Abstract
Clotrimazole and bifonazole are highly effective antifungal agents against mucosal Candida albicans infections. Here we examined the effects of low levels of clotrimazole and bifonazole on the ability of C. albicans to adhere, invade, and damage vaginal epithelial cells. Although adhesion and invasion were not affected, damage was greatly reduced upon azole treatment. This clearly indicates that low levels of azoles influence specific activities of C. albicans during distinct stages of vaginal epithelium infections.
TEXT
The human pathogenic fungus Candida albicans is commonly found on mucosal surfaces, such as the vagina. Here, the fungus may persist as a harmless colonizer but can also cause disease. Around 75% of all women experience at least one episode of vulvovaginal candidiasis (VVC) in their lifetime (23). Some predisposing factors for VVC, such as diabetes or pregnancy, have been identified; however, VVC is also common among otherwise healthy women (8). Fungal adhesion to the epithelium is a prerequisite for colonization, and infections are characterized by the invasion of vaginal epithelial cells (7, 22, 25). Additionally, hyphal formation contributes to symptomatic vaginal infections by damaging epithelial tissue (9, 24, 30). Therefore, a number of factors may be responsible for VVC, but the exact pathogenicity mechanisms of vaginal Candida infections remain poorly understood.
Imidazoles, which block fungal ergosterol biosynthesis, have been proven to be effective against vaginal candidiasis (5). However, due to the pharmacokinetic properties of azoles, the major fraction of an applied dose remains on the surface or in the stratum corneum, leading to reduced therapeutic concentrations. Clotrimazole is one of the most commonly used imidazoles for the treatment of VVC and can persist at inhibitory levels in vaginal secretions for up to 3 days following a single treatment (1, 15, 17). Bifonazole also inhibits the ergosterol biosynthetic pathway but has higher fungicidal activity than clotrimazole due to additional inhibition of terpenoid biosynthesis (2). Furthermore, clotrimazole and bifonazole have been shown to affect C. albicans farnesol production and to reduce virulence in vivo (2, 7, 14, 21). In this study we sought to dissect the effects of clotrimazole and bifonazole on C. albicans interactions with vaginal epithelial cells by analyzing adhesion and invasion, as well as epithelial damage, in the presence of these two drugs.
C. albicans strain SC5314 (10, 13) was used for all experiments. Clotrimazole and bifonazole (Bayer AG) stocks were prepared in dimethyl sulfoxide (DMSO) and used at final concentrations of 0, 0.01, 0.1, 1, 10, and 100 μM in the indicated media. C. albicans growth rates in liquid yeast extract-peptone-dextrose (YPD) were determined at 30°C by measuring the optical density at 600 nm every 30 min using an Infinite M200 enzyme-linked immunosorbent assay (ELISA) reader (16). Filamentation of C. albicans was induced with RPMI 1640 (PAA) at 37°C on plastic surfaces. Interactions of C. albicans with the vaginal epithelial cell line A-431 (ACC 91; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Germany) (12) were performed as described previously (26). Briefly, C. albicans was coincubated with A-431 cells for 1, 3, or 24 h in Dulbecco modified Eagle medium (DMEM) supplemented with 1 or 10 μM clotrimazole or bifonazole at 37°C and 5% CO2. Following coincubation, the numbers of adherent (1 h) and invading (3 h) cells were determined by fluorescence microscopy as described previously (26), and morphology was monitored after 24 h. Epithelial cell damage was measured at 24 h by monitoring the release of lactate dehydrogenase (LDH) using the LDH cytotoxicity detection kit (Roche Applied Science), according to the manufacturer's instructions. All experiments were performed in duplicate at least three times. The data were analyzed using either two-way analysis of variance (ANOVA) to compare the relative growth or ANOVA (Dunnett's multiple comparison test) to compare treated versus untreated controls, and P values of <0.05 and <0.01 were considered significant.
