Abstract
The efficacy of ceftobiprole combined with vancomycin was tested against two vancomycin-intermediate Staphylococcus aureus (VISA) strains, PC3 and Mu50, in rats with experimental endocarditis. Animals with infected aortic vegetations were treated for 3 days with doses simulating the kinetics after intravenous administration in humans of (i) the standard dose of ceftobiprole of 500 mg every 12 h (b.i.d.) (SD-ceftobiprole), (ii) a low dose of ceftobiprole of 250 mg b.i.d. (LD-ceftobiprole), (iii) a very low dose of ceftobiprole of 125 mg b.i.d. (VLD-ceftobiprole), (iv) SD-vancomycin of 1 g b.i.d., or (v) LD- or VLD-ceftobiprole combined with SD-vancomycin. Low dosages of ceftobiprole were purposely used to highlight positive drug interactions. Treatment with SD-ceftobiprole sterilized 12 of 14 (86%) and 10 of 13 (77%) vegetations infected with PC3 and Mu50, respectively (P < 0.001 versus controls). In comparison, LD-ceftobiprole sterilized 10 of 11 (91%) vegetations infected with PC3 (P < 0.01 versus controls) but only 3 of 12 (25%) vegetations infected with Mu50 (P > 0.05 versus controls). VLD-ceftobiprole and SD-vancomycin alone were ineffective against both strains (≤8% sterile vegetations). In contrast, the combination of VLD-ceftobiprole and SD-vancomycin sterilized 7 of 9 (78%) and 6 of 14 (43%) vegetations infected with PC3 and Mu50, respectively, and the combination of LD-ceftobiprole and SD-vancomycin sterilized 5 of 6 (83%) vegetations infected with Mu50 (P < 0.05 versus controls and monotherapy). Thus, ceftobiprole monotherapy simulating standard therapeutic doses was active against VISA experimental endocarditis. Moreover, subtherapeutic LD- and VLD-ceftobiprole synergized with ineffective vancomycin to restore efficacy. Hence, combining ceftobiprole with vancomycin broadens the therapeutic margin of these two compounds against VISA infections.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of hospital- and community-acquired infections, against which vancomycin is still considered the agent of choice. However, continuous vancomycin selective pressure in the clinical environment has led to the emergence of vancomycin-intermediate S. aureus (VISA) strains, which have a decreased susceptibility to glycopeptides.
Infections due to VISA are a concern because they are associated with vancomycin therapeutic failure (27). Moreover, only a few treatment alternatives are currently available, mainly daptomycin and linezolid. Daptomycin was approved for the treatment of S. aureus bloodstream infections, including right-sided endocarditis caused by methicillin-susceptible S. aureus (MSSA) and MRSA (19, 38). However, in vitro studies and case reports have suggested an association between decreased susceptibility to vancomycin and decreased susceptibility to daptomycin (10, 34, 37), making the use of daptomycin against VISA infections potentially problematic. Likewise, the use of linezolid against S. aureus bacteremia has been disappointing (8), and its use against infective endocarditis is precluded by the fact that it is not bactericidal (16).
An alternative to such limits is to identify synergistic combinations of existing compounds. For instance, vancomycin has been often used in combination with rifampin or gentamicin to treat infective endocarditis caused by MRSA (32). However, the benefit of these combinations is debated (21). Moreover, the risk of resistance to rifampin and gentamicin among MRSA strains, together with the decreased susceptibility of VISA to vancomycin, limits these therapeutic options. In addition, some investigators have reported synergism between various β-lactams and vancomycin against MRSA and VISA isolates, both in vitro and in rabbits with experimental endocarditis (6, 13, 28, 36). However, other authors reported contradictory observations in vitro, and some animal experiments were unable to corroborate this synergism (1, 14, 21).
The aim of this study was to evaluate the potential synergistic activity of ceftobiprole in combination with vancomycin against two clinical isolates of VISA, both in vitro and in rats with experimental endocarditis. Ceftobiprole is a novel broad-spectrum cephalosporin with potent activity against Gram-positive pathogens, including MRSA and VISA (39), as well as Gram-negative pathogens. It has been reported to be efficacious in monotherapy against experimental endocarditis caused by both types of bacteria (4, 15, 45). It is therefore of interest to evaluate ceftobiprole as a potential partner with vancomycin for the treatment of endocarditis caused by MRSA or VISA.
(This study was presented in part at the 20th ECCMID, 10 to 13 April 2010, Wien, Austria [abstr. O38], and at the 50th ICAAC, 12 to 15 September 2010, Boston, MA [abstr. B062].)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The well-described clinical VISA isolates PC3 (42) and Mu50 (26) were used for in vitro and in vivo studies. MRSA strain COL was included as a control strain for some in vitro experiments. Bacteria were grown at 37°C in brain heart infusion (BHI) (Difco, Becton Dickinson, Sparks, MD). Strains were stored frozen in BHI containing 10% (vol/vol) glycerol at −80°C and subcultured to ensure purity before testing.
Antimicrobials.
Ceftobiprole (batch 08005R25C) and ceftobiprole medocaril (the prodrug of ceftobiprole; batch 5003DR027) were supplied by Johnson and Johnson (Raritan, NJ). Vancomycin was commercially purchased. All other chemicals were reagent-grade commercially available products.
Susceptibility testing.
MICs of ceftobiprole and vancomycin were determined by broth macrodilution in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (7). The MICs were the lowest concentration of antibiotic that yielded no visible growth after 24 h of incubation at 37°C. A minimum of two independent experiments were performed.
Drug interaction.
