Abstract
Fidaxomicin (FDX), a narrow-spectrum antibiotic recently shown to be superior to vancomycin in providing sustained clinical response to Clostridium difficile infection, was investigated along with its major metabolite, OP-1118, with regard to their postantibiotic effects (PAE). FDX was found to have a prolonged PAE (10 h versus ATCC strains and 5.5 h versus a clinical isolate), and OP-1118's PAE was longer than that of the standard comparator, vancomycin (3 versus 0 to 1.5 h, respectively).
TEXT
Clostridium difficile infections (CDI) continue to be a major cause of diarrhea in hospitalized patients undergoing antibiotic treatment and among the elderly in long-term-care facilities. Current standard therapies for CDI are inadequate and often result in disease recurrence that can lead to multiple episodes in some individuals (12, 16). In contrast, fidaxomicin (FDX), a new narrow-spectrum antibacterial agent with no cross-resistance to known antibiotics (1, 2, 6, 7, 8, 9, 10, 13, 18), has been demonstrated to be superior to vancomycin in sustaining a cure without recurrence of CDI (11). Following oral administration (400 mg/day) for 10 days, FDX and its major metabolite, OP-1118 (derived by hydrolysis of the isobutyryl ester located at the 4″ position of fidaxomicin), are detected abundantly (in mg/g concentrations and in approximately a 2:1 ratio, respectively) in stool samples (17). Like FDX, OP-1118 demonstrates narrow-spectrum activity, albeit with a 32-fold increase in MIC relative to that of FDX, for 90% of C. difficile strains (MIC90) tested from phase 3 trials. This study investigates the postantibiotic effects (PAE) of both FDX and OP-1118 in comparison to those of vancomycin and metronidazole against C. difficile ATCC strains (ATCC 9689 and ATCC 43255) and a clinical isolate (LC3).
In order to use drug concentrations above the MIC for the PAE experiments, the MIC values were determined via the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method, which more closely represents the conditions of PAE experiments (4). Lysed blood, which obscures the color of media, was omitted from brucella broth so that the redox indicator resazurin could be used to monitor the anaerobicity. Briefly, microtiter plates with serially diluted drugs were equilibrated inside an anaerobic glove box for a minimum of 3 h. C. difficile was added to each well (105 CFU/well), and following 48 h of incubation at 35°C, the plates were examined for growth. The MIC was determined as the drug concentration where no growth or the most significant reduction of growth was observed. Drug MIC values for 3 strains of C. difficile, shown in Table 1, represent multiple determinations performed on different days. The MIC values did not vary by more than 1 dilution between experiments. Additionally, MIC values versus the quality control C. difficile ATCC 700057 strain were within the CLSI's expected ranges, indicating that the omission of lysed blood from brucella broth did not affect the growth or reproducibility of the MIC values. Both vancomycin and metronidazole demonstrated MIC values of 0.5 to 1 μg/ml versus all 3 strains, whereas the FDX MICs were 0.125 μg/ml versus the clinical isolate and ATCC 43255 and 0.016 to 0.03 μg/ml versus ATCC 9689. OP-1118 demonstrated 16-fold-higher MIC values than FDX versus both ATCC strains.
Table 1.
MICs of fidaxomicin, OP-1118, vancomycin, and metronidazole
| Antibiotic | MIC(s) (μg/ml) for C. difficile straina: |
||
|---|---|---|---|
| ATCC 43255 | ATCC 9689 | LC3 | |
| Fidaxomicin | 0.125 | 0.03, 0.016 | 0.125 |
| OP-1118 | 2 | 0.25 | ND |
| Vancomycin | 1, 0.5 | 1 | 1 |
| Metronidazole | 1, 0.5 | 1, 0.5 | ND |
ND, no data. Where the MIC values from different runs were dissimilar, both values are presented.
Using the standard viable-cell plate count method described by Craig and Gudmundsson (5), the PAE for all antibiotics was determined under anaerobic conditions. Briefly, each organism, grown to log phase, was incubated with an ∼4-fold MIC of antibiotic for 1 h at 35°C. Prior to and after three washing steps (via centrifugation and supernatant removal), samples were withdrawn at different time intervals and plated, in duplicate, onto blood agar plates for a determination of the number of CFU. Additional control treatments, with antibiotic concentrations at 1/100 and 1/1,000 of the amount present during antimicrobial exposure, were also performed to confirm that residual drug did not affect the rate of growth. All antibiotic-bacterium combinations were performed at least in duplicate.
