Abstract
Dihydroartemisinin-piperaquine is a new, highly effective, and well-tolerated combination treatment for uncomplicated falciparum malaria. The lipophilic characteristic of piperaquine suggests that administration together with fat will increase the oral bioavailability of the drug, and this has been reported for healthy volunteers. This pharmacokinetic study monitored 30 adult patients with uncomplicated falciparum malaria for 4.5 months to evaluate the effects of the concomitant intake of fat on the total piperaquine exposure. The fixed-drug combination of dihydroartemisinin-piperaquine was given with water to fasting patients (n = 15) or was coadministered with 200 ml milk containing 6.4 g fat (n = 15). The drug combination was generally well tolerated, and there were no severe adverse effects reported for either group during the study. Total piperaquine exposure (area under the concentration-time curve from zero to infinity [AUC0-∞]; results are given as medians [ranges]) were not statistically different between fed (29.5 h · μg/ml [20.6 to 58.7 h · μg/ml]) and fasting (23.9 h · μg/ml [11.9 to 72.9 h · μg/ml]) patients, but the interindividual variation was reduced in the fed group. Overall, none of the pharmacokinetic parameters differed statistically between the groups. Total piperaquine exposure correlated well with the day 7 concentrations in the fasted group, but the fed group showed a poor correlation. In conclusion, the coadministration of 6.4 g fat did not have any significant effect on piperaquine pharmacokinetics in the treatment of uncomplicated malaria.
INTRODUCTION
Dihydroartemisinin-piperaquine (DHA-PQ) is a new fixed-dose artemisinin-based combination therapy (ACT) for malaria. Dihydroartemisinin-piperaquine has been shown to be very effective in the treatment of uncomplicated falciparum malaria throughout the tropics (2, 8, 14, 25, 30). Piperaquine alone has been used extensively in China, where it was given as mass prophylaxis and used as a treatment in the 1970s and 1980s (5). Despite the long history of piperaquine use, its pharmacokinetic properties have been characterized only in recent years. Piperaquine is a highly lipophilic bisquinoline compound with a large apparent volume of distribution of approximately 500 liters/kg, low oral clearance of approximately 1 liter/h/kg, and a consequently long terminal elimination half-life of more than 20 days (1, 14, 26–28).
The effects of concomitant fat intake on piperaquine pharmacokinetics have been studied in healthy volunteers with various results (12, 19, 24). The fat effect, in enhancing oral bioavailability, has been well documented for other lipid-soluble antimalarials, including halofantrine (17), mefloquine (6), lumefantrine (3, 10), and atovaquone (21). It is recommended that lumefantrine always be taken with fat in the treatment of malaria, which is a significant practical limitation.
The pharmacokinetic properties of many antimalarials differ in patients with malaria compared to those of healthy volunteers, sometimes in different directions. For both mefloquine and quinine, there is a reduction in clearance and apparent volume of distribution and a shorter terminal elimination half-life in malaria patients than in healthy subjects (16, 29, 31–32). Nguyen et al. reported that dihydroartemisinin (when given with piperaquine) showed higher maximal concentrations and drug exposure in patients (698 ng/ml and 1,949 h · ng/ml, respectively) than in healthy volunteers (176 ng/ml and 398 h · ng/ml, respectively). This was explained by a lower oral clearance and apparent oral volume of distribution in patients (1.19 liters/h/kg of body weight and 1.47 liters/kg, respectively) compared to those for healthy volunteers (5.87 liters/h/kg and 8.02 liters/kg, respectively) (18). The protein binding of antimalarials can also be altered in patients with malaria when compared to healthy volunteers (22, 23). It therefore is uncertain if a significant food effect in healthy volunteers would translate into the same effect in patients. The objective of this study was to characterize the disposition of piperaquine when administered with or without fat to patients with uncomplicated falciparum malaria.
MATERIALS AND METHODS
Study design.
