Abstract
In view of the emergence of multidrug-resistant Salmonella strains, there is a need for therapeutic alternatives. To reduce the dose of antibiotic required in order to decrease the associated side effects, the present study was aimed at evaluating the synergism between cryptdin 2 (a Paneth cell antimicrobial peptide) and ampicillin (Amp) against Salmonella enterica serovar Typhimurium. The synergy was evaluated in terms of the fractional bactericidal concentration (FBC) index, time-kill assay results (in vitro), macrophage functions, i.e., intracellular killing, lipid peroxidation, superoxide dismutase activity, and generation of nitrite (ex vivo), and decreases in CFU of salmonellae in livers, spleens, and small intestines of infected mice treated with cryptdin 2 and/or Amp (in vivo). In vitro synergism between the two agents was observed on the basis of the FBC index and time-kill assays. When the agents were used in combination, ex vivo studies revealed an enhanced effect on macrophage functions, particularly exhibiting a synergetic effect in terms of SOD levels. In vivo synergy was indicated by larger log unit decreases in all target organs of mice treated with the combination than those for the drugs used alone. These results point toward the possible use of cryptdin 2 as an adjunct to ampicillin and may help in developing alternate strategies to combat Salmonella infections.
INTRODUCTION
Emergence of Salmonella strains resistant to first-line antibiotics, as well as increased MICs of quinolones, is tending to be a serious problem limiting the possibilities for effective treatment of human Salmonella infections (15, 33, 34). Moreover, frequent and lengthy use of antibiotics usually results in alteration of the intestinal commensal flora and leads to chronic toxicity. Recent studies have demonstrated that the Gram-negative bacterium Salmonella enterica serovar Typhimurium has nine functional drug efflux pumps (26). The expression of this multidrug efflux system has been indicated to decrease cellular drug accumulation by altering cell permeability, thereby offering resistance to quinolones and some β-lactams (27). It provides an impetus to efforts to identify and exploit alternative antibacterial therapies against Salmonella infections.
In this context, development of cationic antimicrobial peptides (AMPs) for the treatment of salmonellosis has recently become a major area of investigation (41). Among naturally occurring AMPs, defensins form a unique family of cysteine-rich cationic polypeptides with 3 or 4 disulfide bridges (22, 39). Mouse enteric alpha-defensins called cryptdins are broad-spectrum AMPs due to their ability to kill various bacteria (8, 12, 14, 23, 28), parasites (9), and enveloped viruses (35) in vitro. Recently, we demonstrated that cryptdin 2 possesses a strong in vivo therapeutic potential against murine salmonellosis without exhibiting any toxicity as indicated by liver and kidney function tests (29). Additionally, it was found to exhibit very low cytotoxicity toward macrophages, even at a concentration twice the minimal bactericidal concentration (MBC) (29).
It has also been reported that Paneth cell cryptdins are natural pore-forming peptides and may be capable of mediating the transport of therapeutic molecules inside the target cell (19). Hence, the use of cryptdins can be perceived as a promising solution to the growing problem of resistance to conventional antibiotics in Salmonella, particularly by facilitating the entry of drugs into the cell. Combination therapy with antibiotics and defensins can therefore be used to increase the in vivo activity as well as to broaden the antimicrobial spectrum (13). Earlier, several AMPs were used in combination with conventional drugs against various bacteria, fungi, and viruses (10, 16, 21, 31, 42). However, until now, to the best of our knowledge, no information has been available on the combined activity of cryptdins and conventional anti-Salmonella drugs. The present study was therefore planned to evaluate the in vitro, ex vivo, and in vivo synergistic effects, if any, of cryptdin 2, a mouse Paneth cell alpha-defensin, in combination with ampicillin (Amp), an antibiotic conventionally used against Salmonella.
MATERIALS AND METHODS
Bacterial strain and growth medium.
Salmonella Typhimurium NCTC74, procured from Central Research Institute, Kasauli, India, was used in the present study. This strain was maintained on MacConkey agar medium and has been used in earlier studies as both a virulent and a reference strain (29). Overnight cultures were harvested by centrifugation (3,783 × g, 10 min), washed once with 10 mM sodium phosphate-buffered saline (PBS, pH 7.2), and resuspended in PBS to a final concentration of approximately 1 × 107 cells/ml.
Animals.
BALB/c mice (18 to 22 g) of either sex (4 to 5 weeks old), obtained from Central Animal House, Panjab University, Chandigarh, India, were housed under standard conditions with free access to food and water ad libitum. Throughout the study, the guidelines of the Institutional Animal Ethics Committee, Panjab University, Chandigarh, India, were followed.
Synthetic cryptdin 2 and ampicillin.
A chemically synthesized peptide with the amino acid sequence LRDLVCYCRTRGCKRRERMNGTCRKGHLMYTLCCR, identical to the sequence of mouse Paneth cell cryptdin 2, with disulfide linkages at CysI-CysVI, CysII-CysIV, and CysIII-CysV, was obtained from Taurus Scientific. It was suspended in 0.01% acetic acid, stored as a stock solution at 100 mg/liter at −20°C, and used within 3 weeks. Amp powder was procured from Sigma-Aldrich.
In vitro activity of cryptdin 2 and Amp against Salmonella. (i) MBCs.