Studies of interaction between ceftobiprole and vancomycin were performed by Etest and population analysis. For Etest, a 0.5 McFarland standard of each isolate was inoculated on plain BHI agar (BHIA) plates and on BHIA plates containing 0.5 times the MIC of ceftobiprole (1 μg/ml for PC3 and 0.5 μg/ml for Mu50) or 0.5 times the MIC of vancomycin (4 μg/ml for both PC3 and Mu50). Etest strips of ceftobiprole (Johnson and Johnson, Raritan, NJ) and Etest strips of vancomycin (AB Biodisk, Solna, Sweden) were then placed on plain BHIA plates and on vancomycin- or ceftobiprole-containing agar plates, respectively. The plates were then incubated for 24 h at 37°C. Positive interaction was defined as an enhancement in the zone of inhibition surrounding the Etest strip on BHIA plates containing vancomycin or ceftobiprole compared to the zone size around Etest strips placed on BHIA plates containing no antibiotic.
For population analysis, series of bacterial inocula (between 102 and 109 CFU) of the test strains were serially diluted and spread onto BHIA plates supplemented with increasing concentrations of ceftobiprole or vancomycin alone or with the partner drug incorporated in the agar at a fixed concentration of 0.5 times the MIC for each organism. The plates were then incubated for 48 h at 37°C before the colonies were enumerated. The population curve was drawn by calculating and plotting the number of cells giving rise to colonies against the concentration of the drug.
Time-kill studies.
Drug-induced killing and bactericidal synergy between ceftobiprole and vancomycin were assessed by time-kill studies. Experiments were performed in flasks containing 10 ml of prewarmed Mueller-Hinton broth (MHB) (Becton Dickinson) without antibiotic or supplemented with ceftobiprole or vancomycin alone at 0.5 and 1 times the MIC for each organism or with the combination of both antibiotics. Flasks were inoculated with the test strains at a final concentration of 106 CFU/ml. A 100-μl aliquot was removed from the flask at 0, 4, and 24 h, serially diluted in saline, and plated on antibiotic-free agar plates to determine colony counts. The dilution method avoided potential carryover of the drugs. The lower limit of detection was 1 log10 CFU/ml. All experiments were repeated on two or three independent occasions.
Bactericidal activity was defined as a ≥3-log10 decrease in colony counts below the starting inoculum after 24 h of exposure to the drugs. Synergism was defined as a ≥2 log10 increased killing at 24 h by the drug combination over that of the more active drug tested alone and as a ≥2 log10 decrease in colony count below the starting inoculum. Indifference was defined as a <2 log10 change (increase or decrease) in colony counts with the combination in comparison with that with the most active single antimicrobial alone. Antagonism was defined as a ≥2 log10 increase in colony counts afforded by the drug combination compared to the most active drug used alone.
PBP analysis.
Penicillin binding proteins (PBPs) were determined in membrane fractions of bacterial lysates as described previously (20). Membranes containing PBPs were first incubated (20 min at 37°C) with either ceftobiprole (1 times the MIC) or vancomycin (0.5 times the MIC) alone or with ceftobiprole (1 times the MIC) plus vancomycin (0.5 times the MIC) and then were labeled with 1 μg/ml Bocillin FL (Invitrogen, Carlsbad, CA) for 1 h at 37°C as previously described (24, 47). After incubation, the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a NuPAGE 7% Tris-acetate precast gel (Invitrogen). The Bocillin FL-labeled PBPs were visualized by direct scanning of the gels with a Typhoon Trio+ imager (Amersham Biosciences). Intensities of the bands were quantified by densitometry using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Cell wall preparation and muropeptide analysis.
Cell walls were purified from bacteria collected in the exponential growth phase (optical density at 600 nm [OD600] of 0.6) as previously described (31). Briefly, organisms were grown in BHI broth supplemented or not with 0.5 times the MIC of ceftobiprole or vancomycin alone or together. Subinhibitory concentrations were used in order to allow bacterial growth. The peptidoglycan was purified and digested with mutanolysin (Sigma, Buchs, Switzerland), and the muropeptides were separated by reverse-phase high-performance liquid chromatography (HPLC) as described by de Jonge et al. (12). The degree of muropeptide cross-linking was calculated by quantification of the relative amounts of monomers, dimers, trimers, and oligomers in the muropeptide digest as described by Snowden and Perkins (44).
Experimental endocarditis.
Animal experiments were carried out in accordance with Swiss legislation. The production of catheter-induced aortic vegetations and the installation of the infusion pump device to deliver the antibiotics were performed in female Wistar rats (Charles River Laboratories, L'Arbresle, France) as described previously (18, 25). The administration of drug combinations necessitated the use of two infusion pumps (one for each drug) (46). Valve infections were induced 24 h after catheterization by intravenous (i.v.) challenge of the animals with 0.5 ml of saline containing 106 CFU of the test organisms (strains PC3 or Mu50). This inoculum consistently ensured endocarditis in 100% of the control animals as determined in pilot studies.
Antibiotics were delivered at changing flow rates, via the infusion pump, at doses that simulated in rats the drug kinetics in sera of humans during treatment with (i) the standard dose of ceftobiprole of 500 mg given i.v. every 12 h (b.i.d.) (SD-ceftobiprole) (40), (ii) a low dose of ceftobiprole of 250 mg i.v. every 12 h (LD-ceftobiprole) (41); (iii) a very low dose of ceftobiprole of 125 mg i.v. every 12 h (VLD-ceftobiprole) (40), (iv) SD-vancomycin of 1 g i.v. every 12 h (2), or (v) the combination of LD-ceftobiprole or VLD-ceftobiprole with SD-vancomycin. This required total drug amounts (per kilogram of body weight over a period of 12 h) of 17.2 mg ceftobiprole medocaril for SD-ceftobiprole, 5.88 mg for LD-ceftobiprole, and 1.22 mg for VLD-ceftobiprole and 23.2 mg of SD-vancomycin. SD-ceftobiprole was used to test the efficacy of the recommended dosage in humans. LD-ceftobiprole and VLD-ceftobiprole were subtherapeutic regimens purposely chosen to detect possible synergies with vancomycin. Each experiment included a control group of untreated animals.