This was a randomized open-label comparative parallel group study of dihydroartemisinin-piperaquine given with or without fat for the treatment of uncomplicated falciparum malaria in adult patients. The study was conducted at the northwestern border of Thailand (Shoklo Malaria Research Unit), which is an area with low and seasonal malaria transmission. All patients attending the clinic were screened to assess their eligibility for enrollment in the study. Eligible patients were aged 16 to 65 years, were not pregnant, had microscopic confirmation of asexual stages of falciparum or mixed infections (parasite threshold, ≥80 parasites/μl blood), and had no signs of severe malaria. Full informed written consent was obtained from each patient. Exclusion criteria were a positive urine test for pregnancy (beta human chorionic gonadotropin [β-HCG]), ≥4% red blood cell asexual-stage falciparum parasitemia, treatment with dihydroartemisinin-piperaquine within the past 4 months, known hypersensitivity to artemisinins or piperaquine, or hematocrit of less than 30%. Patients were randomized in blocks of five and received the drug with or without fat. Investigators and recruited patients were not blinded to the allocation; however, this was concealed from scientists performing the drug quantification. Thirty subjects were recruited (i.e., 15 in each arm). A questionnaire containing concomitant illness and drug history and time since the last meal was completed for each patient. Symptoms before clinic attendance were documented. On admission, all patients provided a capillary blood sample for malaria thick and thin films, PCR genotyping, and hematocrit evaluation. Thick and thin blood films stained with Giemsa were read, and parasite density was estimated by counting the number of asexual parasites per 1,000 red blood cells or 500 white blood cells, assuming a red blood cell count and white blood cell count of 400,0000 and 8,000/μl, respectively. Three milliliters of venous blood was taken for biochemistry.
Ethical review committee approval.
Approval of the study was granted by the Faculty of Tropical Medicine Mahidol University Ethical Committee, Bangkok, Thailand, and the Oxford Tropical Research Ethics Committee (OXTREC), United Kingdom.
Drug administration and follow-up.
Dihydroartemisinin-piperaquine tablets (Duo-Cotecxin; Beijing Holley-Cotec Pharmaceuticals Co., Ltd., China) containing 40 mg of dihydroartemisinin and 320 mg of piperaquine tetraphosphate were given in a weight-based total regimen of approximately 7 mg/kg dihydroartemisinin and 54 mg/kg piperaquine tetraphosphate (equivalent to 29 mg/kg piperaquine base) in three divided doses (planned at 0, 24, and 48 h; actual median times were 0, 18, and 42 h). A 200-ml carton of chocolate milk containing 6.4 g of fat was given with each dose in the fed group. For the fasting group, the first dose was given at enrollment at least 2 h after the last meal and the consecutive two doses after an overnight fast. Each dose was administered under supervision in the clinic. Patients in the fasting group were asked to continue fasting for 3 h after each dose; only water was consumed during this period. Temperature and malaria smear were checked daily until fever and parasites cleared. A food diary was kept for the 3 days of treatment. Once the intensive phase of blood sampling was completed, patients were discharged and returned to the clinic at the appointed times for blood sampling. At weekly visits temperature was recorded, hematocrit measured, and malaria smear examined. In case of parasite recurrence, PCR genotyping was used to determine whether this was a new infection or a true recrudescence (4). A 5-ml blood sample was taken to measure the piperaquine plasma concentration at the time of any recurrent parasitemia. Drugs with antimalarial activity (except chloroquine, which is for the treatment of vivax malaria) were not permitted during follow-up (e.g., cotrimoxazole and doxycycline). Hematinics were prescribed after day 7 if the hematocrit was less than 30%. Mebendazole was prescribed after day 7 if a confirmed diagnosis of worms was made.
Blood sampling.
Patients were admitted to the inpatient ward to complete the initial intense blood sampling following each dose. As sampling was frequent, an intravenous cannula was used. The cannula was flushed with 0.5 ml heparinized saline after use and changed for a new cannula before the last dose (48 h). A blood volume of 0.5 ml was discarded prior to sample collection to avoid drug dilution effects. Blood samples (2 to 5 ml) for the quantification of piperaquine concentrations in plasma were drawn into lithium heparin tubes and collected predose and at 0.5, 1, 2, 3, 4, 7, and 24 h after the first dose, at 1, 3, 4, 5, 7, and 24 h after the second dose, and at 1, 2, 3, 4, 5, 6, 8, and 12 h after the third dose. Additional blood samples were obtained by venipuncture at days 4, 5, 7, 14, 21, 28, 42, 56, 70, 84, 98, 112, and 126 after the first dose. Whole blood was centrifuged (2,000 × g for 10 min) and the plasma stored in liquid nitrogen within 30 min of collection. The samples were transported on dry ice to the Clinical Pharmacology Laboratory MORU at the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Piperaquine quantification.