The anti-Salmonella activity of cryptdin 2 and Amp was monitored by a broth dilution technique. In brief, cells were grown individually in the presence of different concentrations of cryptdin 2 (5 to 20 μg/ml) and Amp (0.5 to 16 μg/ml) in specially designed flat-bottom tubes containing 3 ml of nutrient broth (5.0 g/liter peptone, 5.0 g/liter NaCl, 1.5 g/liter beef extract, 1.5 g/liter yeast extract, pH 7.4 ± 0.2), followed by the addition of an inoculum of 107 cells of S. Typhimurium. Growth was monitored during the mid-log phase by measuring the optical density at 620 nm. In addition, cell cultures from the mid-log phase were diluted appropriately in PBS and plated on MacConkey agar medium. After 16 to 18 h, the number of CFU was enumerated, and the data obtained were used to calculate the MBCs of cryptdin 2 and Amp. The MBC was defined as the concentration at which there was >99% inhibition of growth.
(ii) FBCs.
A checkerboard test was performed in 96-well microtiter trays, using an 8-by-8 well configuration. Twofold serial dilutions of cryptdin 2 and Amp were prepared, with concentrations ranging from 0 to 2 times the MBC. Ten microliters of each cryptdin 2 dilution was added to the wells of a 96-well plate in a vertical orientation, and 10 μl of each Amp dilution was added in a horizontal orientation so that the plate contained various combinations of the two agents. Each well was then supplemented with 80 μl (107 CFU/ml) of S. Typhimurium, and the plate was incubated at 37°C. Wells not containing any antibacterial agent were used as positive growth controls. The fractional bactericidal concentration (FBC) was calculated by dividing the MBC of the combination of cryptdin 2 and Amp by the MBC of cryptdin 2 or Amp alone. The FBC index, obtained by adding both FBCs, was interpreted as indicating a synergistic effect when it was ≤0.5, an additive or indifferent effect when it was >0.5 and ≤2.0, and an antagonistic effect when it was >2.0 (42).
(iii) Time-kill assay.
To determine the bactericidal action of cryptdin 2 and Amp, separately and in combination, S. Typhimurium was exposed to one of the antimicrobial agents or to both simultaneously, and the viable count was monitored. Cryptdin 2 (19 μg/ml) and Amp (4.0 μg/ml), alone and in combination (cryptdin 2 at 5.0 μg/ml plus Amp at 0.5 μg/ml), were added to nutrient broth (5.0 g/liter peptone, 5.0 g/liter NaCl, 1.5 g/liter beef extract, 1.5 g/liter yeast extract, pH 7.4 ± 0.2) containing 107 CFU of S. Typhimurium and then incubated at 37°C. One-hundred-microliter aliquots were withdrawn at 0, 30, 60, 90, and 120 min and spread plated on MacConkey agar plates. The plates were then incubated at 37°C for 24 h for enumeration of CFU.
Ex vivo effect of cryptdin 2 and Amp.
Murine peritoneal macrophages were isolated by a method previously described by us (29). Briefly, peritoneal macrophages were isolated by intraperitoneal elicitation of mice with 1 ml of 3% thioglycolate medium. Two days later, mouse peritoneal cavity fluids were flushed with 8 to 10 ml chilled RPMI 1640 containing 10% fetal calf serum (FCS) and then centrifuged, and macrophages were resuspended in cold RPMI 1640. Cell viability was checked by the trypan blue exclusion method.
Intracellular killing of S. Typhimurium.
Mouse peritoneal macrophages were infected with S. Typhimurium at a multiplicity of infection (MOI) of 1:100 for 90 min. Infected macrophages were washed three times in RPMI 1640 and then treated with cryptdin 2 (19 μg/ml) and Amp (4 μg/ml), separately and in combination at reduced concentrations (cryptdin 2 at 5.0 μg/ml plus Amp at 0.5 μg/ml). After every 30, 60, and 90 min, treated and untreated macrophages were pelleted (2,000 rpm, 10 min) and lysed with 500 μl of 0.25% Triton X-100. Lysates were serially diluted and plated on MacConkey agar medium. After incubation for 24 h at 37°C, the number of CFU was counted. The mean percent intracellular killing was calculated as described earlier (29). Values are expressed as means ± standard deviations (SD) for three independent experiments. Statistical analysis was done by Student's t test, and P values of <0.05 were considered statistically significant.
Interaction of macrophages with S. Typhimurium.
Since Salmonella is an intracellular pathogen, Salmonella-macrophage interactions play a central role in its pathogenesis. S. Typhimurium encounters oxidative stress within the macrophage milieu, mounted in the form of the respiratory burst. The respiratory burst that is concomitant with phagocytosis produces a number of toxic by-products. Therefore, to assess the effects on the extent of lipid peroxidation (LPO) and on superoxide dismutase (SOD) activity and to measure nitric oxide generation, murine peritoneal macrophages were incubated with Salmonella at an MOI of 1:100 in the presence and absence of cryptdin (19 μg/ml) and Amp (4 μg/ml), alone and in combination at reduced concentrations (cryptdin 2 at 5.0 μg/ml plus Amp at 0.5 μg/ml), in a six-well tissue culture plate at 37°C in a CO2 incubator for 18 h. After incubation, lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride [PMSF]) was added (1:1 ratio) to all the wells, and the plate was further incubated at 4°C for 20 min. Reaction mixtures from each well were centrifuged (2,000 rpm, 15 min), and supernatants thus obtained were used to study the following.