Therapy was started at 12 h after bacterial challenge and lasted for 3 days. Control rats were killed at the onset of treatment in order to determine both the frequency and the severity of infection at the start of therapy, and treated rats were killed 8 h after the end of the last antibiotic dose. Aortic vegetations and spleens were removed, weighed, homogenized in 1 and 2 ml of saline, respectively, and serially diluted before being plated on BHI agar plates for determination of colony counts. This process reduced the potential antibiotic carryover. Quantitative blood cultures were performed in parallel. Plates were incubated for 48 h at 37°C to determine the number of viable organisms remaining in the vegetations. The lower limits of detection of growth were 2 log10 CFU/g of vegetation and 1 log10 CFU/g of spleen.
In order to detect in vivo selection of ceftobiprole- or vancomycin-resistant subpopulations during therapy, 0.1-ml samples from each vegetation homogenate were plated directly onto BHI agar plates supplemented with increasing concentrations of ceftobiprole or vancomycin as in the population analysis profiles. Viable counts were plotted against the ceftobiprole or vancomycin concentration and compared with the profiles of bacteria from vegetations of control animals.
Pharmacokinetic studies.
When used alone, the concentrations of ceftobiprole and vancomycin in the serum of rats were determined by an agar diffusion assay with antibiotic medium 1 (Difco) and Bacillus subtilis ATCC 6633 as the indicator organism. Standard curves were determined using pooled rat serum. The limits of detection of the assays were 0.25 μg/ml for ceftobiprole and 0.7 μg/ml for vancomycin. The linearities of the standard curves were assessed with a regression coefficient of ≥0.995 for both ceftobiprole and vancomycin. Intraplate and interplate variations were ≤10%. When administered in combination, ceftobiprole was determined by an agar diffusion assay with antibiotic medium 1 (Difco) and Escherichia coli ATCC 25922 (which is resistant to vancomycin) as the indicator organism, and vancomycin was measured by fluorescent polarization immunoassay. The detection limits in these assays were 0.5 μg/ml for ceftobiprole and 0.75 μg/ml for vancomycin.
Statistical analysis.
The degrees of muropeptide cross-linking of peptidoglycan purified from bacteria exposed to various drugs or drug combinations were compared by analysis of variance (ANOVA) with Bonferroni's correction for multiple-comparison tests. The rates of valve, spleen, and blood infections of the various groups and the differences in mortality were compared by Fisher's exact test. Median bacterial counts in vegetations in the control and SD-ceftobiprole groups were compared by the Mann-Whitney rank sum test. The SD-ceftobiprole 500-mg monotherapy arm was not used as a comparator with the other monotherapy or bitherapy groups, since it was not tested in combination studies. Median bacterial counts in vegetations in the control and the other groups were compared by the Kruskal-Wallis test with Dunn's correction for multiple-comparison groups. For statistical comparisons, culture-negative vegetations and spleens were considered to contain 2 and 1 log10 CFU/g, respectively, the limits of detection. A P value of <0.05 was considered significant.
RESULTS
MICs, drug interaction, and time-kill experiments.
The MICs of ceftobiprole and vancomycin were 2 and 8 μg/ml for VISA PC3 and 1 and 8 μg/ml for VISA Mu50, respectively. The MICs of ceftobiprole and vancomycin for MRSA COL were 1 and 2 μg/ml, respectively.
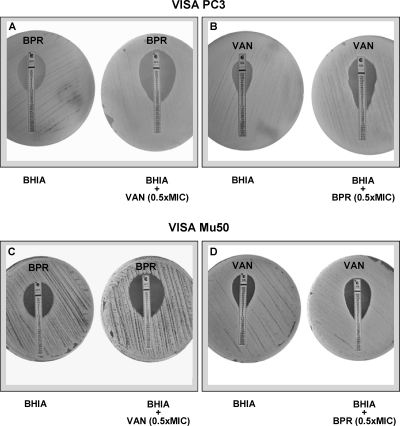
Figures 1 and 2 present the interaction of ceftobiprole with vancomycin for the two VISA strains tested. Combinations of ceftobiprole and vancomycin demonstrated a positive interaction against both strains PC3 and Mu50. Indeed, by Etest, an enhanced zone of inhibition surrounding the ceftobiprole Etest strip was observed in the presence of vancomycin (at a concentration of 0.5 times the MIC), which corresponded to increases of 8-fold and 2-fold in the ceftobiprole susceptibilities of PC3 and Mu50, respectively (Fig. 1A and C). The positive interaction also operated in the symmetric setting, i.e., an enhanced zone of inhibition surrounding the vancomycin Etest strip was observed when plating was on 0.5 times the MIC of ceftobiprole (Fig. 1B and D). This corresponded to increases in vancomycin susceptibility of 8-fold and 4-fold for strains PC3 and Mu50, respectively. For MRSA COL, the susceptibilities to ceftobiprole and vancomycin in the presence of the partner drug, at a fixed concentration of 0.5 times the MIC, increased by 4-fold and 2-fold, respectively (data not shown).
Fig. 1.
Positive interaction between ceftobiprole (BPR) and vancomycin (VAN) by Etest testing. A 0.5 McFarland standard of VISA PC3 (A and B) or VISA Mu50 (C and D) was inoculated on plain BHI agar plates (BHIA) and on BHIA plates containing 0.5 times the MIC of vancomycin (A and C) or 0.5 times the MIC of ceftobiprole (B and D). Etest strips of ceftobiprole (A and C) or vancomycin (B and D) were then placed on the plates, and the plates were incubated at 37°C for 24 h. A marked increase in zone size around Etest ceftobiprole and vancomycin strips was observed in the presence of the partner drug incorporated in the agar.
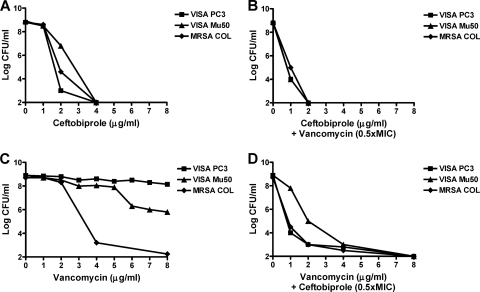
Fig. 2.