Bifonazole concentrations of 0.01 and 0.1 μM and clotrimazole concentrations of 0.01 μM had no significant influence on growth or filamentation of C. albicans under the conditions tested. Treatment of C. albicans with 100 μM of either drug almost completely blocked yeast growth (data not shown). Concentrations of 10 μM (both azoles) and 1 μM (clotrimazole only) inhibited yeast growth, resulting in generation times of over 4.3 h, compared to 2.5 h for the untreated control (Table 1). Both of the azoles had an even stronger effect on filamentous growth: bifonazole (10 to 100 μM) and clotrimazole (0.1 to 100 μM) treatment strongly reduced the formation of long hyphae in RPMI 1640 from 47 μm in the untreated control to around 18 μm in the presence of azoles after 3 h of hyphal induction (Table 1). Bifonazole at 1 μM moderately inhibited filamentation (Table 1). Even after longer incubation periods of 24 h, concentrations of 10 and 100 μM for both azoles and 1 μM for clotrimazole had blocked the ability of C. albicans to form long filaments (data not shown). These data are in agreement with previous findings showing that clotrimazole and bifonazole inhibit both yeast growth and filamentation of C. albicans (4, 19, 27) and that hyphae are more effectively inhibited by these azoles than yeast cells (18). Based on the observed inhibitory effects on yeast growth and filamentation, we used clotrimazole and bifonazole at concentrations of 1 or 10 μM to analyze the effect on C. albicans-vaginal epithelial cell interactions.
Table 1.
Summarized phenotypes of C. albicans wild-type cells treated with 1 μM or 10 μM bifonazole or clotrimazole, respectively, compared to those of the untreated wild-type control
| Phenotypea | Time point | Untreated control | Treatmentb |
|||
|---|---|---|---|---|---|---|
| Bifonazole |
Clotrimazole |
|||||
| 1 μM | 10 μM | 1 μM | 10 μM | |||
| Yeast growth (generation time [h]) | Log phase | 2.53 ± 0.12 | 2.69 ± 0.07 | 5.20 ± 1.69* | 4.26 ± 1.08* | 6.23 ± 3.41** |
| Filament length (RPMI) (μm) | 3 h | 46.9 ± 17 | 33 ± 9.2* | 17.5 ± 3.5** | 19.1 ± 6.1** | 18.5 ± 7.2** |
| Filament length (vaginal cells) (μm) | 3 h | 46.1 ± 3.8 | 27.3 ± 14.2** | 23.3 ± 11.0** | 19.2 ± 7.8** | 16.9 ± 5.3** |
| Adhesion (%) | 1 h | 100 | 109.6 ± 34 | 99.6 ± 18.7 | 94 ± 26.9 | 109 ± 37.4 |
| Invasion (%) | 3 h | 100 | 92.2 ± 6.7 | 101.5 ± 35 | 91.3 ± 30.5 | 91.8 ± 29.3 |
| Damage (ng/ml) | 24 h | 157.0 ± 23.8 | 37.3 ± 47.5** | 4.6 ± 22.6** | −0.7 ± 12.8** | −3.7 ± 9.0** |
Yeast growth in liquid YPD presented as generation time (in h) during exponential growth phase. Filament length (in μm) was determined either following induction in RPMI in liquid cultures after 3 h or on vaginal epithelial cells in DMEM after 3 h of coincubation. Adhesion to and invasion of vaginal epithelial cells were compared to 100% adhesion and invasion of the untreated control. Damage was calculated as LDH release in ng/ml by vaginal epithelial cells after 24 h.
*, P < 0.05; **, P < 0.01 (significant differences compared to values of the untreated control).