(i) Extent of LPO.
Lipid peroxidation is the process of oxidative degradation of polyunsaturated fatty acids (PUFAs). The peroxidation of PUFAs is a feature of many types of cell injuries in which free radical intermediates are produced in excess. Lipid peroxidation can cause changes in membrane fluidity and permeability and can increase the rate of protein degradation, which eventually leads to cell lysis. A measure of LPO products, such as malondialdehyde (MDA), is an indication of the extent of damage. Quantitative measurement of lipid peroxidation in the culture supernatants of macrophages was performed as described by Wills (40). The results are expressed as nanomoles of MDA per milligram of protein, using the molar extinction coefficient of the chromophore (1.56 × 105 M−1 cm−1). The protein content of the samples was estimated by the method described by Lowry et al. (20).
(ii) Estimation of nitrite concentration.
Nitric oxide is an important signaling molecule regulating a diverse range of physiological processes. Nitrite levels were measured by a slight modification of the method of Green et al. (11), as described earlier (5), and served as an indicator of nitric oxide production. One-hundred-microliter aliquots of sample were mixed with 400 μl of distilled water and 500 μl of Griess reagent. The reaction mixture was incubated at room temperature for 10 min in the dark, and the optical density was measured at 546 nm. Nitrite was quantified using a standard graph for sodium nitrite. Nitrite levels in the cell-free supernatants of infected macrophages (treated with the agents) were compared with those of control (uninfected) and infected (untreated) macrophages.
(iii) Estimation of SOD activity.
Host cells are protected from oxygen-derived radical injury by naturally occurring free radical scavengers and antioxidant pathways, including superoxide dismutase (SOD). However, the antioxidant defense mechanism fails due to overproduction of free radicals, decreased activities of scavenging enzymes, or both, causing lipid peroxidation. Therefore, SOD activity was assayed according to the method of Kono (17). The reaction was initiated by the addition of 0.5 ml of hydroxylamine hydrochloride to a reaction mixture containing 2 ml of nitroblue tetrazolium (NBT) and 0.1 ml of culture supernatant. The change in absorbance was measured spectrophotometrically at 560 nm. SOD activity is expressed as units of SOD per milligram of protein, where 1 unit of activity is defined as the amount of SOD required to inhibit the rate of reduction by NBT by 50%.
In vivo effect of cryptdin 2 in conjunction with Amp against murine salmonellosis.
Mice were infected with 107 CFU of Salmonella Typhimurium orally. Seven days after the challenge, establishment of Salmonella infection was confirmed by bacterial translocation into the intestines, livers, and spleens of the infected mice. At 7 days postinfection, mice were randomized into 11 groups of five mice each and treated with cryptdin 2 and ampicillin. Cryptdin 2 was injected subcutaneously (s.c.) at a dose of 5 μg/mouse, while Amp was administered s.c. at 16, 32, and 64 mg/kg of body weight, individually and in combination. In addition to these doses, lower concentrations of both the agents in combination were also tested.
Mice in group I were injected s.c. with 0.1 ml sterile saline and served as the control (infected) group. Group II mice were administered 16 mg/kg body weight of ampicillin s.c. Group III mice were administered Amp s.c. at 32 mg/kg body weight. Group IV mice received 64 mg/kg body weight of Amp s.c. Group V mice were administered cryptdin 2 s.c. at a single dose of 5 μg/mouse. Group VI mice were coadministered cryptdin 2 (5 μg/mouse; s.c.) and Amp (16 mg/kg body weight; s.c.). Group VII mice were coadministered cryptdin 2 (5 μg/mouse; s.c.) and Amp (32 mg/kg body weight; s.c.). Group VIII mice were coadministered cryptdin 2 (5 μg/mouse; s.c.) and Amp (64 mg/kg body weight; s.c.). Group IX mice were coadministered cryptdin 2 (2.5 μg/mouse; s.c.) and Amp (8 mg/kg body weight; s.c.). Group X mice were coadministered cryptdin 2 (2.5 μg/mouse; s.c.) and Amp (16 mg/kg body weight; s.c.). Group XI mice were coadministered cryptdin 2 (2.5 μg/mouse; s.c.) and Amp (32 mg/kg body weight; s.c.). At 48 h posttherapy, mice were sacrificed and their livers, small intestines, and spleens were removed aseptically. Tissues were weighed, and 10% homogenates were prepared in PBS. Serial 10-fold dilutions of each homogenate were plated on MacConkey agar medium for enumeration of CFU in different groups.
Statistical analysis.
Data are expressed as means ± standard deviations for three to five independent experiments. Statistical analysis was done by Student's unpaired t test and one-way analysis of variance (ANOVA), followed by pairwise comparison procedures (Tukey test), using Jandel Sigma Stat statistical software, version 2.0. In all cases, statistical significance was defined as having a P value of <0.05.
RESULTS
In vitro activity of cryptdin 2 and Amp against Salmonella. (i) Minimum bactericidal concentrations.
Cryptdin 2 and Amp decreased the CFU of Salmonella Typhimurium in vitro in a concentration-dependent manner. When Salmonella Typhimurium cells were incubated with 0.5, 1, 1.5, 2, 4, 8, and 16 μg/ml of Amp, no visible growth was observed at 4 μg/ml (10.77 μmol/liter), indicating this concentration as the MBC of Amp against S. Typhimurium. Similarly, the MBC of cryptdin 2 against Salmonella was evaluated to be 19 μg/ml (4.47 μmol/liter), as reported earlier (29).