The postantibiotic effect was calculated using the following formula: PAE = T − C, where T is the time required for the titer to increase 1 log10 over the postwashing titer in the presence of antibiotic and C is the time required for the titer to increase 1 log10 over the postwashing titer in the absence of antibiotic. Modal values of multiple experiments were used for T and C. No attempts were made to interpolate between time points when the 1-log increase occurred between time points. In most cases, the log10 increase was called at the nearest time point. When the time points were far apart (i.e., the endpoint was reached overnight), the endpoint is presented as limits.
Recovery kinetics following incubation of ATCC 9689 (6 experiments) and ATCC 43255 (5 experiments) with OP-1118 and metronidazole are summarized in Table 2. Fidaxomicin recovery kinetics were also determined for comparison to results of earlier studies that yielded PAE values in excess of 12 h (3). While OP-1118 (at 4× the mean MIC) demonstrated PAE of 3 h versus both ATCC strains, the metronidazole PAE could not be reliably determined. When used at 2 μg/ml (at 2.6× the mean MIC), the metronidazole PAE versus strain ATCC 9689 was 3 h but at 4 μg/ml (at 5.3× the mean MIC), the metronidazole PAE against strain ATCC 43255 could not be determined, because metronidazole rapidly lowered the viability of cells to below the level of quantitation.
Table 2.
Recovery kinetics for C. difficile ATCC 9689 and ATCC 43255 after OP-1118 or metronidazole exposurea
| Strain | Antibiotic | Time to increase 1 log (h) | Duration of PAE (h) |
|---|---|---|---|
| ATCC 9689 | None (control) | 3 | NA |
| OP-1118 | 6 | 3 | |
| Metronidazole | 6 | 3 | |
| ATCC 43255 | None (control) | 3 | NA |
| OP-1118 | 6 | 3 | |
| Metronidazole | NC | NC |
The PAE is based on the mode obtained from a minimum of five separate experiments. NA, not applicable; NC, not calculable (the number of CFU/ml was too low for quantitation).
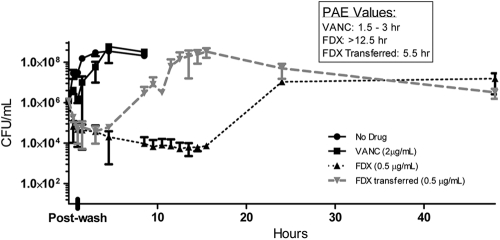
Fidaxomicin, included as another control in the above-described experiments, resulted in a slow recovery, consistent with earlier observations. At a 4×-MIC exposure, C. difficile ATCC 43255 recovery required overnight incubation, and C. difficile ATCC 9689 did not consistently recover following exposure to 0.125 μg/ml fidaxomicin. Since fidaxomicin is a hydrophobic compound that may adsorb nonspecifically to plastic surfaces, we surmised that the unusually long fidaxomicin PAE may actually be an artifact of nonspecific binding of sub-MIC drug concentrations followed by a slow release back into the medium. In fact, for MIC determination via the broth microdilution method, we have found that without pipette tip changes (during serial dilution of a compound), MIC values tend to be 2- to 4-fold lower than if the drug dilution is performed with repeated tip changes, indicating possible drug carryover through pipette tips. To eliminate any remaining drug during recovery, cells (following drug removal) were transferred to a new test tube and their recovery was compared to that of cells that remained in the original tube. Indeed, upon transfer to a new tube, the strains recovered faster, with times to recovery of up to 9.5 to 12.5 h for the ATCC strains (Fig. 1 and 2) and 5.5 h for the LC3 clinical strain (Fig. 3). In contrast, PAE values for cells remaining in their original tubes were above 12.5 h for LC3 and ATCC 43255 and could not be determined for ATCC 9689, indicating the presence of residual fidaxomicin in the original tubes. Vancomycin, used as a control (at 4× the MIC for the ATCC strains and at 2× the MIC for the clinical isolate), performed as expected, with a PAE of 0 to 1.5 h. While it is possible that the higher FDX PAE value (up to 24 h) represents the sub-MIC PAE phenomenon that has been reported for other drugs and organisms (15), we did not conduct further investigation to confirm a sub-MIC PAE effect.
Fig. 1.
Recovery kinetics of C. difficile ATCC 9689 following a 1-h exposure to fidaxomicin (OPT-80) and vancomycin (VANC). The data represent average values from two separate runs.
Fig. 2.