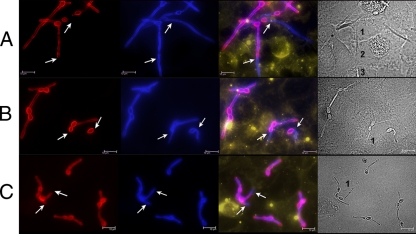
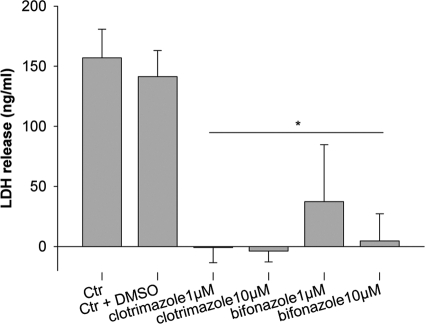
Irrespective of azole treatment, more than 98% of yeast cells formed germ tubes following 3 h of incubation while in contact with epithelial cells; however, treatment with clotrimazole or bifonazole strongly reduced the average filament length, similar to RPMI-induced filamentation (Fig. 1; Table 1). Approximately 5 to 15% (ranging from 2.92 × 103 to 1.49 × 104) of inoculated yeast cells adhered tightly to vaginal epithelium cells after 1 h. Adhesion was not affected by azole treatment, with or without azole pretreatment (Table 1 and data not shown), resulting in adherence of 5.81 × 103 to 7.46 × 103 cells. Similarly, azole treatment did not influence the invasion of epithelial cells by C. albicans (Fig. 1; Table 1). However, the invading (intracellular) portions of azole-treated fungal cells were considerably shorter than those without treatment and did not undergo interepithelial invasion (penetration from one epithelial cell to another adjacent cell) (Fig. 1). Therefore, although clotrimazole and bifonazole strongly reduced the formation of long filaments of C. albicans, these azoles did not influence adhesion to or initial invasion into vaginal epithelial cells. In contrast, both azoles had a striking effect on the ability of C. albicans to elicit cellular damage. Coincubation of untreated C. albicans with vaginal epithelial cells resulted in the release of 157 ng/ml LDH after 24 h (Fig. 2). The addition of 10 μM bifonazole, 1 μM clotrimazole, or 10 μM clotrimazole prevented epithelial damage (Fig. 2; Table 1). Bifonazole at a lower concentration (1 μM) reduced epithelial damage by 76% compared to the untreated control (Fig. 2; Table 1).
Fig. 1.
Representative fluorescent pictures of C. albicans invasion into epithelial cells. (A) Untreated; (B) treated with 1 μM bifonazole; (C) treated with 1 μM clotrimazole. C. albicans hyphae are stained differentially: first panel (stained red), extracellular part of C. albicans hyphae; second panel (stained blue), extracellular and internalized C. albicans hyphae; third panel, overlay of first and second panels (extracellular parts appear pink), epithelial cell membranes (stained yellow); fourth panel, differential interference contrast (DIC) images. Arrows mark internalized hyphae. Numbers indicate epithelial cells invaded by C. albicans. Note that all hyphae have the capacity to invade epithelial cells but that only untreated C. albicans hyphae penetrate through the first epithelial cell. Bar = 10 μm.
Fig. 2.
Damage of vaginal epithelial cells is inhibited by bifonazole and clotrimazole. Vaginal epithelial cell damage was determined by measuring the LDH release. Bifonazole at 1 μM inhibited damage compared to untreated infections (control [Ctr]) or infections with DMSO only (Ctr + DMSO); 10 μM bifonazole and 1 μM/10 μM clotrimazole blocked damage entirely. *, significant difference compared to the untreated control (P < 0.01).
Together, these data demonstrate that low levels of clotrimazole and bifonazole, which had only moderate effects on yeast growth, did not affect adhesion or primary invasion but prevented vaginal epithelial cell damage by C. albicans.
For the establishment of C. albicans colonization and infection of epithelial surfaces, adhesion to host cells is a prerequisite. During interaction with epithelial cells, adhesion and hyphal formation are linked: contact and adhesion stimulates hyphal formation, and hyphal formation enhances adhesion (9, 29, 30). Interestingly, although treatment with clotrimazole or bifonazole resulted in shorter germ tubes, the actual percentage of yeast cells, which formed germ tubes, was not affected (data not shown). Moreover, these short germ tubes retained full adhesive potential (Table 1). This is in accordance with results obtained by Odds and Webster (20), which showed that sub-MICs of ketoconazole and clotrimazole have no significant influence on C. albicans adhesion to vaginal epithelial cells. However, in other studies, sublethal concentrations of ketoconazole and/or fluconazole were shown to reduce the adhesion of C. albicans to buccal epithelial cells (6) or endothelial cells (11), suggesting an azole- and/or host cell type-specific effect.