(ii) Fractional bactericidal concentrations.
The combination of cryptdin 2 and Amp was found to be highly effective in vitro, as evidenced by the reduced MBCs of 5 μg/ml and 0.5 μg/ml for cryptdin 2 and Amp, respectively. The FBC index was calculated to be 0.388, which indicated in vitro synergy between the two agents.
(iii) Time-kill assay.
After 120 min, cryptdin 2 (19 μg/ml) and Amp (4 μg/ml) administered separately gave significant decreases (P < 0.01), of 4.11 and 3.26 log units, respectively, compared to the control (120 min). However, when they were used in combination (cryptdin 2 at 5 μg/ml and Amp at 0.5 μg/ml), a decrease of 5.32 log units was observed after 120 min (P < 0.05), indicating an in vitro synergetic effect (Fig. 1).
Fig. 1.
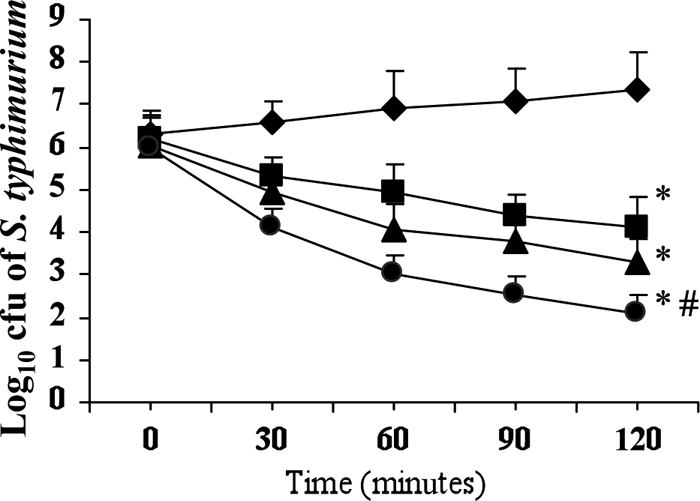
Log10 CFU of Salmonella Typhimurium NCTC74 at various time intervals in the presence of ampicillin alone at 4 μg/ml (■), cryptdin 2 alone at 19 μg/ml (▴), and both drugs in combination, i.e., Amp at 0.5 μg/ml plus cryptdin 2 at 5 μg/ml (●). Values are expressed as means ± SD for three independent experiments. *, P < 0.01 versus log10 CFU of Salmonella after 120 min in the absence of any antibacterial agent (control; ♦); #, P < 0.05 versus log10 CFU of Salmonella after 120 min in the presence of Amp (4 μg/ml) (■).
Activities of cryptdin 2 and Amp against intracellular Salmonella.
The mean percent intracellular killing in untreated macrophages was found to be 17.1% ± 3.4%, 27.6% ± 3.67%, and 43.4% ± 5.2% at 30, 60, and 90 min, respectively. On the other hand, the mean percent intracellular killing in the presence of cryptdin 2 (19 μg/ml) alone at 30, 60, and 90 min was 31.5% ± 4.98% (P < 0.05), 46% ± 5.16% (P < 0.05), and 61.8% ± 8.57%, respectively, compared to that in untreated cells. Similarly, when infected macrophages were treated with Amp (4 μg/ml) alone, the mean percent intracellular killing was 30.2% ± 4.23% (P < 0.05), 40.7% ± 3.95% (P < 0.05), and 59.2% ± 6.17% at 30, 60, and 90 min, respectively. A higher percentage of intracellular killing was observed when infected macrophages were treated with cryptdin 2 (5 μg/ml) in conjunction with Amp (0.5 μg/ml) at lower doses, indicating ex vivo synergy. In this case, the mean percent killing was found to be 43.4% ± 5.5% (P < 0.05), 57.8% ± 6.09% (P < 0.01), and 82.8% ± 10.05% (P < 0.001) at 30, 60, and 90 min, respectively. These results indicate that both cryptdin 2 and Amp might act in conjunction with macrophage antibacterial effectors to lead to enhanced killing of intracellular salmonellae.
Estimation of lipid peroxidation levels.
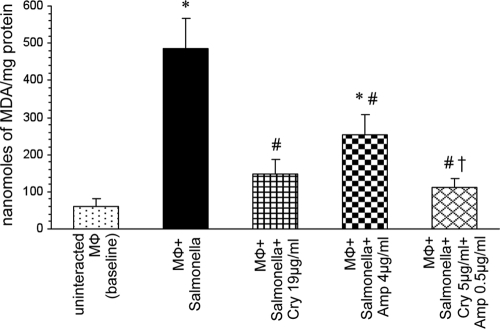
Significant decreases in the levels of MDA were observed when macrophages were incubated with S. Typhimurium in the presence of cryptdin 2 (P < 0.01) or Amp (P < 0.05) alone compared to those in macrophages incubated with Salmonella in the absence of any antimicrobial agent. However, a much larger reduction in MDA levels was observed (P < 0.01) when macrophages were incubated in the presence of both cryptdin 2 and Amp (Fig. 2).
Fig. 2.