Population analysis of strains VISA PC3 and VISA Mu50. Large inocula (ca. 109 CFU) of the tests strains were spread onto BHI agar plates supplemented with increasing concentrations of ceftobiprole alone (A) or vancomycin alone (C) or combined with vancomycin (B) or ceftobiprole (D) incorporated in the agar at a fixed concentration of 0.5 times the MIC for each organism. The plates were incubated at 37°C for 48 h before the colonies were enumerated.
The positive interaction between ceftobiprole and vancomycin could also be demonstrated by population analysis (Fig. 2). When ceftobiprole was combined with vancomycin incorporated in the agar at a fixed concentration of 0.5 times the MIC, the growth of the less susceptible subpopulation was prevented, as indicated by a shift to the left of the population analysis curve. Moreover, when vancomycin was combined with ceftobiprole incorporated in the agar at a fixed concentration of 0.5 times the MIC, a virtual disappearance of vancomycin-resistant subpopulations was observed, and the two VISA isolates displayed a profile close to that of the vancomycin-susceptible MRSA COL strain.
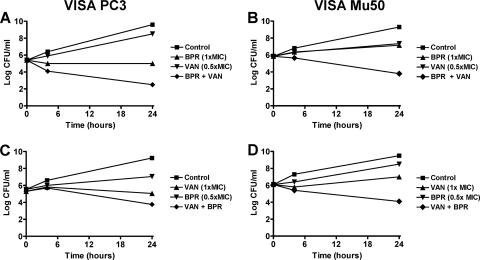
To test whether this positive interaction translated into bactericidal synergy, time-kill assays were performed using ceftobiprole and vancomycin concentrations of 0.5 and 1 times the MIC. The rates of in vitro killing are presented in Fig. 3. At these low concentrations, ceftobiprole or vancomycin alone did not display significant bactericidal effects (i.e., <1.5 log kill at any time point). In contrast, the combination of ceftobiprole at 1 times the MIC plus vancomycin at 0.5 times the MIC was synergistic against both PC3 and Mu50, as demonstrated by killing at 24 h of ≥3 and ≥2.5 log10 CFU/ml, respectively, compared with single agents used alone and the initial inoculum (Fig. 3A and B). The combination of ceftobiprole at 0.5 times the MIC and vancomycin at 1 times the MIC was positive, yet formally indifferent, against PC3 (loss of <2 log10 CFU/ml at 24 h for the combination compared with vancomycin and the initial inoculum) and was synergistic against Mu50, with a loss of viability of ≥2.6 log10 CFU/ml at 24 h for the combination compared with each single agent and the initial inoculum (Fig. 3C and D). The combination of ceftobiprole and vancomycin at concentrations of 0.5 times the MIC for each strain was indifferent against both isolates (data not presented).
Fig. 3.
Time-kill curves for VISA PC3 (left panel) and VISA Mu50 (right panel) after incubation with ceftobiprole (BPR) or vancomycin (VAN) at concentrations of 0.5 and 1 times the MIC alone or in combination.
PBP analysis.
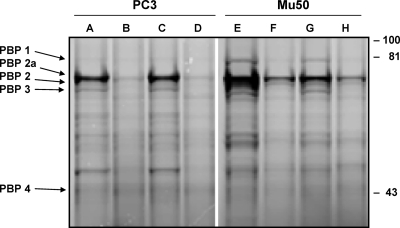
Figure 4 depicts PBP labeling with Bocillin FL after preincubation of plasma membranes of strains PC3 and Mu50 with or without the tested drugs. In the absence of drug preincubation, PBPs 1, 2a, 2, and 3 were readily detected, while PBP 4 was poorly labeled (Fig. 4, lanes A and E). Preincubation with 1 times the MIC of ceftobiprole saturated the “normal” PBPs, and decreased PBP2a labeling by approximately 4-fold for PC3 and 2-fold for Mu50 (Fig. 4, lanes B and F). In contrast, no changes in Bocillin FL labeling were observed in membranes preincubated with 0.5 times the MIC of vancomycin (Fig. 4, lanes C and G), and vancomycin did not significantly modify the ability of ceftobiprole to block the PBP labeling when both drugs were used in combination (Fig. 4, lanes D and H).
Fig. 4.
PBP patterns of VISA strains PC3 and Mu50. Membrane preparations were incubated with no antibiotic (lanes A and E), ceftobiprole at 1 times the MIC alone (lanes B and F), vancomycin at 0.5 times the MIC alone (lanes C and G), or the combination of ceftobiprole and vancomycin (lanes D and H) and labeled with Bocillin FL. The samples were resolved by SDS-polyacrylamide gel electrophoresis, and the Bocillin FL-labeled PBPs were visualized by scanning of the gels using a Typhoon Trio+ imager. Numbers at right are molecular masses in kilodaltons.
Muropeptide analysis.
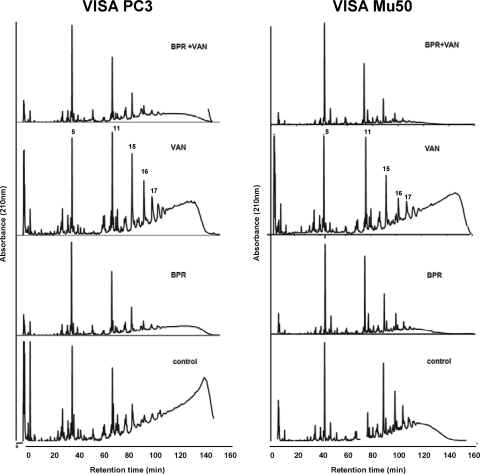
Figure 5 shows the muropeptide fingerprints generated by muramidase digestion of peptidoglycan purified from isolates PC3 and Mu50 grown in the absence or the presence of subinhibitory concentrations of ceftobiprole or vancomycin alone or with the combination of the two drugs. The degrees of cross-linking for PC3 and Mu50 (73% and 57%, respectively) decreased by ca. 10% following exposure to vancomycin, as judged by a decrease in the proportion of the oligomer peaks and an increase in the proportion of monomer peaks. After exposure to ceftobiprole alone, the HPLC profiles of both PC3 and Mu50 showed a significant reduction (to 52% and 46%, respectively) of the amount of cross-linking (P < 0.005). After exposure to the combination of ceftobiprole and vancomycin, cell wall cross-linking was not further reduced and was 55% and 43% for PC3 and Mu50, respectively.