Piperaquine plasma samples were quantified using high-throughput liquid chromatography-tandem mass spectrometry (LC-MS) (15). Plasma samples were analyzed by first adding internal standard (D6 PQ; a piperaquine stable isotope-labeled internal standard) dissolved in a low-pH buffer to plasma (50 μl) to disrupt protein binding and compensate for method variations. The buffered plasma samples were extracted on a mixed-phase cation (MPC) deep-well 96-SPE (solid-phase extraction) plate. The dried SPE eluates were reconstituted and analyzed by liquid chromatography with MS/MS detection on a Gemini (4- by 2-mm internal diameter) column using a mobile phase containing acetonitrile-ammonium bicarbonate (pH 10; 2.5 mM) (85:15, vol/vol) at a flow rate of 0.5 ml/min. PQ concentrations were quantified using an API 5000 triple quadrupole mass spectrometer (Applied Biosystems/MDS SCIEX, Foster City, CA) with a Turbo V ionization source interface operated in the positive-ion mode. Quantification was performed using selected reaction monitoring for m/z transitions from 535/288 and 541/294 for PQ and D6 PQ, respectively. The LC system was an Agilent 1200 system (Agilent Technologies, Santa Clara, CA). Data acquisition and quantification were performed using Analyst 1.4 (Applied Biosystems/MDS SCIEX, Foster City, CA). The coefficient of variation for the quality control samples during analysis were 4.4, 3.6, and 3.6% at 4.5, 20, and 400 ng/ml, respectively. The lower limit of quantification was 1.2 ng/ml.
Pharmacokinetic and statistical analysis.
Pharmacokinetic parameters of piperaquine were determined by noncompartmental analysis using WinNonlin version 5.3 (Pharsight Corporation, CA). The total exposure of piperaquine (AUC0-∞) was calculated using the linear trapezoid method for increasing concentrations and the logarithmic trapezoidal method for declining concentrations. The area from the last observed concentration to infinity was extrapolated for each patient as Clast/λZ. The terminal elimination half-life (t1/2) was estimated by log-linear regression in the terminal phase using an average of seven observed concentrations. The maximum plasma piperaquine concentration (Cmax) and time to Cmax (Tmax) for each dose were taken directly from the observed data. The apparent volume of distribution (VZ) and oral clearance (CL) were computed individually and reported. All reported pharmacokinetic parameters of the two treatment groups were compared using a nonparametric Wilcoxon rank-sum test (Mann-Whitney) in STATA v10.0 (StataCorp, TX). Day 7 piperaquine concentrations were plotted against total and fractional piperaquine exposure. Fractional exposure was evaluated starting at the time of the last dose (i.e., 42 h), 24 h after last dose (i.e., 66 h) when dihydroartemisinin is completely eliminated, and day 7. This was done to assess the day 7 concentration relationship with different stages of the elimination phase.
RESULTS
The demographics did not differ significantly between the fasting and the fed groups. Both groups had similar parasitemia on enrollment, with median (range) parasites/μl blood of 8 × 103 (0.45 × 103 to 140 × 103) for the fasting group and 8 × 103 (0.35 × 103 to 60 × 103) for the fed group. The median body weight (kg) was 52 (39 to 62) and 53 (45 to 73) in fasting and fed patients, respectively. The median age (years) was 39 (18 to 55) and 28 (19 to 41) in the fasting and fed group, respectively. There were two female patients in each group. The median total dose (mg/kg) was the same for the two groups, 30.4 (27.7 to 32.6) and 30.4 (27.7 to 32.2), expressed as piperaquine base in fed and fasting subjects, respectively. The drug generally was well tolerated, and there were no severe adverse effects reported for either group during the study. A total number of eight patients (five in the fasting group and three in the fed group) had recurrent malaria during the 126 days of follow-up. All of these were classified as new infections by PCR genotyping.