Effect on MDA levels of macrophages (Mφ) incubated with S. Typhimurium in the presence of Amp and cryptdin (Cry) alone and in combination. Values are expressed as means ± SD for four independent experiments. Statistical analysis was done using one-way ANOVA. *, P < 0.01 versus uninfected macrophages (baseline); #, P < 0.05 versus macrophages incubated with Salmonella; †, P < 0.05 versus macrophages incubated with Salmonella in the presence of Amp.
Estimation of nitrite levels.
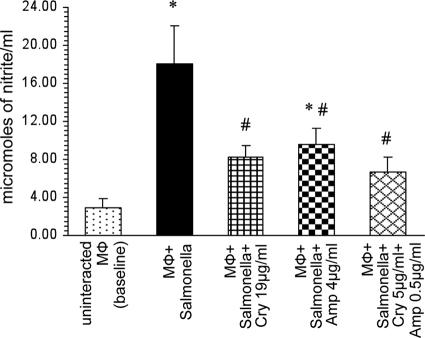
A significant decrease (P < 0.05) in the level of nitrite produced by macrophages was observed in the presence of cryptdin 2 compared to that in macrophages incubated with Salmonella in the absence of any antimicrobial agent. Similarly, nitrite levels of infected macrophages were significantly reduced in the presence of Amp also, but to a lesser extent (P < 0.05) (Fig. 3). However, a more pronounced effect (P < 0.05) was observed when macrophages were incubated with Salmonella in the presence of both cryptdin 2 and Amp (Fig. 3).
Fig. 3.
Effect on nitrite levels of macrophages (Mφ) incubated with S. Typhimurium in the presence of Amp and cryptdin (Cry) alone and in combination. Values are expressed as means ± SD for four independent experiments. Statistical analysis was done using one-way ANOVA. *, P < 0.05 versus uninfected macrophages (baseline); #, P < 0.05 versus macrophages incubated with Salmonella.
Estimation of SOD levels.
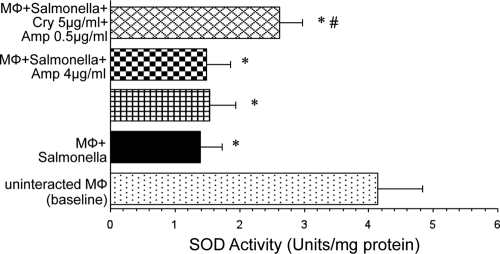
Interaction of macrophages with Salmonella induced a significant decrease in the SOD activity in the culture supernatant of macrophages (P < 0.01). No significant change in SOD activity was observed in the presence of cryptdin 2 or Amp alone compared to that in normally interacting macrophages in the absence of any antimicrobial agent. However, supplementation of the reaction mixture with both cryptdin 2 and Amp significantly restored the SOD activity (P < 0.05) (Fig. 4).
Fig. 4.
Effect on activity of superoxide dismutase of macrophages (Mφ) incubated with S. Typhimurium in the presence of Amp and cryptdin (Cry) alone and in combination. Values are expressed as means ± SD for four independent experiments. Statistical analysis was done using one-way ANOVA. *, P < 0.05 versus uninfected macrophages (baseline); #, P < 0.05 versus macrophages incubated with Salmonella.
Therapeutic potential and synergetic efficacy of cryptdin 2 and Amp against experimental salmonellosis.
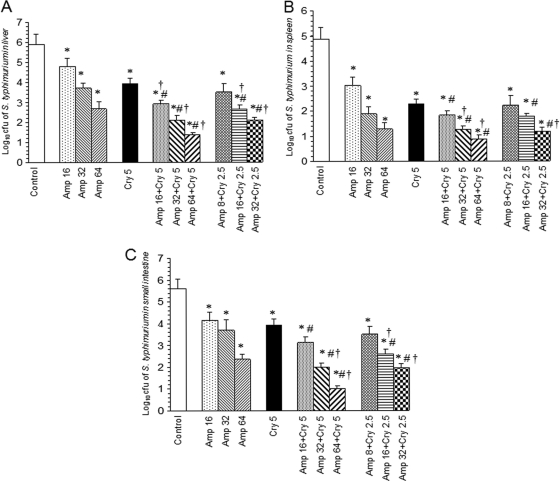
The therapeutic efficacy of cryptdin 2 and Amp alone and in conjunction was investigated in terms of reduction in the number of salmonellae in different target organs of mice infected with Salmonella Typhimurium after 48 h of chemotherapy. In the livers of treated mice, log unit decreases in bacterial loads were 1.1, 2.2, and 3.23 for 16, 32, and 64 mg Amp/kg body weight, respectively, while a 1.96-log-unit decrease was observed after treatment with cryptdin 2. Coadministration of 5 μg of cryptdin 2 with 16, 32, and 64 mg Amp/kg body weight gave higher log unit decreases of 3, 3.79, and 4.52, respectively (Fig. 5A). In spleens of mice, log unit decreases in bacterial loads were observed to be 1.86, 2.98, 3.57, and 2.59 after treatment with 16, 32, and 64 mg Amp/kg body weight and with cryptdin 2 (5 μg), respectively. In this case also, the adjunct therapy of 5 μg cryptdin 2 with 16, 32, and 64 mg Amp/kg body weight was found to be more effective, as higher log unit decreases of 3.4, 3.61, and 3.98, respectively, were observed (Fig. 5B). In the small intestines, cryptdin 2 (5 μg/mouse) alone gave a decrease in bacterial loads of 1.67 log units compared to untreated controls. There were log unit decreases of 1.43, 1.92, and 3.25 in the number of salmonellae in small intestines of mice treated with 16, 32, and 64 mg Amp/kg body weight, respectively (Fig. 5C). However, when 5 μg of cryptdin 2 was used in combination with 16, 32, and 64 mg Amp/kg body weight, the log unit decreases in intestinal bacterial loads were found to be 2.47, 3.62, and 4.58 units, respectively. Interestingly, when the mice were treated with half of each Amp dose in conjunction with half the dose of cryptdin 2 (2.5 μg/mouse), the log unit decreases in the numbers of salmonellae in all target organs were observed to be comparable to the decreases observed when both agents were used together at 2-fold higher concentrations.