Moreover, the short hyphae produced by azole-treated C. albicans also invaded vaginal epithelial cells to the same degree as untreated C. albicans cells (Table 1), indicating that azole treatment does not prevent initial epithelial invasion. Significantly, these azole-treated invasive hyphae were unable to damage vaginal epithelial cells, clearly demonstrating that initial fungal invasion is not sufficient to cause epithelial damage. Importantly, these data imply that clotrimazole and bifonazole are capable of interfering with cellular damage caused by C. albicans after invasion of vaginal epithelia. Consistent with these findings, fluconazole and voriconazole have been shown to reduce tissue destruction in reconstituted esophageal epithelia when administered early after C. albicans infection (3). Although primary invasion was not affected by azole treatment, we did observe differences in the invasion patterns following treatment, as follows: secondary invasion of adjacent epithelial cells was not observed in the presence of clotrimazole or bifonazole (Fig. 1). After the initial invasion into an epithelial cell, C. albicans hyphae do not persist within this primary epithelial cell (like some facultative intracellular bacteria) but further penetrate through the first epithelial cell and into adjacent epithelial cells (B. Wächtler and B. Hube, unpublished data). Such interepithelial invasion properties have been shown to depend on hyphal extension and to be essential for damage. We therefore speculate that inhibition of hyphal extension is responsible for the observed reduction in epithelial damage upon clotrimazole and bifonazole treatment.
Taken together, low levels of the two azoles, clotrimazole and bifonazole, have strong inhibitory effects on a distinct stage of C. albicans-vaginal epithelial cell infection: the ability to cause damage. Therefore, we conclude that treatment with clotrimazole or bifonazole, at levels which have only a moderate effect on fungal growth, may alleviate disease symptoms in vivo by preventing epithelial damage and possibly by reducing the attraction of further tissue-damaging neutrophils (28). Our data also suggest that targeting a distinct fungal virulence attribute or stage of infection is a reasonable and realistic antifungal strategy.
Acknowledgments
This work was funded by Bayer Laboratories and supported with the resources and facilities at the Hans Knoell Institute, Jena, Germany.
We thank H. Schoeler and S. Boerner for assistance during laboratory work.
Footnotes
Published ahead of print on 11 July 2011.
REFERENCES
- 1. Berg D., Buchel K. H., Plempel M., Zywietz A. 1986. Action mechanisms of cell-division-arresting benzimidazoles and of sterol biosynthesis-inhibiting imidazoles, 1,2,4-triazoles, and pyrimidines. Mykosen 29:221–229 [DOI] [PubMed] [Google Scholar]
- 2. Berg D., Regel E., Harenberg H. E., Plempel M. 1984. Bifonazole and clotrimazole. Their mode of action and the possible reason for the fungicidal behaviour of bifonazole. Arzneimittelforschung 34:139–146 [PubMed] [Google Scholar]
- 3. Bernhardt J., Bernhardt H., Knoke M., Ludwig K. 2004. Influence of voriconazole and fluconazole on reconstituted multilayered oesophageal epithelium infected by Candida albicans. Mycoses 47:330–337 [DOI] [PubMed] [Google Scholar]
- 4. Blanco M. T., et al. 1992. In vitro studies of activities of some antifungal agents against Candida albicans ATCC 10231 by the turbidimetric method. Antimicrob. Agents Chemother. 36:898–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edelman D. A., Grant S. 1999. One-day therapy for vaginal candidiasis. A review. J. Reprod. Med. 44:543–547 [PubMed] [Google Scholar]
- 6. Ellepola A. N., Panagoda G. J., Samaranayake L. P. 1999. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol. Immunol. 14:358–363 [DOI] [PubMed] [Google Scholar]
