (40 mg/kg of body weight subcutaneously [s.c.] once daily) during the 4 days (days 1 to 4) when the central venous catheter infection was developing.
Terminal harvests and preparation and plating of catheters, tissues, and fluids.
At the end of each experiment, the rats were euthanized by carbon dioxide asphyxiation, and the central venous catheters and selected tissues or fluids were collected by use of sterile techniques. Most often, tissues and fluids of rats infected with MRSE were harvested 7 days after the end of ALT to look for the persistence of catheter sterilization; tissues and fluids of rats infected with MSSA were harvested 24 h after the end of ALT to determine the degree of sterilization in tissues and fluids soon after the end of therapy. Cardiac blood was collected first via cardiac puncture, serially diluted, and plated. The heart, lungs, kidneys (paired), and spleen were transferred individually into 5 ml sterile distilled water (dH2O); the vena cava was collected along with the distal portion of the central venous catheter (see below), separated from the catheter tip, and placed into 3 ml sterile dH2O. All organs and the vena cava were homogenized and processed for CFU/ml counts by standard methods.
The terminal portion (approximately 3 cm) of the central venous catheter just proximal to the right atrium was collected, along with the attached vena cava, and kept on ice during processing; the vena cava was dissected from the catheter by use of sterile techniques and processed as described above. The harvested catheter was measured and then cut into 1- to 2-mm sections, transferred into 5 ml sterile dH2O, and placed on ice. The sterile dH2O containing the catheter sections was sonicated for 10 min and then vortexed for 1 min to dislodge adherent bacteria. The catheter supernatant was serially diluted, plated, and incubated for CFU/ml counts by standard methods.
Antibiotic lock treatment.
All studies of ALT against mature central venous catheter biofilms were started at 5 days postinoculation. ALT consisted of the once-daily instillation of the test agent (volume of 0.2 ml) into the catheter for 30 min (for the MSSA model) or 18 h (for the MRSE models), followed by a heparin saline flush and lock (0.3 ml; 400 U/ml). The shorter ALT period tested for MSSA was intended to match the standard 30-min daily infusion time for daptomycin. Blood samples for CFU/ml determinations were obtained through the catheter prior to ALT. In most experiments, systemic doses of antibiotics (2 ml/kg administered s.c.) were given concurrently with ALT to mimic the clinical paradigm for catheter-related infections. Daptomycin and vancomycin lock solutions contained 5 mg/ml each. Daptomycin was dosed systemically at 40 mg/kg subcutaneously once daily, approximating the total area under the concentration-time curve (AUC) obtained for people dosed at 6 mg/kg/day; vancomycin was dosed systemically at 50 mg/kg subcutaneously twice daily, to approximate the standard human dose.
Stability of daptomycin and heparin in lactated Ringer's solution.
We investigated the stability of daptomycin in a lactated Ringer's vehicle (54 μg/ml calcium [Ca2+]) containing added heparin. Aliquots (35 ml) of lactated Ringer's solution containing daptomycin (5 and 25 mg/ml) and USP-grade heparin sodium (0, 5, 500, or 5,000 U/ml) were stored at 37°C and tested after 0, 4, 6, 12, and 24 h. Daptomycin concentrations were determined by using a validated high-pressure liquid chromatography method. Clarity, color changes, and the presence and/or absence of particulate matter were assessed by visual inspection. Turbidity, pH, and heparin activity were determined by compendial methods (46).
Data analysis.
A correlation analysis of CFU/ml data from matched central venous catheter blood draws and harvested central venous catheters was performed by using a linear regression least-squares-fit analysis with Microsoft Excel.
Daily catheter blood draws were compared among different treatment groups by using a repeated-measures two-way analysis of variance (ANOVA) with a Bonferroni posttest to compare replicate means (GraphPad Prism, version 5.02). Terminal blood and organ bacterial loads were compared by using a two-way ANOVA with a Bonferroni posttest, and rates of sterility from different treatment groups were compared by using a one-way ANOVA (Kruskal-Wallis test), followed by Dunn's multiple-comparison test (GraphPad Prism, version 5.02). Different treatment groups were considered significantly different if P values were less than 0.05.
RESULTS
Antibiotic susceptibilities.
The three strains of S. epidermidis used in these central venous catheter infection models were susceptible to both daptomycin (MICs = 0.5 to 1.0 μg/ml) and vancomycin (MICs = 1.0 to 2.0 μg/ml) (Table 1). Methicillin-susceptible S. aureus strain ATCC 25923 was susceptible to daptomycin (MIC = 1.0 μg/ml) (Table 1).