Fig. 5.
Log10 CFU of S. Typhimurium in livers (A), spleens (B), and small intestines (C) of infected mice after 48 h of therapy with cryptdin 2 (Cry) and ampicillin (Amp), separately and in combination. Values are expressed as means ± SD for two independent experiments. Statistical analysis was done using one-way ANOVA. *, P < 0.05 versus log10 CFU of S. Typhimurium in untreated mice (control); #, P < 0.05 versus log10 CFU of S. Typhimurium in mice treated with Amp (16 mg/kg body weight); †, P < 0.05 versus log10 CFU of S. Typhimurium in mice treated with cryptdin (5 μg/mouse).
DISCUSSION
A strategy to overcome the problem of emerging drug resistance in Salmonella strains is to avoid overuse and misuse of antibiotics (1, 24). In this regard, the use of cationic antimicrobial peptides in conjunction with conventional antibiotics might prove useful, as this would reduce the dose of antibiotics required to treat the disease, thereby decreasing the associated side effects. In light of these facts, the present study aimed at evaluating the synergetic effect of ampicillin in conjunction with cryptdin 2 against Salmonella Typhimurium.
In vitro synergy was evidenced from the FBC index and the time-kill assay, as a larger antibacterial effect was exhibited when the two agents were used against Salmonella in combination at concentrations much lower than their individual MBCs. This can be attributed to an increased permeability of the Salmonella cell membrane created by cryptdin 2, thereby leading to an increased diffusion of Amp into the intracellular targets. These observations are consistent with previous studies pertaining to the synergetic actions of α-helical peptide p18, reproductive tract beta-defensins, HNP-1, magainin, cathelicidins, and several other novel cationic peptides with commonly used antibiotics against Escherichia coli, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterobacter cloacae (7, 16, 32, 36, 37).
Salmonella has been reported to establish a niche within macrophages where it replicates and causes systemic disease. The dynamic interplay between host responses and bacterial virulence mechanisms determines the outcome of the infection. At this stage, antibacterial agents may directly or indirectly modulate the natural phagocyte-bacterium interactions (18). Keeping this in view, the ex vivo effect of cryptdin 2 and Amp on macrophage functions was evaluated in terms of intracellular killing, extent of lipid peroxidation, superoxide dismutase activity, and generation of reactive nitrogen intermediates (RNIs).
Treatment of infected macrophages with cryptdin 2 and Amp individually exhibited more killing of intracellular salmonellae than that by untreated macrophages. Interestingly, the killing effect was found to be more pronounced when both agents were used in combination to treat intracellular salmonellae. These results are in concordance with earlier reports wherein it was proposed that ampicillin kills intracellular salmonellae within mouse macrophages owing to its internalization by cells through pinocytosis (6, 18). The fact that alpha-defensins may enhance phagocytosis by interacting directly with the pathogen may account for the higher killing level observed when macrophages were treated with cryptdin 2 (29). In support of our findings, defensins have been reported to enable macrophages to inhibit intracellular proliferation of Listeria (2).
There is evidence that Salmonella infection results in excessive production of reactive oxygen species, thereby leading to lipid peroxidation and finally to tissue damage (25, 30). Interaction of macrophages with Salmonella in the presence of cryptdin 2 in conjunction with Amp decreased the extent of LPO and restored the SOD level. In particular, the SOD activity appeared to be enhanced synergistically in the presence of the combination. These results indicate that these agents might have decreased LPO by scavenging the free radicals and by upregulating the antioxidant activity, thus counteracting the oxidative stress. In agreement with our findings, it has been reported that antibacterial agents, including defensins, may directly scavenge the oxidants produced as a result of phagocyte-bacterium interaction (18). It is also possible that cryptdin 2 might contribute to inhibition of quorum sensing (in terms of bacterial density due to direct killing), thereby rendering the number of surviving salmonellae insufficient to activate macrophages.
Nitric oxide (NO) is an important signaling molecule that acts in many tissues to regulate a diverse range of physiological processes (25). However, excessive amounts of NO are potentially toxic and have been implicated in numerous pathological situations and chronic inflammation (3). RNIs such as nitrites are known to be the end products of oxidative metabolism of labile nitric oxide, and their quantification is regarded as an indicator of NO generation (5). A significantly increased level of nitrite in macrophages infected with Salmonella might combine with superoxide anions to form peroxynitrites, which further stimulate the production of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), contributing to tissue injury. However, when macrophages were infected with Salmonella in the presence of cryptdin 2 alone or in combination with ampicillin, significant decreases in nitrite levels were observed. It might be possible that these agents neutralize the Salmonella-induced release of nitric oxide in macrophages, as reported earlier (43). Additionally, the lower levels of nitrite observed in the macrophages treated with cryptdin 2 might be attributed to the anti-inflammatory activity of cryptdin, as has been reported for various other AMPs (4, 12).