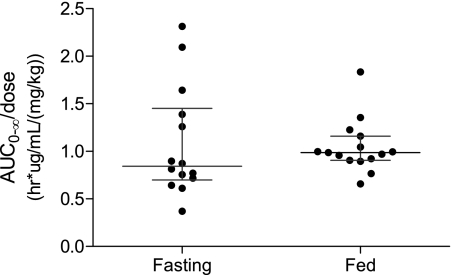
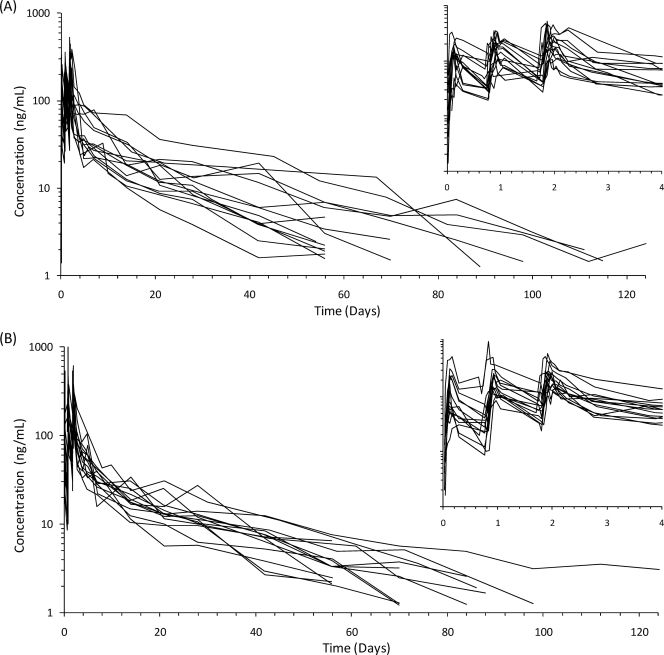
Piperaquine pharmacokinetics were well described by the obtained data. One patient in the fasting group was lost to follow-up at 2 weeks and was excluded from all calculations. The median (range) extrapolated area after the last measured concentration was low (4.5%; 2.3 to 24.8%). No significant pharmacokinetic differences were evident between the fasting and the fed groups (Table 1 and Fig. 1-2). Maximum piperaquine plasma concentrations (median [range], in ng/ml) after each successive dose did not differ between the fed group (128 [31 to 535], 185 [84.2 to 519], and 257 [147 to 615]) and the fasting group (143 [37 to 333], 217 [67.3 to 356], and 236 [105 to 524]). The highest concentration observed in the fasting patients was 524 ng/ml, and the highest concentration observed in the fed subjects was 615 ng/ml. The accumulation of piperaquine with each successive dose was similar in the two groups (Fig. 2). Dose-normalized (i.e., mg/kg) total piperaquine exposure (median [range]; in h · μg/ml/[mg/kg]) were not statistically different (P = 0.32) between fed (0.99 [0.66 to 1.83]) and fasting (0.84 [0.37 to 2.31]) patients (Fig. 1).
Table 1.
Piperaquine pharmacokinetic parameters for fasting and fed groups following a 3-day fixed oral treatment of piperaquine phosphate (54 mg/kg) and dihydroartemisinin (7 mg/kg)
| Parameterc | Pharmacokinetic result by sample group |
P | |||
|---|---|---|---|---|---|
| Fasting (n = 14) |
Fed (n = 15) |
||||
| Median (range) | Means ± SD | Median (range) | Means ± SD | ||
| Cmax 1st dose (ng/ml) | 143 (37–333) | 153 ± 75.1 | 128 (31–535) | 179 ± 149 | 0.760 |
| Cmax 2nd dose (ng/ml) | 217 (67.3–356) | 207 ± 98.7 | 185 (84.2–519) | 220 ± 120 | 0.930 |
| Cmax 3rd dose (ng/ml) | 236 (105–524) | 296 ± 149 | 257 (147–615) | 305 ± 151 | 0.711 |
| Tmax 1st dose (h) | 3 (2–7) | 3.29 ± 1.33 | 3 (2–7) | 3.20 ± 1.26 | 0.698 |
| Tmax 2nd dose (h) | 4 (1–7) | 4.29 ± 2.13 | 4 (3–7) | 3.93 ± 1.10 | 0.684 |
| Tmax 3rd dose (h) | 4 (1–12) | 4.30 ± 2.55 | 3 (3–8) | 3.93 ± 1.75 | 0.359 |
| CL/F (liter/h) | 67.1 (19.1–116.9) | 58.4 ± 26.3 | 53.2 (28.3–94.3) | 55.4 ± 15.4 | 0.458 |
| CL/Fa (liter/h/kg) | 1.19 (0.43–2.72) | 1.18 ± 0.60 | 1.01 (0.54–1.52) | 1.01 ± 0.23 | 0.316 |
| V/F (liter) | 34,000 (14,800–55,000) | 34,400 ± 13,000 | 38,400 (17,700–89,500) | 44,000 ± 21,000 | 0.239 |
| V/Fa (liter/kg) | 700 (335–1246) | 689 ± 258 | 769 (340–1444) | 796 ± 323 | 0.458 |
| t1/2 (days) | 17.5 (12.6–29.1) | 18.4 ± 5.52 | 21.4 (12.3–42.6) | 22.7 ± 7.87 | 0.116 |
| AUC0-last (h · μg/ml) | 22.4 (11.4–71.8) | 31.0 ± 17.3 | 28.6 (18.1–57.3) | 29.9 ± 9.39 | 0.571 |
| AUC0-∞ (h · μg/ml) | 23.9 (11.9–72.9) | 32.3 ± 17.5 | 29.5 (20.6–58.7) | 31.9 ± 9.28 | 0.458 |
| AUC0-∞/doseb (h · μg/ml/[mg/kg]) | 0.84 (0.37–2.31) | 1.08 ± 0.58 | 0.99 (0.66–1.83) | 1.04 ± 0.28 | 0.316 |
Body weight normalized.