Table 1.
MICs for Staphylococcus epidermidis and Staphylococcus aureus strains used in the rat central venous catheter biofilm model
| Strain | MIC (μg/ml)a |
|
|---|---|---|
| Daptomycin | Vancomycin | |
| S. epidermidis ATCC 35984 | 0.5 | 1.0 |
| S. epidermidis ATCC 49461 | 1.0 | 2.0 |
| S. epidermidis ATCC 700565 | 0.5 | 1.0 |
| S. aureus ATCC 25923 | 1.0 | ND |
ND, not done.
Stability of daptomycin in lactated Ringer's solution with heparin.
The concentration of daptomycin in the Ringer's solution-heparin vehicle decreased by ≤10% when stored for 24 h at 37°C (Table 2). The decrease occurred primarily at between 6 and 24 h of storage and was similar regardless of the initial concentration of daptomycin or of heparin. No significant changes in clarity, color, particulate matter, turbidity, pH, or heparin activity were observed (data not shown).
Table 2.
Stability of daptomycin in lactated Ringer's solution with heparin
| Heparin concn (U/ml) | Daptomycin concn (mg/ml) (% of initial value) with storage duration (h) of: |
||||
|---|---|---|---|---|---|
| 0 | 4 | 6 | 12 | 24 | |
| 0 | 5.1 (100) | 4.9 (97) | 4.9 (97) | 4.8 (95) | 4.6 (90) |
| 5 | 5.1 (100) | 5.0 (99) | 5.0 (99) | 4.9 (96) | 4.6 (90) |
| 500 | 5.1 (100) | 5.0 (99) | 5.0 (98) | 4.9 (96) | 4.6 (90) |
| 5,000 | 5.1 (100) | 5.1 (99) | 5.0 (98) | 5.0 (96) | 4.7 (91) |
| 0 | 26.8 (100) | 26.5 (99) | 26.0 (97) | 25.4 (95) | 24.0 (90) |
| 5 | 26.3 (100) | 26.3 (100) | 26.1 (99) | 25.3 (96) | 23.9 (91) |
| 500 | 26.3 (100) | 26.2 (100) | 26.1 (99) | 25.2 (96) | 24.2 (92) |
| 5,000 | 27.1 (100) | 26.6 (98) | 26.8 (99) | 25.1 (92) | 24.6 (90) |
Treatment of catheter-related infections caused by S. epidermidis. (i) Optimizing daptomycin ALT.
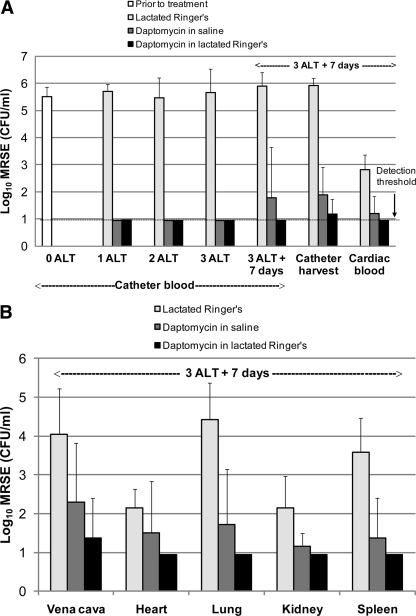
Daptomycin is a calcium-dependent antibacterial agent, working optimally in vitro with levels of free calcium ions at physiological concentrations (∼50 μg/ml). We investigated this phenomenon in the treatment of catheter-related infections. Figure 2 shows the effects of ALT with daptomycin (5 mg/ml for 18 h) for 3 days in rats with central venous catheter infections caused by S. epidermidis ATCC 49461. Daptomycin was formulated in either 0.9% saline or lactated Ringer's solution (containing 54 μg/ml calcium [Ca2+]) for ALT. ALT with lactated Ringer's solution alone was used as a control. Animals that received daptomycin ALT also received parenteral daptomycin (40 mg/kg s.c. daily) for 3 days. Both daptomycin ALT formulations rapidly cleared detectable MRSE from catheter blood samples; 100% of rats treated with daptomycin in either saline or lactated Ringer's solution had no detectable bacteria in their catheter blood draws after only 2 days of ALT (5 out of 5 rats for both formulations) (Fig. 2A).
Fig. 2.