Fig. 5.
HPLC elution profiles of muropeptides isolated from VISA strains PC3 and Mu50. Peptidoglycan was purified from bacteria grown in the absence or presence of subinhibitory concentrations of ceftobiprole (BPR) or vancomycin (VAN) alone or with the combination of the two drugs and digested with muramidase, and the muropeptides were separated by HPLC. Peaks 5, 11, 15, 16, and 17 represent the monomeric muropeptides. The HPLC profile eluting with retention times of greater than 100 min (i.e., after peak 17) represents highly cross-linked oligomeric components.
Pharmacokinetic studies.
The peak (30-min) and trough (12-h) concentrations (mean ± standard deviation for 3 to 12 individual animals) of simulated human doses of ceftobiprole in rat serum were, respectively, 45.1 ± 9.5 μg/ml and 3.9 ± 0.9 μg/ml for SD-ceftobiprole (500 mg), 19.4 ± 4.4 μg/ml and 0.8 ± 0.2 μg/ml for LD-ceftobiprole (250 mg), and 11.3 ± 2.9 μg/ml and 0.6 ± 0.2 μg/ml for VLD-ceftobiprole (125 mg). The peak and trough concentrations (mean ± standard deviation for 3 individual animals) of vancomycin were 58.9 ± 6.9 μg/ml and 6.8 ± 0.6 μg/ml, respectively. These values for ceftobiprole and vancomycin in rats were very close to the peak and trough values reported in humans, i.e., 40.6 and 1.3 μg/ml for SD-ceftobiprole (500 mg), 19.5 and 0.8 μg/ml for LD-ceftobiprole (250 mg), and 52.5 and 5.2 μg/ml for vancomycin, respectively (2, 40, 41). No reduction in ceftobiprole and vancomycin levels in serum with the combination of the two drugs was observed.
Therapy of experimental endocarditis.
The results for therapy of experimental endocarditis are shown in Table 1. Treatment with SD-ceftobiprole simulating human kinetics of 500 mg b.i.d. sterilized 93% and 77% of vegetations infected with strains PC3 and Mu50, respectively, and reduced the vegetation counts by >6 log10 CFU/g compared to those in untreated controls (P < 0.001). The high efficacy of the SD-ceftobiprole (500-mg) regimen did not allow us to study its potential synergism with vancomycin. Thus, further treatments used lower doses of the drug.
Table 1.
Treatment of experimental endocarditis induced by VISA strains PC3 and Mu50
| VISA strain and treatment groupa | No. of sterile samples/total no. (%) in: |
Median (range) log10 CFU/g in: |
||
|---|---|---|---|---|
| Vegetation | Spleen | Vegetation | Spleen | |
| PC3 | ||||
| Control | 0/14 (0) | 0/14 (0) | 8.46 (5.06–9.42) | 4.70 (4.11–5.76) |
| SD-ceftobiprole 500 | 13/14 (93)b | 1/14 (7) | 2.00 (2.00–3.20)b | 2.21 (1.00–2.97)b |
| SD-vancomycin | 0/7 (0) | 0/7 (0) | 7.38 (5.99–9.56) | 5.59 (4.87–6.67) |
| LD-ceftobiprole 250 | 10/11 (91)c | 0/11 (0) | 2.00 (2.00–4.38)c | 2.99 (1.57–4.11)c |
| VLD-ceftobiprole 125 | 1/12 (8) | 0/12 (0) | 8.09 (2.00–9.42) | 4.72 (2.28–6.69) |
| LD-ceftobiprole 250 + SD-vancomycin | NDe | ND | ND | ND |
| VLD-ceftobiprole 125 + SD-vancomycin | 7/9 (78)d | 0/9 (0) | 2.00 (2.00–8.06)d | 2.87 (2.30–3.14)d |
| Mu50 | ||||
| Control | 0/14 (0) | 0/14 (0) | 8.23 (7.02–9.16) | 4.71 (3.96–5.68) |
| SD-ceftobiprole 500 | 10/13 (77)b | 5/13 (38)b | 2.00 (2.00–3.86)b | 2.33 (1.00–3.61)b |
| SD-vancomycin | 0/8 (0) | 0/8 (0) | 9.03 (5.56–9.34) | 4.96 (3.94–6.41) |
| LD-ceftobiprole 250 | 3/12 (25) | 3/12 (25) | 5.31 (2.00–9.27)c | 3.99 (1.00–5.56) |
| VLD-ceftobiprole 125 | 0/15 (0) | 0/15 (0) | 8.87 (5.71–9.38) | 5.42 (4.38–6.72) |
| LD-ceftobiprole 250 + SD-vancomycin | 5/6 (83)d | 1/6 (17) | 2.00 (2.00–4.78)d | 1.95 (1.00–3.04)d |
| VLD-ceftobiprole 125 + SD-vancomycin | 6/14 (43)d | 4/14 (28) | 5.09 (2.00–8.07)d | 2.50 (1.00–5.64)d |
SD-ceftobiprole 500, LD-ceftobiprole 250, and VLD-ceftobiprole 125, simulation in rats of human pharmacokinetics following 500, 250, and 125 mg i.v. every 12 h, respectively. SD-vancomycin, simulation of 1 g i.v. every 12 h.
P < 0.001 versus controls.
P < 0.01 versus controls and vancomycin.
P < 0.05 versus controls and single-drug therapy alone.
ND, not done.