The results of in vivo studies also indicated synergism between Amp and cryptdin 2, as more clearance of salmonellae from all target organs was observed when both agents were used in combination than the bactericidal effect observed when the agents were used alone at the same doses. These results are in concordance with earlier reports suggesting a synergetic effect of antimicrobial peptides with β-lactam antibiotics (16, 36). The most interesting finding of our study was that the addition of cryptdin 2 not only potentiated the effectiveness of ampicillin but also reduced the therapeutic dosage of both agents to half (from 5 μg to 2.5 μg per mouse of cryptdin 2 and from 16, 32, and 64 mg/kg to 8, 16, and 32 mg/kg of Amp) while maintaining the increased therapeutic efficacy, compared with that of the drugs alone, against experimental salmonellosis. In the present study, a significantly lower concentration of cryptdin 2 (5 μg per mouse) was found to clear Salmonella infection in vivo than that required for in vitro anti-Salmonella activity (19 μg/ml), in concordance with our earlier report (29). The therapeutic efficacy of cryptdin 2 might be attributed to the property of antimicrobial-immunomodulatory duality exhibited by host defense peptides. Earlier, various AMPs were also shown to possess a broad range of immunomodulatory properties, including chemoattraction of immune cells, induction of chemokines, and resolution of infections (4, 12, 14, 38). Thus, it can be hypothesized that cryptdin 2 may act as an antibacterial as well as immunostimulatory molecule in vivo in Salmonella-infected mice, where it is recognized as an effector molecule of innate immunity. The implication of the present study is that cryptdin 2 can be used as a possible adjunct to ampicillin for superior treatment of salmonellosis. These observations seem to be significant, as reducing the therapeutic concentrations of antibiotics may be a valuable strategy for avoiding the development of emerging antibiotic resistance. In addition to the findings of the present study, cryptdin 2 has also been observed to possess antibacterial activity against other Gram-negative organisms, such as Yersinia enterocolitica (MBC, 21 μg/ml) and E. coli (MBC, 10 μg/ml), as well as against Staphylococcus aureus (MBC, 12 μg/ml) and Entamoeba histolytica (minimum amoebicidal concentration, 4 μg/ml) (unpublished data). In light of these data, the peptide seems to be a promising broad-spectrum antimicrobial agent.
ACKNOWLEDGMENTS
This work was supported by the Indian Council of Medical Research (ICMR), New Delhi, India, under the extramural research scheme [grant 5/8-1(207)/D/2005/ECD-II].
We declare that there are no conflicts of interest.
Footnotes
Published ahead of print on 20 June 2011.
REFERENCES
- 1. Aarestrup F. M., et al. 2001. Effect of abolishment of the use of antimicrobial agents for growth promoters on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnett E., Lehrer R. I., Pratikhya P., Lu W., Seveau S. 2011. Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. [Epub ahead of print.] doi:10.1111/j.1462-5822.2010.01563.x [DOI] [PubMed] [Google Scholar]
- 3. Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907–916 [DOI] [PubMed] [Google Scholar]
- 4. Bowdish D. M. E., et al. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451–459 [DOI] [PubMed] [Google Scholar]
- 5. Chanana V., Ray P., Rishi D. B., Rishi P. 2007. Reactive nitrogen intermediates and monokines induce caspase-3 mediated macrophage apoptosis by anaerobically stressed Salmonella typhi. Clin. Exp. Immunol. 150:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang H. R., Vladoianu I.-R., Pechere J.-C. 1990. Effects of ampicillin, ceftriaxone, chloramphenicol, pefloxacin and trimethoprim-sulphamethoxazole on Salmonella typhi within human monocyte-derived macrophages. J. Antimicrob. Chemother. 26:689–694 [DOI] [PubMed] [Google Scholar]
- 7. Darveau R. P., et al. 1991. Beta-lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob. Agents Chemother. 35:1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisenhauer P. B., Harwig S. S. S. L., Lehrer R. I. 1992. Cryptdins: antimicrobial defensins of the murine small intestine. Infect. Immun. 60:3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foureau D. M., et al. 2010. TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J. Immunol. 184:7022–7029 [DOI] [PubMed] [Google Scholar]
- 10. Giacometti A., Cirioni O., Barchiesi F., Fortuna M., Scalise G. 1999. In vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 44:641–645 [DOI] [PubMed] [Google Scholar]
- 11. Green L. C., et al. 1982. Analysis of nitrate, nitrite and 15N nitrate in biological fluids. Anal. Biochem. 126:131–138 [DOI] [PubMed] [Google Scholar]
- 12. Hancock R. E. W., Sahl H.-G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 13. Ibezim E. C., et al. 2006. A study of the in-vitro interaction of co-trimoxazole and ampicillin using the checker board method. Afr. J. Biotechnol. 5:1284–1288 [Google Scholar]
- 14. Inoue R., et al. 2008. Postnatal changes in the expression of genes for cryptdins 1–6 and the role of luminal bacteria in cryptdin gene expression in mouse small intestine. FEMS Immunol. Med. Microbiol. 52:407–416 [DOI] [PubMed] [Google Scholar]