Dose normalized (i.e., mg/kg).
AUC0-last, observed area under the plasma concentration time curve from zero time to last measured concentration; F, oral bioavailability.
Fig. 1.
Predicted dose-normalized AUC0-∞ for fasting and fed groups, with bars representing median values with interquartile ranges.
Fig. 2.
Plasma piperaquine concentration-time profiles following a once-daily oral administration of dihydroartemisinin-piperaquine phosphate (total doses of 7 and 54 mg/kg, respectively) for 3 days in 14 fasting patients (A) and 15 fed patients (B) with uncomplicated falciparum malaria.
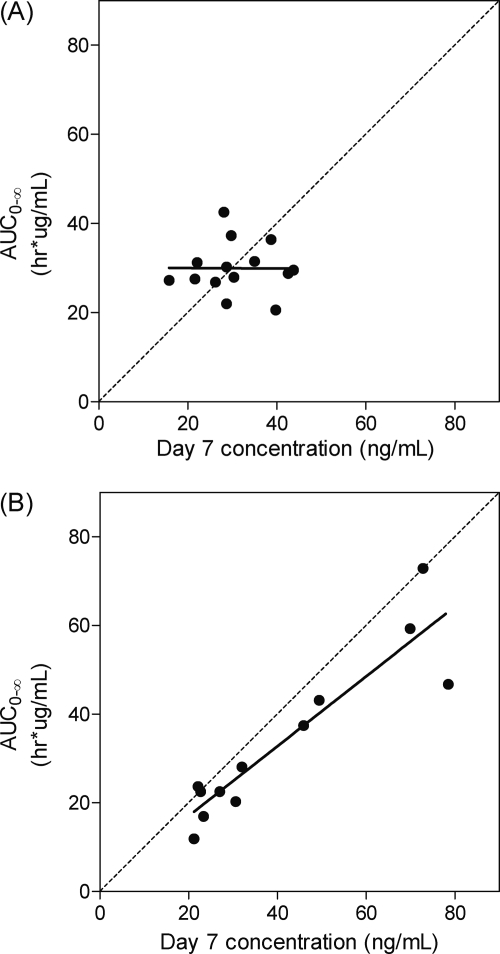
A single-time-point concentration in the elimination phase of slowly eliminated antimalarials has been suggested previously as a good predictor for therapeutic success (33). Price et al. defined a venous day 7 piperaquine concentration of 30 ng/ml as a good cutoff value for therapeutic efficacy (20). Correlation between piperaquine day 7 concentrations and total piperaquine exposure was investigated for the fed and fasted groups individually (Fig. 3). Two patients in the fasting group and one patient in the fed group were excluded from this analysis, since they did not have blood samples drawn at day 7. The plots showed that total piperaquine exposure was well correlated with the day 7 concentrations in the fasted group, but the fed group showed a poor correlation (Fig. 3). Fractional piperaquine exposure (i.e., from 42 h, 66 h, day 7, and onward) versus day 7 concentrations were plotted to evaluated correlations in different stages of the elimination phase and showed similar correlations (data not shown).
Fig. 3.
Correlation between day 7 concentration and total exposure of piperaquine. (A) Fed group; (B) fasting group. Solid line, line of regression; dashed line, line of identity.
DISCUSSION
Optimizing dosing regimens is essential for improving antimalarial treatment. The pharmacokinetic properties of piperaquine were well captured and characterized with the applied sampling scheme, and the pharmacokinetic parameters for piperaquine obtained in this study are similar to those previously published for piperaquine, e.g., body weight-adjusted clearance values of approximately 1 liter/h/kg, body weight-adjusted volume of distribution of approximately 740 liters/kg, a terminal elimination half-life of approximately 20 days, and a dose-normalized AUC of approximately 1 h · μg/ml/(mg/kg) for fasting subjects (14, 26–28).