Treatment with doses simulating human pharmacokinetics of LD-ceftobiprole (250 mg) sterilized 91% of animals inoculated with PC3 and reduced the vegetation counts by >6 log10 CFU/g compared to those in untreated controls (P < 0.001). However, this regimen was ineffective against Mu50 (only 25% of valves were sterile). Both VLD-ceftobiprole (125 mg) and SD-vancomycin failed to cure the animals. In sharp contrast, the combination of LD-ceftobiprole with SD-vancomycin successfully treated 83% of vegetations infected with Mu50, and VLD-ceftobiprole plus SD-vancomycin was successful against both PC3 and Mu50 (78% and 43% culture-negative vegetations, respectively; P < 0.05 compared to controls and either drug given alone). No ceftobiprole- or vancomycin-resistant subpopulations were detected in vegetation homogenates of treated animals during the study period.
Table 1 also shows the results for spleen tissue cultures. In general, spleens remain infected even after effective antibiotic therapy of the valves, although with decreased bacterial numbers. Treatment with SD-ceftobiprole significantly decreased bacterial densities in the spleens infected with both test isolates (P < 0.001 compared to controls). Likewise, all combination regimens significantly (P < 0.05) decreased median bacterial densities in the spleens relative to both untreated controls and monotherapy. On the other hand, monotherapy with LD-ceftobiprole, VLD-ceftobiprole, and SD-vancomycin was ineffective, with the exception of LD-ceftobiprole against VISA PC3.
None of the control animals at the start of therapy had sterile blood cultures. Available animals were also analyzed for positive blood cultures at the end of treatment. Blood cultures were sterile in 93 to 100% of animals treated with SD-ceftobiprole (P < 0.001 versus untreated controls), 78 to 100% of animals treated with LD-ceftobiprole (P < 0.01 versus untreated controls), 20 to 33% of animals treated with VLD-ceftobiprole (P > 0.05 versus untreated controls), and 0 to 25% of animals treated with SD-vancomycin (P > 0.05 versus untreated controls). LD-ceftobiprole plus SD-vancomycin sterilized 100% of blood cultures in rats challenged with Mu50 (P < 0.0001 versus controls and monotherapy), and VLD-ceftobiprole plus SD-vancomycin sterilized 80 to 100% of blood cultures (P < 0.05 versus controls and single drug therapy).
DISCUSSION
New β-lactams exhibiting good anti-MRSA activity have an improved affinity for PBP 2a (3, 23, 33). This makes them active against MRSA, VISA, and even high-level-vancomycin-resistant S. aureus (VRSA). Anti-MRSA activity is mediated by the ability to inhibit both normal PBPs and PBP 2a, and anti-VISA and anti-VRSA activities take advantage of the fact that β-lactams act via antibacterial mechanisms that are different from that of vancomycin. However, while blocking PBP 2a may overcome resistance in MRSA, VISA, and VRSA, it may be risky to limit the antimicrobial strategy to this unique approach. Indeed, one could underestimate the ability of the bacterium to develop further resistance to the new β-lactams, for instance, by introducing new mutations in the regulatory or structural genes of PBP 2a (30). This risk needs to be prevented.
One strategy for resistance prevention could be combining β-lactams with vancomycin. Indeed, while VISA strains are resistant to most β-lactams and vancomycin, it was recently observed that combining both types of drugs together restored their activity against these organisms in vitro and partially in vivo (6, 14). Thus, when β-lactams (e.g., nafcillin) and vancomycin were applied together against VISA, the mechanisms of resistance to these two drugs became mutually exclusive.
Here we investigated the potential benefit of such a strategy by combining ceftobiprole with vancomycin against VISA PC3 and VISA Mu50 clinical isolates. Of note, ceftobiprole monotherapy was active when administered to animals at doses mimicking the treatment recommended in human (i.e., 500 mg b.i.d.), thus confirming its intrinsic activity against VISA (4, 15, 45). On the other hand, it was not effective when used at low or very low doses, but these subtherapeutic doses could be remediated by combination with vancomycin at an inactive dose. This synergism was also apparent in population analysis profiles in vitro, where low doses of one drug could prevent the growth of subpopulations resistant to the partner drugs, and by the fact that low-dose drug combinations prevented the emergence of resistant escape mutants during therapy in vivo.
Synergy between subtherapeutic doses of ceftobiprole and vancomycin against MRSA was also suggested in a recent meeting abstract (17). Nevertheless, the exact mechanism of this synergism is as yet incompletely elucidated. It was proposed that alterations in the cell wall that confer vancomycin resistance made these isolates simultaneously more susceptible to β-lactams (43). Alternatively, Howe et al. suggested that high concentrations of β-lactams may cause inhibition of synthesis of proteins essential for the expression of vancomycin resistance, thus resulting in synergy (28). Eventually, the mutual exclusion of both resistance mechanisms could also result from complementary interactions of the drugs with peptidoglycan assembly. The logic of this argument is as follows: (i) the VISA phenotype is associated with a thickened peptidoglycan carrying an increased proportion of free d-alanine–d-alanine termini, which trap incoming vancomycin; (ii) the free d-alanine–d-alanine is synonymous with decreased PBP-mediated transpeptidation, resulting in a less cross-linked and (probably) mechanically weaker peptidoglycan; and (iii) additional weakening of peptidoglycan by blockage of remaining PBPs with β-lactams further decreases peptidoglycan stability and results in cell death. In this model it is critical that the β-lactams block all of the essential staphylococcal PBPs for the synergism to occur. Thus, β-lactams with increased PBP 2a affinity are likely to be more efficient than β-lactams with affinity restricted to the “normal” staphylococcal PBPs in the combination with vancomycin.