Daptomycin efficacy optimized by a formulation of daptomycin ALT solution. Rats were infected with methicillin-resistant Staphylococcus epidermidis (MRSE) strain ATCC 49461 and monitored for 4 days to confirm the establishment of a mature biofilm. Groups of rats were then treated for 18 h daily for three consecutive days with ALT solutions as indicated (n = 6 rats/group). Rats receiving daptomycin ALT also received systemic daptomycin at 40 mg/kg daily via subcutaneous injection on each day of ALT. Animals were monitored for 7 days following the end of ALT, and tissues and fluids were then harvested for determinations of bacteremia and catheter-related metastatic infections. (A) Bacterial titers (means and standard deviations) in central venous catheter blood draws before (0 ALT), during (1 ALT and 2 ALT), and after (3 ALT) ALT, including terminal central venous catheter harvesting and cardiac blood draws 7 days following the cessation of ALT (3 ALT + 7 days). (B) Bacterial titers (means and standard deviations) measured at metastatic sites of infection, including the vena cava (surrounding the central venous catheter), heart, lung, kidneys, and spleen, 7 days following the end of 3 days of ALT.
The persistence of clearance from central venous catheters and tissues harvested 7 days after the last ALT treatment was evaluated (Fig. 2). One week after dosing, daptomycin ALT in lactated Ringer's solution achieved bacterial clearance rates in central venous catheter blood draws and harvested catheters of 100% (5/5 rats) and 83% (5/6), respectively, while daptomycin ALT in saline had clearance rates of only 80% (4/5) and 50% (3/6). Daptomycin in lactated Ringer's solution sterilized significantly more harvested catheters than did lactated Ringer's solution alone (P < 0.01 by one-way ANOVA Kruskal-Wallis test), while daptomycin in saline did not (P > 0.05). Furthermore, daptomycin in the lactated Ringer's formulation sterilized blood and tissue samples out to 1 week after dosing (except the vena cava, where 5/6 rats were sterilized), while daptomycin ALT in saline failed to consistently clear secondary infections (Fig. 2B). Although this calcium dependence of daptomycin ALT was not tested for other bacterial strains, all subsequent studies using ALT with daptomycin were formulated in lactated Ringer's solution.
(ii) Daptomycin versus vancomycin in ALT against S. epidermidis.
The efficacies of vancomycin and daptomycin ALT, in combination with the systemic dosing of vancomycin and daptomycin, respectively, were compared by using three different strains of S. epidermidis with this model. Figure 3 shows the results of two representative experiments comparing daptomycin and vancomycin in the model, using ATCC 49461 or ATCC 700565 as the infecting strain (individual results combined). Each symbol represents the bacterial count from a single animal in this scatter plot: symbols containing a black dot are from animals infected with ATCC 700565, while symbols without the black dot are from animals infected with ATCC 49461. Individual results from studies with two different MRSE strains are plotted together to illustrate the consistency of the results using different strains (Fig. 3); similar results were achieved with a third MRSE strain (data not shown). As described above, daptomycin (in lactated Ringer's solution) or vancomycin (in saline) was administered via ALT for 18 h daily (5 mg/ml) and systemically (daptomycin at 40 mg/kg once daily along with daptomycin ALT and vancomycin at 50 mg/kg twice daily along with vancomycin ALT). Both daptomycin and vancomycin caused a rapid elimination of MRSE from central venous catheter blood samples for both strains (Fig. 3A). Although the difference was not statistically significant, daptomycin appeared to act slightly faster. Vancomycin sterilized the catheter blood from only 3/6 rats after 1 day of ALT, 4/6 rats after 2 days of ALT, and 6/6 rats after 3 days of ALT; daptomycin sterilized 6/7 rats after 1 day of ALT, 7/7 rats after 2 days of ALT, and 8/8 rats after 3 days of ALT. Daptomycin treatment sterilized significantly more catheters than did lactated Ringer's solution after 1, 2, and 3 days of ALT; vancomycin treatment achieved significance in catheter clearance compared with lactated Ringer's solution after 3 days of ALT (Kruskal-Wallis test followed by Dunn's multiple-comparison test). One week after the end of dosing, both daptomycin and vancomycin maintained 100% central venous catheter clearance for both strains, as measured by central venous catheter blood draws, and harvested central venous catheters were largely cleared (9 out of 10 rats [90%] for daptomycin and 8 out of 8 rats [100%] for vancomycin). Furthermore, combined ALT and systemic dosing with either daptomycin or vancomycin cleared metastatic infections in most organs (Fig. 3B).
Fig. 3.