Recovery kinetics of C. difficile ATCC 43255 following a 1-h exposure to fidaxomicin (OPT-80) and vancomycin (VANC). The data represent average values from two separate runs.
Fig. 3.
Recovery kinetics of C. difficile LC3 following a 1-h exposure to fidaxomicin (OPT-80) and vancomycin (VANC). The data represent average values from two separate runs.
A possible mechanism for such a long suppression of growth by FDX may be due to either specific binding to RNA polymerase or nonspecific binding to bacterial cell components or both; by this/these means, fidaxomicin may be able to remain inside the cell and exert its antimicrobial activity. If so, only after slow dissociation and diffusion of drug from the bacteria would the cell be allowed to undergo repair followed by transcription and translation of proteins, leading to bacterial growth and recovery. A similar impact is not expected for the metabolite OP-1118, which is significantly less hydrophobic (it has an approximately 100-fold-higher solubility in water) and has a 32-fold-greater MIC90 than fidaxomicin against C. difficile.
In summary, our findings of shorter PAE for vancomycin and metronidazole than for fidaxomicin and its metabolite are consistent with a previous publication (14). Our study further demonstrates that FDX and its metabolite have long PAE. In clinical studies, fidaxomicin dosed twice daily was equivalent to vancomycin dosed four times daily in achieving a resolution of symptoms and was superior in preventing recurrences of CDI.
Acknowledgments
This work was supported by a Public Health Service grant from the National Institutes of Health (5R44 AI 63692-04 [New Treatment for C. difficile-Associated Diarrhea]).
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Ackermann G., Loffler B., Adler D., Rodloff A. C. 2004. In vitro activity of OPT-80 against Clostridium difficile. Antimicrob. Agents Chemother. 48:2280–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babakhani F., Shangle S., Robert N., Sears P., Shue Y. K. 2004. Resistance development, cross-resistance, and synergy studies of OPT-80, abstr. E-2047. 44th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 3. Babakhani F. K., et al. 2004. Antimicrobial activity and postantibiotic effect of OPT-80, a new macrocyclic compound, against Clostridium difficile, abstr. E-2048. 44th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 4. CLSI 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria; 7th ed Approved standard M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Craig W. A., Gudmundsson S. 1996. “Postantibiotic effect,” p. 296–329 In Lorian V. (ed.), Antibiotics in laboratory medicine, 4th ed Williams and Wilkins Co., Baltimore, MD [Google Scholar]
- 6. Credito K. L., Applebaum P. C. 2004. Activity of OPT-80, a novel macrocycle, compared with those of eight other agents against selected anaerobic species. Antimicrob. Agents Chemother. 48:4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finegold S. M., et al. 2004. In vitro activity of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob. Agents Chemother. 48:4898–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hecht D. W., et al. 2007. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 51:2716–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karlowsky J. A., Laing N. M., Zhanel G. G. 2008. In vitro activity of OPT-80 tested against clinical isolates of toxin producing Clostridium difficile. Antimicrob. Agents Chemother. 52:4163–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louie T. J., Emery J., Krulicki W., Byrne B., Mah M. 2009. OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob. Agents Chemother. 53:261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Louie T. J., et al. 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364:22–23 [DOI] [PubMed] [Google Scholar]
- 12. McFarland L. V. 2005. Alternative treatments for Clostridium difficile: what really works? J. Med. Microbiol. 54:101–111 [DOI] [PubMed] [Google Scholar]
- 13. Miller M. 2010. Fidaxomicin (OPT-80) for the treatment of Clostridium difficile infection. Expert Opin. Pharmacother. 11:1569–1578 [DOI] [PubMed] [Google Scholar]
- 14. Odenholt I., Walder M., Wullt M. 2007. Pharmacodynamic studies of vancomycin, metronidazole, and fusidic acid against Clostridium difficile. Chemotherapy 53:267–274 [DOI] [PubMed] [Google Scholar]
- 15. Odenholt-Tornqvist I., Lowdin E., Cars O. 1992. Postantibiotic sub-MIC effects of vancomycin, roxithromycin, sparfloxacin, and amikacin. Antimicrob. Agents Chemother. 9:1852–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rupnik M., Wilcox M. H., Gerding D. N. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature 7:526–536 [DOI] [PubMed] [Google Scholar]
- 17. Shue Y. K., et al. 2008. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob. Agents Chemother. 52:1391–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tannock G. W., et al. 2010. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 156:3354–3359 [DOI] [PubMed] [Google Scholar]