Here we had a glimpse of ceftobiprole and vancomycin blockage of PBP labeling by Bocillin FL and of the effects of these drugs on muropeptide cross-linkage as analyzed by HPLC. Ceftobiprole readily blocked PBP labeling, including labeling of PBP 2a (11). In contrast, vancomycin had no effect on PBP labeling when used either alone or in combination with ceftobiprole. Although this last result was expected, it excluded any unforeseen interaction between ceftobiprole (which mimics d-alanine–d-alanine) and vancomycin, which could interfere with PBP blockage. Another hypothesis, alluded to above, is that both drugs could synergize to decrease d-alanine–d-alanine cross-linkage. However, peptidoglycan analyses showed that while sub-MIC concentrations of ceftobiprole significantly decreased cross-linkage (by increasing the ratio of monomers), sub-MIC concentrations of vancomycin did not, and combining both drugs was not more effective than using ceftobiprole alone in this regard. Therefore, one is left with another possibility, that the mutual benefit of both drugs would result from the summation of transpeptidase inhibition, by ceftobiprole, and transglycosidase inhibition, by vancomycin. This is reminiscent of the synergism between PBP 2a blockage and moenomycin, which specifically blocks transglycosidase (35). Although not formally tested here (which would require measurement of the length of the glycan chains in addition to the transpeptidation activity), this is logical and should be used as a further working hypothesis.
The present observations may also help clarify some contradictory results previously reported with β-lactam–vancomycin combinations against MRSA and VISA. For instance, Climo et al. (6) and Rochon-Edouard et al. (36) reported a synergistic in vitro effect of combinations of different β-lactam antibiotics and vancomycin against clinical MRSA and VISA isolates. In addition, Climo et al. (6) have shown that the combination of nafcillin and vancomycin significantly reduced vegetation colony counts in experimental endocarditis caused by VISA strains compared to either treatment alone. On the other hand, some studies have reported indifference or antagonism of some β-lactam–vancomycin combinations in vitro (1, 22, 28), and synergism could not be demonstrated with oxacillin and vancomycin in a mouse peritonitis model (14).The discrepancy regarding the effects of β-lactam–vancomycin combinations against MRSA and/or VISA could be related to the binding affinities of β-lactams for PBPs, including PBP 2a, the major determinant for methicillin resistance in MRSA and VISA. For instance, synergism was stronger when β-lactams with relatively high PBP 2a affinities, i.e., amoxicillin-clavulanate, ampicillin-sulbactam, or nafcillin (5, 20), were used as partner drugs (6, 36). The present study with ceftobiprole extends these observations and suggests that blocking PBP 2a in addition to the normal PBPs might be necessary for optimal synergy.
Moreover, the utilization of strains expressing different heterogeneous resistance phenotypes toward β-lactams could also account for some discordant results. The fact that in the present study ceftobiprole given alone at subtherapeutic doses (with human-like pharmacokinetics of 250 mg i.v. every 12 h) was effective against VISA PC3 but not against VISA Mu50 supports this hypothesis. Indeed, the PC3 isolate presents a highly heterogeneous phenotype toward oxacillin (inhibited by <1 μg/ml) (42) and displayed more ceftobiprole-susceptible subpopulations than the Mu50 strain.
In conclusion, the combination of ceftobiprole and vancomycin was synergistic against VISA in vitro and in rats with endocarditis. These results, as well as those observed against MRSA in similar experiments (17), suggested that this combination therapy might be useful for the treatment of multiresistant S. aureus infections in humans. In particular, it increases the margin of treatment efficacy, and consequently the efficacy and safety in severe infections such as infective endocarditis, where drug penetration at the infection site might be impaired (9). Further studies examining the potential role of this combination are warranted. Whether or not other novel cephalosporins with activity against MRSA and VISA, i.e., ceftaroline (29), would have the same synergistic properties in combination with vancomycin remains to be evaluated.
ACKNOWLEDGMENTS
We thank Laurenz Kellenberger for useful comments on the manuscript.
This study was funded in part by a research grant from Johnson & Johnson Laboratories.
We have no conflicts to disclose.
Footnotes
Published ahead of print on 5 July 2011.
REFERENCES
- 1. Aritaka N., Hanaki H., Cui L., Hiramatsu K. 2001. Combination effect of vancomycin and beta-lactams against a Staphylococcus aureus strain, Mu3, with heterogeneous resistance to vancomycin. Antimicrob. Agents Chemother. 45:1292–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blouin R. A., Bauer L. A., Miller D. D., Record K. E., Griffen W. O., Jr 1982. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob. Agents Chemother. 21:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bush K., Heep M., Macielag M. J., Noel G. J. 2007. Anti-MRSA beta-lactams in development, with a focus on ceftobiprole: the first anti-MRSA beta-lactam to demonstrate clinical efficacy. Expert Opin. Invest. Drugs 16:419–429 [DOI] [PubMed] [Google Scholar]
- 4. Chambers H. F. 2005. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:884–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chambers H. F., Sachdeva M. 1990. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 161:1170–1176 [DOI] [PubMed] [Google Scholar]
- 6. Climo M. W., Patron R. L., Archer G. L. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CLSI 2006. Performance standards for antimicrobial susceptibility testing, 16th ed Approved standard M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Cosgrove S. E., Fowler V. G., Jr 2008. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46(Suppl. 5):S386–S393 [DOI] [PubMed] [Google Scholar]
- 9. Cremieux A. C., et al. 1989. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J. Infect. Dis. 159:938–944 [DOI] [PubMed] [Google Scholar]
- 10. Cui L., Tominaga E., Neoh H. M., Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies T. A., et al. 2007. Binding of ceftobiprole and comparators to the penicillin-binding proteins of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 51:2621–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jonge B. L., Chang Y. S., Gage D., Tomasz A. 1992. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J. Biol. Chem. 267:11255–11259 [PubMed] [Google Scholar]