Comparative efficacies of vancomycin and daptomycin ALT (both at 5 mg/ml) for 18 h daily for three consecutive days against two different strains of MRSE, biofilm-forming strains ATCC 700565 and ATCC 49461. (A) Bacterial titers in central venous catheter blood draws before (0 ALT), during (1 ALT and 2 ALT), and after (3 ALT) ALT, including terminal central venous catheter blood draws, catheter harvesting, and cardiac blood draws 7 days following the cessation of ALT (3 ALT + 7 days). (B) Bacterial titers measured at metastatic sites of infection, including the vena cava (surrounding the central venous catheter), heart, lung, kidneys, and spleen.
Treatment of catheter-related infections caused by S. aureus.
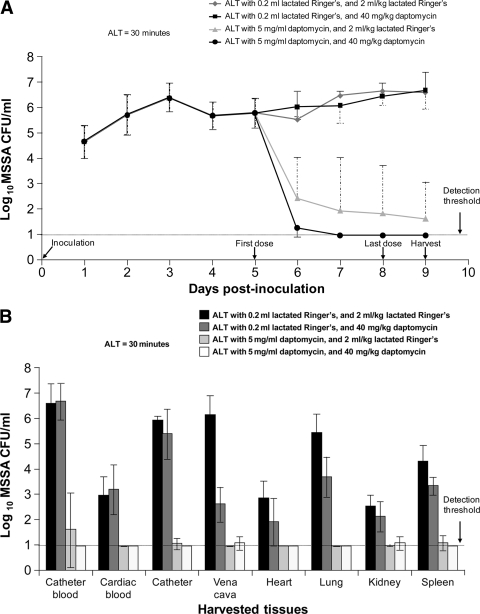
In order to maintain rats with a mature S. aureus infection prior to ALT, rats inoculated with S. aureus ATCC 25923 were dosed with 40 mg/kg daptomycin s.c. daily from day 1 through day 4. Starting on day 5, three daptomycin dosing paradigms were evaluated to determine their relative efficacies against established in vivo MSSA central venous catheter infections: systemic daptomycin (40 mg/kg s.c.) alone, 30-min daptomycin ALT (5 mg/ml in lactated Ringer's solution) alone, or combined systemic and ALT dosing with daptomycin. Daptomycin systemic dosing alone, at doses achieving AUCs similar to those for people dosed at 6 mg/kg/day, had no discernible effect on central venous catheter biofilms (Fig. 4A) or on bacterial loads associated with catheter-related bacteremia (cardiac blood) and showed only limited effects on reducing the bacterial counts in the vena cava (P < 0.001), heart (not significant), lungs (P < 0.01), or spleen (not significant; determined by two-way ANOVA with Bonferroni posttests) (Fig. 4B). In contrast, one 30-min daptomycin ALT alone significantly reduced the central venous catheter burden (reduction of >3 log10 CFU/ml; P < 0.001) but failed to clear central venous catheters completely (Fig. 4A). Combined daptomycin ALT and systemic daptomycin dosing proved to be the most effective treatment paradigm tested against S. aureus central venous catheter infections. Central venous catheter infections were cleared after two combined dosing sessions (Fig. 4A), and bacteria were not detected in harvested central venous catheters at 24 h after four daily combined dosing treatments (P < 0.001) (Fig. 4B). Finally, while ALT treatment alone led to low levels of staphylococcal burden in catheter-related bacteremia and all tissues with catheter-related metastatic infections (with clearance achieved only in the heart), combined ALT and systemic treatments cleared catheter-related bacteremia and all tested metastatic sites of infection in the heart, lungs, and spleen, with a low remaining bacterial burden in the vena cava and kidneys (Fig. 4B).
Fig. 4.
ALT and parenteral daptomycin treatment, separately and together, of methicillin-susceptible Staphylococcus aureus (MSSA) central venous catheter infection. (A) Daily central venous catheter blood draws were used to monitor S. aureus ATCC 25923 biofilms in rats (means ± standard deviations). CFU/ml data before dosing are presented as a single group (days 1 to 5 following inoculation; n = 18). ALT for only 30 min daily was initiated along with systemic dosing for four consecutive days starting on day 5. ALT with 5 mg/ml daptomycin, combined with either systemic lactated Ringer's solution (2 ml/kg s.c.) or systemic daptomycin (40 mg/kg s.c.), resulted in a significant decrease in the bacterial burden from daily drawn catheter blood from day 6 (after only one dose) through harvesting on day 9 (P < 0.001 versus both groups with ALT with lactated Ringer's solution). (B) Bar graph showing bacterial titers (means ± standard deviations) for catheter blood, cardiac blood, harvested catheter, vena cava, heart, lung, kidneys, and spleen 24 h following the end of four daily ALT treatments. Tissues and fluids from rats receiving ALT with 5 mg/ml daptomycin had significantly decreased bacterial loads compared with those from rats receiving ALT with lactated Ringer's solution and systemic lactated Ringer's solution (P value of <0.001, except for kidneys, with a P value of <0.01).