Food interaction.
In this study, we concluded that the coadministration of 200 ml milk containing 6.4 g fat with piperaquine does not significantly affect the rate or extent of piperaquine absorption and, thus, the total exposure of piperaquine in patients with uncomplicated malaria. This is in contradiction to other findings that show that the absorption of piperaquine in healthy volunteers is significantly increased if given with the concomitant intake of fat (19, 24). These studies have been done in healthy Vietnamese and Caucasian subjects and compared to Burmese malaria patients, and they used much larger amounts of fat. In the present study, there was a trend toward higher (21%) total dose-normalized piperaquine exposure for fed patients than for fasting patients, but it did not reach statistical significance (median, 18.3 and 15.1 h · μg/ml/g, respectively; P = 0.458). The study by Nguyen et al. (17 g fat, 14 subjects) showed a significant increase in piperaquine exposure (AUC0-∞ after the last dose following 500 mg piperaquine phosphate/day for 3 days) of 63% (median, 24.1 h · μg/ml/g for fed patients and 14.7 h · μg/ml/g for fasting patients) (19). The study by Sim et al. with the largest amount of fat (53 g fat, 16 subjects) showed the largest increase (also significant) of 98% (geometric mean, 25.5 h · μg/ml/g for fed patients and 12.9 h · μg/ml/g for fasting patients) after a single oral dose (500 mg) of piperaquine phosphate (24). Reported piperaquine exposures for these studies were dose adjusted for comparison as reported mean (or median) AUC0-∞/mean dose. It should be noted that the study by Nguyen et al. gives an underestimation of the true exposure, since the contributing exposure from the first and second doses were not estimated.
Febrile patients with acute malaria usually are anorectic and often nauseated and do not tolerate large quantities of fat very well. Vomiting is common, particularly in young children. Data from studies on healthy volunteers when drugs are coadministered with food are difficult to translate into patient settings, as it is not always feasible to administer a drug with fat and certainly not in a controlled amount. For instance, one healthy-volunteer study provided a fat-rich McDonalds meal, while another provided a more reasonable meal of noodle soup. In this study, what was considered a reasonable volume of milk (200 ml) was given. This is easier for patients to consume and also is easier to control, but milk may still be poorly tolerated by nauseated patients. The loss of drug by vomiting undoubtedly will reduce efficacy. Ashley et al. showed that only 1.3 g of fat, corresponding to 40 ml of milk, was necessary to maximize the absorption of lumefantrine, another lipophilic antimalarial drug, in healthy volunteers (3). Clearly, the absorption of piperaquine in this formulation is augmented much less by the coadministration of fat.
Another publication reports piperaquine pharmacokinetics when given with 17 g fat in 32 healthy Vietnamese subjects. The median (80% central range) piperaquine exposure during the first 24 h (AUC0-24) increased in the fed state by 29% (2.2 versus 1.7 h · mg/liter), while total piperaquine exposure decreased by 9% (20.9 versus 23.1 h · mg/liter) (12). Piperaquine is a very difficult drug to quantify, and this paper also reports exceptionally high variability in pharmacokinetic parameters. These results could be explained by problems with the analytical method, especially at low concentrations. The maximal piperaquine concentration was 63% higher in the fed group, and the piperaquine exposure during the first 24 h was 29% higher, findings which are in agreement with other studies, even though the difference for exposure did not reach statistical significance. Piperaquine exposure during the first 24 h would have much less variability than total piperaquine exposure and is less affected by the bioanalytical method since concentrations are higher.
Taken together, the available data suggest that fat increases oral piperaquine bioavailability but that significant increases in piperaquine absorption are found only when coadministered with amounts of fat that are unlikely to be well tolerated in the treatment of acute malaria.
The lipophilic characteristic of piperaquine limits its solubility in water, and it is predominantly bound to lipoproteins in the blood (7). The effect of fat coadministration on drug absorption also could depend on the correlation between the Tmax for the lipids and the Tmax for the drug, as discussed for halofantrine (13). It was shown that the absorption of triglycerides (TG) varied across subjects and that the fed/fasted ratio for TG was strongly correlated with the fed/fasted ratio for halofantrine (13).
Effect of disease.