- 15. Izumiya H., et al. 2011. Whole-genome analysis of Salmonella enterica serovar Typhimurium T000240 reveals the acquisition of a genomic island involved in multidrug resistance via IS1 derivatives on the chromosome. Antimicrob. Agents Chemother. 55:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalita A., Verma I., Khuller G. K. 2004. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J. Infect. Dis. 190:1476–1480 [DOI] [PubMed] [Google Scholar]
- 17. Kono Y. 1978. Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 186:189–195 [DOI] [PubMed] [Google Scholar]
- 18. Labro M. T. 2000. Interference of antibacterial agents with phagocyte functions: immunomodulation or immuno-fairy tales. Clin. Microbiol. Rev. 13:615–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lencer W. I., et al. 1997. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc. Natl. Acad. Sci. U. S. A. 94:8585–8589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lowry O. H., Rosenbrough N. J., Farr A. L., Randell R. J. 1951. Protein measurement with Folin's phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 21. Luders T., Berkemo G. A., Finland G., Nissen-Meyer J., Nes I. F. 2003. Strong synergy between a eukaryotic antimicrobial peptide and bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 69:1797–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mastroianni J. R., Ouellette A. J. 2009. α-Defensins in enteric innate immunity: functional Paneth cell α-defensins in mouse colonic lumen. J. Biol. Chem. 284:27848–27856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masuda K., Sakai N., Nakamura K., Yoshioka S., Ayabe T. 2010. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J. Innate Immun. [Epub ahead of print.] doi:10.1159/000322037 [DOI] [PubMed] [Google Scholar]
- 24. McEwen S. A., Fedorka-Cray P. J. 2002. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 34:S93–S106 [DOI] [PubMed] [Google Scholar]
- 25. Moncada S., Palmer R. M., Higgs E. A. 1991. Nitric oxide: physiology, patho-physiology and pharmacology. Pharmacol. Rev. 43:109. [PubMed] [Google Scholar]
- 26. Nikaido E., Yamaguchi A., Nishino K. 2008. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J. Biol. Chem. 283:24245–24253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piddock L. J. V., White D. G., Gensberg K., Pumbwe I., Griggs D. J. 2000. Evidence for an efflux pump mediating multiple antibiotic resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 44:3118–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Preet S., Rishi P. 2010. Antimicrobial activity of Paneth-cell derived cryptdin-2 against selected pathogens. Am. J. Biomed. Sci. 2:13–22 [Google Scholar]
- 29. Preet S., Verma I., Rishi P. 2010. Cryptdin-2: a novel therapeutic agent for experimental Salmonella typhimurium infection. J. Antimicrob. Chemother. 65:991–994 [DOI] [PubMed] [Google Scholar]
- 30. Rishi P., et al. 2006. Are the increases in local tumour necrosis factor and lipid peroxidation observed in pre-starved mice infected with Salmonella typhimurium markers of increased liver damage? Microbes Infect. 8:1695–1701 [DOI] [PubMed] [Google Scholar]
- 31. Scott M. G., Hancock R. E. W. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407–431 [PubMed] [Google Scholar]
- 32. Shin S. Y., et al. 2002. Salt resistance and synergistic effect with vancomycin of alpha-helical antimicrobial peptide P18. Biochem. Biophys. Res. Commun. 290:558–562 [DOI] [PubMed] [Google Scholar]
- 33. Su L. H., Chiu C. H., Chu C., Ou J. T. 2004. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin. Infect. Dis. 39:546–551 [DOI] [PubMed] [Google Scholar]
- 34. Suh Y. C. 2010. Plasmid-encoded multidrug resistance: a case study of Salmonella and Shigella from enteric diarrhea sources among humans. Biol. Res. 43:141–148 [PubMed] [Google Scholar]
- 35. Tanabe H., Ouellette A. J., Cocco M. J., Robinson W. E. 2004. Differential effects on human immunodeficiency virus type 1 replication by α-defensins with comparable bactericidal activities. J. Virol. 78:11622–11631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ulvatne H., Karoliussen S., Stiberg T., Rekdal O., Svendsen J. S. 2001. Short antibacterial peptides and erythromycin act synergically against Escherichia coli. J. Antimicrob. Chemother. 48:203–208 [DOI] [PubMed] [Google Scholar]
- 37. Vaara M., Poro M. 1996. Group of peptides that act synergistically with hydrophobic antibiotics against Gram-negative enteric bacteria. Antimicrob. Agents Chemother. 40:1801–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Welling M. M., et al. 1998. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J. Clin. Invest. 102:1583–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wiesner J., Vilcinskas A. 2010. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1:440–464 [DOI] [PubMed] [Google Scholar]
- 40. Wills E. D. 1966. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 99:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiradharma N., et al. 2011. Synthetic cationic amphiphilic α-helical peptides as antimicrobial agents. Biomaterials 32:2204–2212 [DOI] [PubMed] [Google Scholar]
- 42. Yenugu S., Narmadha G. 2010. The human male reproductive tract antimicrobial peptides of the HE2 family exhibit potent synergy with standard antibiotics. J. Pept. Sci. 16:337–341 [DOI] [PubMed] [Google Scholar]
- 43. Zughaier S. M., Shafer W. M., Stephens D. S. 2005. Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell. Microbiol. 7:1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]