DISCUSSION
We optimized an in vivo rat model of central venous catheter infection and used the model to examine the potential utility of daptomycin ALT for the treatment of the catheter infection and associated disseminated infections. This model employed several unique techniques. First, quantitative daily blood cultures were used to verify the development of the infection and to evaluate the efficacy of treatment. Second, our model system demonstrated an improved daptomycin ALT efficacy by formulating the drug in lactated Ringer's solution, which has physiological concentrations of Ca2+ (54 μg/ml), which are lacking in saline. Bookstaver and colleagues (4) also demonstrated previously that daptomycin in saline was inferior to daptomycin in lactated Ringer's solution in their in vitro model. The greater efficacy is likely due to the presence of calcium in lactated Ringer's solution and the calcium-dependent activity of daptomycin. The slight decrease in the potency of daptomycin in lactated Ringer's solution after 6 h suggests that daptomycin for ALT should be prepared within 6 h of ALT administration.
Finally, this study demonstrated the optimized dosing paradigm for established MSSA central venous catheter biofilm infection to be daptomycin ALT with concurrent daptomycin systemic dosing, compared to daptomycin ALT alone or systemic daptomycin therapy alone. The ability of daptomycin to provide rapid bacterial eradication after 1 dose of ALT suggests that daptomycin rapidly penetrates the biofilm layer (38, 44) and at a concentration that is above the MIC. This was achieved with only 30 min of daptomycin ALT daily in our rat central venous catheter infection model. This rapid clearance of the pathogen will likely be important clinically, especially for immunocompromised and critically ill patients. It should be emphasized that we tested this with only one strain of S. aureus in our model, so its applicability to central venous catheter infections from other S. aureus strains has not been demonstrated.
ALT has been used successfully in hemodialysis, cancer, and critically ill patient populations to prevent as well as to treat catheter-related infections (37, 40, 42, 49). Benefits of ALT include a reduction in catheter removal rates compared with those associated with a heparin lock alone (49), improvement in infection-free survival rates for patients treated with ALT compared with a historical matched cohort of patients who received routine catheter replacement (37), success in salvaging infected catheters in chronically catheterized cancer patients (37), and a reduced risk of catheter-related bacteremia compared with a flush solution (40).
Antibiotics such as linezolid and vancomycin have displayed limited activity in eradicating staphylococci embedded within a biofilm (12, 16, 20, 34, 38, 41, 48). In an in vivo catheter-related infection model in rabbits, a lock solution of 2 mg/ml vancomycin showed limited efficacy against one of two S. aureus strains tested, whereas ALT with 1 mg/ml ciprofloxacin demonstrated activity against both strains (6). Interestingly, the combination of 2,500 IU/ml heparin with vancomycin or ciprofloxacin reduced the effectiveness of each antibiotic alone for one strain or the other (6). One in vitro biofilm model found undetectable levels of S. epidermidis ATCC 35984 after 10 days of exposure to 10 mg/ml vancomycin, compared with 3 days for 2 mg/ml linezolid; 10 mg/ml gentamicin did not cause an eradication after 10 days (12). The lack of effectiveness of gentamicin, as well as of cefazolin, nafcillin, and erythromycin, over 5 days was confirmed in another in vitro biofilm model; alternately, vancomycin, ciprofloxacin, and rifampin were effective in this model (28). However, the results of in vitro biofilm studies are not consistent. Another investigation found that the eradication of S. aureus ATCC 29213 and clinical strains of S. epidermidis was not achieved by using simulated dosing regimens of linezolid (600 mg every 12 h) or vancomycin (1 g every 12 h) in a one-compartment catheter biofilm model (43). In addition, bacterial release from the biofilm persisted over the 48 h of the study (48).