Drug exposures for fed and fasting patients in this study (n = 30) were similar to those reported for fasting healthy volunteers in previous studies (19, 24). In the present study, the total piperaquine exposure for fasting patients was (median) 15.1 h · μg/ml/g. The study by Nguyen et al. in healthy volunteers showed a total piperaquine exposure of (median) 14.7 h · μg/ml/g for the fasting group (although this is an underestimation, as described above) (19), and the study by Sim et al. (also with healthy volunteers) showed a total piperaquine exposure of (geometric mean) 12.9 h · μg/ml/g for the fasting group (24).
Half-life.
Data for terminal elimination half-lives described in a detailed study of healthy volunteers suggest that the true elimination half-life previously was underestimated due to limited sampling and assay sensitivity issues and could be as long as 40 to 50 days (27). The half-life presented in this study is approximately 20 days. This is likely to be an accurate estimate of the true half-life, since the method used quantifies as little as 1.2 ng/ml, and patients were monitored for up to 126 days.
Variability.
Although there were no significant differences for maximal piperaquine concentration between the two groups, the fasting group (49% coefficient of variation [CV]) had less variability than the fed group (83% CV) in the drug concentrations reached after the first dose. However, following the second and third doses the differences in variation were much less between the two groups. The data for dose-normalized total piperaquine exposure measured to the last concentration and extrapolated to infinity indicates that variability is almost half in the fed group what it is in the fasting group. Thus, the concomitant intake of a sufficient amount of fat might lead to less variability and higher exposure.
For many of the subjects, piperaquine displayed multiple absorption peaks, which have been described before (12, 24). Enterohepatic recirculation has been proposed as a possible explanation (24), but data from rat studies do not support this theory, since the biliary excretion of piperaquine was found to be very low (28). This phenomenon could be related to the chemical characteristics of piperaquine that allow its solubility properties to vary with pH environment in the gastrointestinal tract.
Conclusion.
There were no significant differences in any pharmacokinetic parameters when piperaquine was administered to fasting patients or coadministered with 200 ml milk containing 6.4 g fat. These data, combined with data from other reports, indicate that to increase the oral bioavailability of piperaquine significantly, a substantial amount of coadministered fat is required. Whether the type of fat is important, and whether there are differences between the effects of solid and liquid foods, are unanswered questions. The reduced interindividual variability in piperaquine exposure with fat coadministration presumably reflects absorption stability and argues for further studies with fat and drug formulation. Current dose regimens have been built on evidence gained from clinical trials where dihydroartemisinin-piperaquine was administered without specific fat coadministration. In our experience, administering amounts of fat larger than that used here (6.4 g) in cases of acute malaria is likely to be poorly tolerated; furthermore, giving dihydroartemisinin-piperaquine with large amounts of fat might lead to very high drug concentrations in some patients, with an attendant risk of significant cardiotoxicity (reflected by QT prolongation), which is not seen with current fat-free dose administration. Concomitant food intake does not appear to affect the disposition of artemisinin (9), while it does seem to reduce the Cmax but not the AUC of artesunate and dihydroartemisinin (11). Taken together, these data do not support the coadministration of dihydroartemisinin-piperaquine with fat.
ACKNOWLEDGMENTS
This study was part of the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme (077166/Z/05/Z) and the PKPDia collaboration, both supported by the Wellcome Trust of Great Britain.