- 7. Farrell S. M., Hawkins D. F., Ryder T. A. 1983. Scanning electron microscope study of Candida albicans invasion of cultured human cervical epithelial cells. Sabouraudia 21:251–254 [PubMed] [Google Scholar]
- 8. Fidel P. L., Jr., Sobel J. D. 1996. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 9:335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filler S. G., Sheppard D. C. 2006. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fonzi W. A., Irwin M. Y. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghannoum M. A., Filler S. G., Ibrahim A. S., Fu Y., Edwards J. E., Jr 1992. Modulation of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob. Agents Chemother. 36:2239–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giard D. J., et al. 1973. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 51:1417–1423 [DOI] [PubMed] [Google Scholar]
- 13. Gillum A. M., Tsay E. Y., Kirsch D. R. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 14. Hornby J. M., Nickerson K. W. 2004. Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 48:2305–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lebherz T., Guess E., Wolfson N. 1985. Efficacy of single- versus multiple-dose clotrimazole therapy in the management of vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 152:965–968 [DOI] [PubMed] [Google Scholar]
- 16. Lis M., Liu T. T., Barker K. S., Rogers P. D., Bobek L. A. 2010. Antimicrobial peptide MUC7 12-mer activates the calcium/calcineurin pathway in Candida albicans. FEMS Yeast Res. 10:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendling W., Plempel M. 1982. Vaginal secretion levels after 6 days, 3 days and 1 day of treatment with 100, 200 and 500 mg vaginal tablets of clotrimazole and their therapeutic efficacy. Chemotherapy 28(Suppl. 1):43–47 [DOI] [PubMed] [Google Scholar]
- 18. Niimi M., Kamiyama A., Tokunaga M., Tokunaga J., Nakayama H. 1985. Germ tube-forming cells of Candida albicans are more susceptible to clotrimazole-induced killing than yeast cells. Sabouraudia 23:63–68 [PubMed] [Google Scholar]
- 19. Odds F. C., Cockayne A., Hayward J., Abbott A. B. 1985. Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J. Gen. Microbiol. 131:2581–2589 [DOI] [PubMed] [Google Scholar]
- 20. Odds F. C., Webster C. E. 1988. Effects of azole antifungals in vitro on host/parasite interactions relevant to Candida infections. J. Antimicrob. Chemother. 22:473–481 [DOI] [PubMed] [Google Scholar]
- 21. Plempel M., Berg D. 1984. Reduction of the in vivo virulence of Candida albicans by pretreatment with subinhibitory azole concentrations in vitro. Dermatologica 169(Suppl. 1):11–18 [DOI] [PubMed] [Google Scholar]
- 22. Powell B. L., Drutz D. J. 1983. Confirmation of corticosterone and progesterone binding activity in Candida albicans. J. Infect. Dis. 147:359. [DOI] [PubMed] [Google Scholar]
- 23. Sobel J. D. 1997. Vaginitis. N. Engl. J. Med. 337:1896–1903 [DOI] [PubMed] [Google Scholar]
- 24. Sobel J. D., Muller G., Buckley H. R. 1984. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect. Immun. 44:576–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobel J. D., Muller G., McCormick J. F. 1985. Experimental chronic vaginal candidosis in rats. Sabouraudia 23:199–206 [DOI] [PubMed] [Google Scholar]
- 26. Wachtler B., Wilson D., Haedicke K., Dalle F., Hube B. 2011. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One 6:e17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. White T. C., Holleman S., Dy F., Mirels L. F., Stevens D. A. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yano J., Lilly E., Barousse M., Fidel P. L., Jr 2010. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect. Immun. 78:5126–5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zakikhany K., et al. 2007. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell. Microbiol. 9:2938–2954 [DOI] [PubMed] [Google Scholar]
- 30. Zhu W., Filler S. G. 2010. Interactions of Candida albicans with epithelial cells. Cell. Microbiol. 12:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]