Daptomycin ALT has been investigated with in vitro biofilm models. In an in vitro model with Hickman catheter segments, daptomycin (1 mg/ml) formulated in lactated Ringer's solution with heparin (5,000 units/ml) was among the best ALTs against methicillin-resistant S. aureus (MRSA) (4). In an in vitro central venous catheter infection model, both daptomycin and vancomycin catheter lock solutions were effective in eradicating S. epidermidis at 72 h; furthermore, daptomycin was effective in eliminating S. aureus, while vancomycin was not (27). The rapid effectiveness of daptomycin is supported further by the observation that daptomycin eradicated MRSA embedded in a biofilm after 3 days of 4-h daily exposures (38). Using this model, daptomycin was shown to act faster than minocycline, tigecycline, linezolid, vancomycin, and rifampin against in vitro central venous catheter biofilm infections (38). The efficacy of daptomycin compared to those of other antibiotics was also demonstrated against biofilm-forming strains of staphylococci in an antibiotic lock model and vascular graft model (15). In the antibiotic lock model, for example, daptomycin and rifampin were effective at eradicating staphylococcal adherence by day 4, compared to day 7 for linezolid, gentamicin, and vancomycin, while ceftriaxone failed to eradicate staphylococcal adherence by day 10.
Daptomycin ALT has not been studied clinically. However, a recent case report demonstrated that daptomycin treatment of catheter-related MSSA acute endocarditis led to a cure after the failure of 3 weeks of high-dose cefazolin treatment to eradicate a tricuspid valve vegetation (10). Another case report showed that high-dose, prolonged therapy with daptomycin resolved both pacemaker-induced S. aureus acute bacterial endocarditis and persistent S. aureus bacteremia resulting from an infected coronary stent (9). A third case report also demonstrated the efficacy of daptomycin concurrently administered systemically and via ALT for 14 days in a patient with S. epidermidis bacteremia who had failed vancomycin therapy (18). A fourth case report, however, demonstrated the failure of daptomycin to eradicate S. epidermidis despite 5 weeks of endovenous treatment and 6 weeks of ALT treatment with daptomycin (19).
The major limitation of this study is that a rat model was used. Although we optimized the conditions employed previously by Ulphani and Rupp (45), these optimized conditions may not be the same as the complex conditions found within human biofilms. Another limitation is that the 5-mg/ml concentration of daptomycin used for ALT in this study may not be optimal, although it was based on previous investigations that used concentrations ranging from 0.5 mg/ml to 40 mg/ml (4, 15, 27, 38).
Conclusion.
Concurrent ALT and systemic therapy with daptomycin or vancomycin were equally effective in clearing MRSE-infected central venous catheters and maintaining clearance of the catheters for 1 week posttherapy. This suggests that daptomycin ALT with concurrent systemic therapy could be effective for eradicating staphylococcal central venous catheter biofilm infections. If so, the occurrence of catheter-related bacteremia or catheter-related metastatic infections might be reduced. Clinical studies of daptomycin ALT are warranted.
ACKNOWLEDGMENTS
Tongchuan Li, Shuxin Zhang, Anu Arya, Xi-Xian Zhang, Shellie Bertolami, and Lawrence I. Mortin are employees of Cubist Pharmaceuticals, Inc. Andrew D. G. Van Praagh and Liping Chen are former employees of Cubist. The study was sponsored by Cubist Pharmaceuticals, Inc.
We thank Kimberly Lindfield of Cubist Pharmaceuticals for assistance with the statistical analyses. Phase Five Communications, Inc., New York, provided editorial services, which were funded by Cubist Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Bagnall-Reeb H. 2004. Evidence for the use of the antibiotic lock technique. J. Infus. Nurs. 27:118–122 [DOI] [PubMed] [Google Scholar]
- 2. Bestul M. B., Vandenbussche H. L. 2005. Antibiotic lock technique: review of the literature. Pharmacotherapy 25:211–227 [DOI] [PubMed] [Google Scholar]
- 3. Bleyer A. J. 2007. Use of antimicrobial catheter lock solutions to prevent catheter-related bacteremia. Clin. J. Am. Soc. Nephrol. 2:1073–1078 [DOI] [PubMed] [Google Scholar]
- 4. Bookstaver P. B., Williamson J. C., Tucker B. K., Raad I. I., Sherertz R. J. 2009. Activity of novel antibiotic lock solutions in a model against isolates of catheter-related bloodstream infections. Ann. Pharmacother. 43:210–219 [DOI] [PubMed] [Google Scholar]