Footnotes
Published ahead of print on 27 June 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Ahmed T., et al. 2008. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J. Clin. Pharmacol. 48:166–175 [DOI] [PubMed] [Google Scholar]
- 2. Ashley E. A., et al. 2005. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41:425–432 [DOI] [PubMed] [Google Scholar]
- 3. Ashley E. A., et al. 2007. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop. Med. Int. Health 12:195–200 [DOI] [PubMed] [Google Scholar]
- 4. Brockman A., et al. 1999. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am. J. Trop. Med. Hyg. 60:14–21 [DOI] [PubMed] [Google Scholar]
- 5. Chen L., Qu F. Y., Zhou Y. C. 1982. Field observations on the antimalarial piperaquine. Chin. Med. J. 95:281–286 [PubMed] [Google Scholar]
- 6. Crevoisier C., Handschin J., Barre J., Roumenov D., Kleinbloesem C. 1997. Food increases the bioavailability of mefloquine. Eur. J. Clin. Pharmacol. 53:135–139 [DOI] [PubMed] [Google Scholar]
- 7. Davis T. M., Hung T. Y., Sim I. K., Karunajeewa H. A., Ilett K. F. 2005. Piperaquine: a resurgent antimalarial drug. Drugs 65:75–87 [DOI] [PubMed] [Google Scholar]
- 8. Denis M. B., et al. 2006. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop. Med. Int. Health 11:1800–1807 [DOI] [PubMed] [Google Scholar]
- 9. Dien T. K., et al. 1997. Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrob. Agents Chemother. 41:1069–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ezzet F., van Vugt M., Nosten F., Looareesuwan S., White N. J. 2000. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob. Agents Chemother. 44:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitoussi S., et al. 2009. Bioavailability of a co-formulated combination of amodiaquine and artesunate under fed and fasted conditions. A randomised, open-label crossover study. Arzneimittel-Forschung 59:370–376 [DOI] [PubMed] [Google Scholar]
- 12. Hai T. N., Hietala S. F., Van Huong N., Ashton M. 2008. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 107:145–149 [DOI] [PubMed] [Google Scholar]
- 13. Humberstone A. J., Porter C. J., Edwards G. A., Charman W. N. 1998. Association of halofantrine with postprandially derived plasma lipoproteins decreases its clearance relative to administration in the fasted state. J. Pharm. Sci. 87:936–942 [DOI] [PubMed] [Google Scholar]
- 14. Hung T. Y., et al. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br. J. Clin. Pharmacol. 57:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindegardh N., Annerberg A., White N. J., Day N. P. 2008. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of piperaquine in plasma stable isotope labeled internal standard does not always compensate for matrix effects. J. Chromatogr. 862:227–236 [DOI] [PubMed] [Google Scholar]
- 16. Looareesuwan S., et al. 1987. Studies of mefloquine bioavailability and kinetics using a stable isotope technique: a comparison of Thai patients with falciparum malaria and healthy Caucasian volunteers. Br. J. Clin. Pharmacol. 24:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milton K. A., Edwards G., Ward S. A., Orme M. L., Breckenridge A. M. 1989. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br. J. Clin. Pharmacol. 28:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen D. V., et al. 2009. Pharmacokinetics and ex vivo pharmacodynamic antimalarial activity of dihydroartemisinin-piperaquine in patients with uncomplicated falciparum malaria in Vietnam. Antimicrob. Agents Chemother. 53:3534–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen T. C., et al. 2008. Short report: pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 79:620–623 [PubMed] [Google Scholar]
- 20. Price R. N., et al. 2007. Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria. Antimicrob. Agents Chemother. 51:4090–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rolan P. E., et al. 1994. Examination of some factors responsible for a food-induced increase in absorption of atovaquone. Br. J. Clin. Pharmacol. 37:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silamut K., Molunto P., Ho M., Davis T. M., White N. J. 1991. Alpha 1-acid glycoprotein (orosomucoid) and plasma protein binding of quinine in falciparum malaria. Br. J. Clin. Pharmacol. 32:311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silamut K., White N. J., Looareesuwan S., Warrell D. A. 1985. Binding of quinine to plasma proteins in falciparum malaria. Am. J. Trop. Med. Hyg. 34:681–686 [DOI] [PubMed] [Google Scholar]
- 24. Sim I. K., Davis T. M., Ilett K. F. 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob. Agents Chemother. 49:2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smithuis F., et al. 2006. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet 367:2075–2085 [DOI] [PubMed] [Google Scholar]
- 26. Tarning J., et al. 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob. Agents Chemother. 52:1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarning J., et al. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob. Agents Chemother. 49:5127–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tarning J., et al. 2008. Pharmacokinetics and metabolism of the antimalarial piperaquine after intravenous and oral single doses to the rat. J. Pharm. Sci. 97:3400–3410 [DOI] [PubMed] [Google Scholar]
- 29. Trenholme G. M., Williams R. L., Rieckmann K. H., Frischer H., Carson P. E. 1976. Quinine disposition during malaria and during induced fever. Clin. Pharmacol. Ther. 19:459–467 [DOI] [PubMed] [Google Scholar]
- 30. Valecha N., et al. 2010. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 5:e11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White N. J. 1985. Clinical pharmacokinetics of antimalarial drugs. Clin. Pharmacokinet. 10:187–215 [DOI] [PubMed] [Google Scholar]
- 32. White N. J., et al. 1982. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated Falciparum malaria. Am. J. Med. 73:564–572 [DOI] [PubMed] [Google Scholar]
- 33. White N. J., van Vugt M., Ezzet F. 1999. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin. Pharmacokinet. 37:105–125 [DOI] [PubMed] [Google Scholar]