- 13. Domaracki B. E., Evans A. M., Venezia R. A. 2000. Vancomycin and oxacillin synergy for methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 44:1394–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domenech A., et al. 2005. Experimental study on the efficacy of combinations of glycopeptides and beta-lactams against Staphylococcus aureus with reduced susceptibility to glycopeptides. J. Antimicrob. Chemother. 56:709–716 [DOI] [PubMed] [Google Scholar]
- 15. Entenza J. M., Hohl P., Heinze-Krauss I., Glauser M. P., Moreillon P. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falagas M. E., Manta K. G., Ntziora F., Vardakas K. Z. 2006. Linezolid for the treatment of patients with endocarditis: a systematic review of the published evidence. J. Antimicrob. Chemother. 58:273–280 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez J., et al. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-061.2010. [Google Scholar]
- 18. Fluckiger U., et al. 1994. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob. Agents Chemother. 38:2846–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fowler V. G., Jr., et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665 [DOI] [PubMed] [Google Scholar]
- 20. Franciolli M., Bille J., Glauser M. P., Moreillon P. 1991. Beta-lactam resistance mechanisms of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 163:514–523 [DOI] [PubMed] [Google Scholar]
- 21. Garau J., Bouza E., Chastre J., Gudiol F., Harbarth S. 2009. Management of methicillin-resistant Staphylococcus aureus infections. Clin. Microbiol. Infect. 15:125–136 [DOI] [PubMed] [Google Scholar]
- 22. Goldstein F. W., et al. 2004. False synergy between vancomycin and beta-lactams against glycopeptide-intermediate Staphylococcus aureus (GISA) caused by inappropriate testing methods. Clin. Microbiol. Infect. 10:342–345 [DOI] [PubMed] [Google Scholar]
- 23. Guignard B., Entenza J. M., Moreillon P. 2005. Beta-lactams against methicillin-resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 5:479–489 [DOI] [PubMed] [Google Scholar]
- 24. Hebeisen P., et al. 2001. In vitro and in vivo properties of Ro 63–9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heraief E., Glauser M. P., Freedman L. R. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiramatsu K., et al. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135–136 [DOI] [PubMed] [Google Scholar]
- 27. Howden B. P., Davies J. K., Johnson P. D., Stinear T. P., Grayson M. L. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, includ-ing vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23:99–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howe R. A., Wootton M., Bennett P. M., MacGowan A. P., Walsh T. R. 1999. Interactions between methicillin and vancomycin in methicillin-resistant Staphylococcus aureus strains displaying different phenotypes of vancomycin susceptibility. J. Clin. Microbiol. 37:3068–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacqueline C., et al. 2011. Comparison of ceftaroline fosamil, daptomycin and tigecycline in an experimental rabbit endocarditis model caused by methicillin-susceptible, methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. J. Antimicrob. Chemother. 66:863–866 [DOI] [PubMed] [Google Scholar]
- 30. Katayama Y., Zhang H. Z., Chambers H. F. 2004. PBP 2a mutations producing very-high-level resistance to beta-lactams. Antimicrob. Agents Chemother. 48:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Majcherczyk P. A., et al. 1999. Digestion of Streptococcus pneumoniae cell walls with its major peptidoglycan hydrolase releases branched stem peptides carrying proinflammatory activity. J. Biol. Chem. 274:12537–12543 [DOI] [PubMed] [Google Scholar]
- 32. Moreillon P., Que Y. A. 2004. Infective endocarditis. Lancet 363:139–149 [DOI] [PubMed] [Google Scholar]
- 33. Page M. G. 2006. Anti-MRSA beta-lactams in development. Curr. Opin. Pharmacol. 6:480–485 [DOI] [PubMed] [Google Scholar]
- 34. Patel J. B., Jevitt L. A., Hageman J., McDonald L. C., Tenover F. C. 2006. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 42:1652–1653 [DOI] [PubMed] [Google Scholar]
- 35. Pinho M. G., de Lencastre H., Tomasz A. 2001. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. U. S. A. 98:10886–10891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rochon-Edouard S., Pestel-Caron M., Lemeland J. F., Caron F. 2000. In vitro synergistic effects of double and triple combinations of beta-lactams, vancomycin, and netilmicin against methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 44:3055–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sader H. S., Jones R. N. 2006. The activity of daptomycin against wild-type Staphylococcus aureus and strains with reduced susceptibility to vancomycin. Clin. Infect. Dis. 43:798–799 (Author reply, 43:799–800.) [DOI] [PubMed] [Google Scholar]
- 38. Sakoulas G., Golan Y., Lamp K. C., Friedrich L. V., Russo R. 2007. Daptomycin in the treatment of bacteremia. Am. J. Med. 120:S21–S27 [DOI] [PubMed] [Google Scholar]
- 39. Saravolatz L. D., Pawlak J., Johnson L. B., Saravolatz L. D., 2nd, Husain N. 2010. In vitro activity of ceftobiprole against meticillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus (VRSA) and daptomycin-non-susceptible S. aureus (DNSSA). Int. J. Antimicrob. Agents 36:478–480 [DOI] [PubMed] [Google Scholar]
- 40. Schmitt-Hoffmann A., et al. 2004. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2576–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitt-Hoffmann A., et al. 2004. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2570–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sieradzki K., Roberts R. B., Haber S. W., Tomasz A. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517–523 [DOI] [PubMed] [Google Scholar]
- 43. Sieradzki K., Tomasz A. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Snowden M. A., Perkins H. R. 1990. Peptidoglycan cross-linking in Staphylococcus aureus. An apparent random polymerisation process. Eur. J. Biochem. 191:373–377 [DOI] [PubMed] [Google Scholar]
- 45. Tattevin P., Basuino L., Bauer D., Diep B. A., Chambers H. F. 2010. Ceftobiprole is superior to vancomycin, daptomycin, and linezolid for treatment of experimental endocarditis in rabbits caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:610–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vouillamoz J., Entenza J. M., Feger C., Glauser M. P., Moreillon P. 2000. Quinupristin-dalfopristin combined with beta-lactams for treatment of experimental endocarditis due to Staphylococcus aureus constitutively resistant to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob. Agents Chemother. 44:1789–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao G., Meier T. I., Kahl S. D., Gee K. R., Blaszczak L. C. 1999. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob. Agents Chemother. 43:1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]