- 5. Burton D. C., Edwards J. R., Horan T. C., Jernigan J. A., Fridkin S. K. 2009. Methicillin-resistant Staphylococcus aureus central-line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 301:727–736 [DOI] [PubMed] [Google Scholar]
- 6. Capdevila J. A., et al. 2001. Lack of antimicrobial activity of sodium heparin for treating experimental catheter-related infection due to Staphylococcus aureus using the antibiotic-lock technique. Clin. Microbiol. Infect. 7:206–212 [DOI] [PubMed] [Google Scholar]
- 7. Christensen G. D., et al. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—8th ed. CLSI document M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Cunha B. A., Eisenstein L. E., Hamid N. S. 2006. Pacemaker-induced Staphylococcus aureus mitral valve acute bacterial endocarditis complicated by persistent bacteremia from a coronary stent: cure with prolonged/high-dose daptomycin without toxicity. Heart Lung 35:207–211 [DOI] [PubMed] [Google Scholar]
- 10. Cunha B. A., Hamid N., Kessler H., Parchuri S. 2005. Daptomycin cure after cefazolin treatment failure of methicillin-sensitive Staphylococcus aureus (MSSA) tricuspid valve acute bacterial endocarditis from a peripherally inserted central catheter (PICC) line. Heart Lung 34:442–447 [DOI] [PubMed] [Google Scholar]
- 11. Cuntz D., et al. 2002. Local antibiotic lock for the treatment of infections related to central catheters in parenteral nutrition in children. J. Parenter. Enteral Nutr. 26:104–108 [DOI] [PubMed] [Google Scholar]
- 12. Curtin J., Cormican M., Fleming G., Keelehan J., Colleran E. 2003. Linezolid compared with eperezolid, vancomycin, and gentamicin in an in vitro model of antimicrobial lock therapy for Staphylococcus epidermidis central venous catheter-related biofilm infections. Antimicrob. Agents Chemother. 47:3145–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dimick J. B., et al. 2001. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch. Surg. 136:229–234 [DOI] [PubMed] [Google Scholar]
- 14. Easom A. 2000. Prophylactic antibiotic lock therapy for hemodialysis catheters. Nephrol. Nurs. J. 27:75. [PubMed] [Google Scholar]
- 15. Edmiston C. E., Jr., et al. 2006. Impact of selective antimicrobial agents on staphylococcal adherence to biomedical devices. Am. J. Surg. 192:344–354 [DOI] [PubMed] [Google Scholar]
- 16. Evans R. C., Holmes C. J. 1987. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob. Agents Chemother. 31:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez-Hidalgo N., Almirante B., Calleja R., Ruiz I., Planes A. M. 2006. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J. Antimicrob. Chemother. 57:1172–1180 [DOI] [PubMed] [Google Scholar]
- 18. Grau S., Gil M. J., Mateu-de Antonio J., Pera M., Marin-Casino M. 2009. Antibiotic-lock technique using daptomycin for subcutaneous injection ports in a patient on home parenteral nutrition. J. Infect. 59:298–299 [DOI] [PubMed] [Google Scholar]
- 19. Guimard T., et al. 2010. Failure of antibiotic-lock technique using daptomycin for subcutaneous injection ports in a patient on home parenteral nutrition. J. Infect. 60:505–507 [DOI] [PubMed] [Google Scholar]
- 20. Hachem R. Y., Hanna H. A., Dvorak T., Chemaly R. F., Raad I. 2005. Activity of daptomycin and tigecycline against clinical isolates causing catheter-related methicillin-resistant Staphylococcus aureus (CR-MRSA) embedded in biofilm, abstr. E-809. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC American Society for Microbiology, Washington, DC [Google Scholar]
- 21. Heinzelmann M., et al. 1997. Phagocytosis and oxidative-burst response of planktonic Staphylococcus epidermidis RP62A and its non-slime-producing variant in human neutrophils. Clin. Diagn. Lab. Immunol. 4:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson D. C., Johnson F. L., Goldman S. 1994. Preliminary results treating persistent central venous catheter infections with the antibiotic lock technique in pediatric patients. Pediatr. Infect. Dis. J. 13:930–931 [DOI] [PubMed] [Google Scholar]
- 23. Kim S. H., et al. 2006. Prevention of uncuffed hemodialysis catheter-related bacteremia using an antibiotic lock technique: a prospective, randomized clinical trial. Kidney Int. 69:161–164 [DOI] [PubMed] [Google Scholar]
- 24. King A., Phillips I. 2001. The in vitro activity of daptomycin against 514 Gram-positive aerobic clinical isolates. J. Antimicrob. Chemother. 48:219–223 [DOI] [PubMed] [Google Scholar]
- 25. Krishnasami Z., et al. 2002. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 61:1136–1142 [DOI] [PubMed] [Google Scholar]
- 26. Krzywda E. A., Andris D. A., Edmiston C. E., Jr., Quebbeman E. J. 1995. Treatment of Hickman catheter sepsis using antibiotic lock technique. Infect. Control Hosp. Epidemiol. 16:596–598 [DOI] [PubMed] [Google Scholar]
- 27. Laplante K. L., Mermel L. A. 2007. In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol. Dial. Transplant. 22:2239–2246 [DOI] [PubMed] [Google Scholar]
- 28. Lee J.-Y., Ko K. S., Peck K. R., Oh W. S., Song J.-H. 2006. In vitro evaluation of the antibiotic lock technique (ALT) for the treatment of catheter-related infections caused by staphylococci. J. Antimicrob. Chemother. 57:1110–1115 [DOI] [PubMed] [Google Scholar]
- 29. Mascio C. T. M., Alder J. D., Silverman J. A. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mermel L. A., et al. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Messing B., Peitra-Cohen S., Debure A., Beliah M., Bernier J. J. 1988. Antibiotic-lock technique: a new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. J. Parenter. Enteral Nutr. 12:185–189 [DOI] [PubMed] [Google Scholar]
- 32. Mortin L. I., et al. 2007. Rapid bactericidal activity of daptomycin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus peritonitis in mice as measured with bioluminescent bacteria. Antimicrob. Agents Chemother. 51:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peters G., Locci R., Pulverer G. 1982. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J. Infect. Dis. 146:479–482 [DOI] [PubMed] [Google Scholar]
- 34. Petersen P. J., et al. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pfaller M. A., Sader H. S., Jones R. N. 2007. Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of Gram-positive cocci collected in North American hospitals (2002–2005). Diagn. Microbiol. Infect. Dis. 57:459–465 [DOI] [PubMed] [Google Scholar]
- 36. Pittet D., Tarara D., Wenzel R. P. 1994. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601 [DOI] [PubMed] [Google Scholar]
- 37. Poole C. V., Carlton D., Bimbo L., Allon M. 2004. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol. Dial. Transplant. 19:1237–1244 [DOI] [PubMed] [Google Scholar]
- 38. Raad I., et al. 2007. Comparative activity of daptomycin, linezolid and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rupp M. E., Archer G. L. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin. Infect. Dis. 19:231–243 [DOI] [PubMed] [Google Scholar]
- 40. Safdar N., Maki D. G. 2006. Use of vancomycin-containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta-analysis of prospective, randomized trials. Clin. Infect. Dis. 43:474–484 [DOI] [PubMed] [Google Scholar]
- 41. Saginur R., et al. 2006. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob. Agents Chemother. 50:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanchez-Munoz A., et al. 2005. Usefulness of antibiotic-lock technique in management of oncology patients with uncomplicated bacteremia related to tunneled catheters. Eur. J. Clin. Microbiol. Infect. Dis. 24:291–293 [DOI] [PubMed] [Google Scholar]
- 43. Segarra-Newnham M., Martin-Cooper E. M. 2005. Antibiotic lock technique: a review of the literature. Ann. Pharmacother. 39:311–318 [DOI] [PubMed] [Google Scholar]
- 44. Steward P. S., Davison W. M., Steenbergen J. N. 2009. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 53:3505–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ulphani J. S., Rupp M. E. 1999. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab. Anim. Sci. 49:283–287 [PubMed] [Google Scholar]
- 46. U.S. Pharmacopeia and National Formulary 2006. <851> Spectrophotometry and light scattering, p. 2770–2775. <791> pH, p. 2730–2731. Heparin sodium assay, p. 1047–1048. (USP29-NF24). U.S. Pharmacopeia and National Formulary, Rockville, MD [Google Scholar]
- 47. Viale P., et al. 2003. Antibiotic lock-technique for the treatment of catheter-related bloodstream infections. J. Chemother. 15:152–156 [DOI] [PubMed] [Google Scholar]
- 48. Wiederhold N. P., Coyle E. A., Raad I. I., Prince R. A., Lewis R. E. 2005. Antibacterial activity of linezolid and vancomycin in an in vitro pharmacodynamic model of gram-positive catheter-related bacteraemia. J. Antimicrob. Chemother. 55:792–795 [DOI] [PubMed] [Google Scholar]
- 49. Yahav D., et al. 2008. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin. Infect. Dis. 47:83–93 [DOI] [PubMed] [Google Scholar]