Abstract
While current data indicate only free (unbound) drug is pharmacologically active and is most predictive of response, pharmacodynamic studies of vancomycin have been limited to measurement of total concentrations. The protein binding of vancomycin is thought to be approximately 50%, but considerable variability surrounds this estimate. The present study sought to determine the extent of vancomycin protein binding, to identify factors that modulate its binding, and to create and validate a prediction tool to estimate the extent of protein binding based on individual clinical factors. This single-site prospective cohort study included hospitalized adult patients treated with vancomycin and with a vancomycin serum concentration determination available. Linear regression was used to predict the free vancomycin concentration (f[vanco]) and to determine the clinical factors modulating vancomycin protein binding. Among the 50 patients in the study, the mean protein binding was 41.5%. The strongest predictor of f[vanco] was the total vancomycin concentration (total [vanco]), and this was modified by dialysis and total protein of ≥6.7 g/dl as covariates. The algebraic expression from the final prediction model was f[vanco] = 0.643 + 0.560 × total [vanco] − {0.067 × total [vanco] × D} − {0.071 × total [vanco] × TP} where D = 1 if dialysis dependent or 0 if not dialysis dependent, and TP = 1 if total protein is ≥6.7 g/dl or 0 if total protein is <6.7 g/dl. The R2 of the final prediction model was 0.959 (P < 0.001). Validation of our model was performed in 13 patients, and the predictive performance was highly favorable (R2 was 0.9, and bias and precision were 0.18 and 0.18, respectively). Prediction models such as ours can be utilized in future pharmacokinetics and pharmacodynamics studies evaluating the exposure-response profile and to determine the pharmacodynamic target of interest as it relates to the free concentration.
INTRODUCTION
Antimicrobial pharmacodynamics describes the relationship between drug exposure and antimicrobial activity. The past 25 years have witnessed tremendous advances in understanding the relationship between antimicrobial pharmacodynamics and microbiological response, and the pharmacodynamic targets associated with maximal effect have been identified for many antimicrobials (8, 9, 14, 16). For vancomycin, the ratio of the area under the serum drug concentration-versus-time curve (AUC) and the MIC, or the AUC/MIC ratio, appears to be the best predictor of response based in part on data from animal models, in vitro studies, and limited human studies (22, 27). Collectively, these data suggest that microbiological success is optimized when the vancomycin total drug AUC/MIC ratio exceeds 400 (22, 26). In clinical practice, since it is not practical to obtain serial vancomycin concentrations within a dosing interval to estimate the AUC, many clinicians use vancomycin trough concentrations as a surrogate for the AUC when optimizing the vancomycin dosing regimen.
One commonality of these vancomycin pharmacodynamic studies is that they examined total vancomycin concentrations (total [vanco]) rather than free or unbound drug (22, 27). While these studies demonstrated a positive correlation between the total vancomycin AUC/MIC ratio and response, there are data that indicate only free drug or unbound drug is pharmacologically active and is most predictive of the response (3, 5, 10, 11, 15, 19, 21, 24, 33). We are not aware of any studies that have assessed whether free vancomycin concentrations (f[vanco]) are predictive of outcomes.
In the absence of pharmacodynamic studies that delineate the relationship between drug exposure and response, it is common practice to multiply the total drug exposure by the extent of protein binding to determine the amount of free drug necessary for response. In order to do this, it is critical to have a reasonable point estimate of the extent of protein binding. While it is assumed that vancomycin is approximately 50% bound (25), protein binding for the agent has not been well characterized in the literature, and estimates have varied considerably (1, 2, 12, 18, 23, 31, 35). In particular, a recent analysis reported a range of percent protein binding to be 12% to 100% in 15 hospitalized adults (4).
Given the variability surrounding the extent of vancomycin protein binding, the purpose of this study was 2-fold: (i) to determine the extent to which vancomycin is protein bound in the blood and (ii) to identify factors that modulate vancomycin protein binding to create and validate a prediction tool for estimating protein binding based on these clinical factors.
MATERIALS AND METHODS
This prospective, noninterventional cohort study was performed at the Albany Medical Center Hospital (AMCH), a 631-bed tertiary-care academic hospital located in upstate New York. The study cohort for development of the prediction tool consisted of 50 consecutive hospitalized adult (age ≥ 18 years) patients being treated with vancomycin for a suspected or documented infection by Gram-positive bacteria and with a vancomycin pharmacokinetic sample collected between 15 June 2010 and 3 July 2010. All blood samples were collected as part of the patient's routine standard of care at AMCH for clinical (nonresearch) purposes. The study was approved by the AMCH institutional review board, and waiver of informed consent was obtained.
Data were collected from patients' medical records by a trained investigator using a structured data instrument. Data elements included age, sex, height, and weight (within 48 h of vancomycin level determination), comorbidities (diabetes mellitus, heart failure, hepatic dysfunction, dialysis, and epilepsy), location (intensive care unit [ICU] versus non-ICU), length of hospitalization prior to sample collection, source of infection, and severity of illness at sample collection (as calculated by means of the APACHE II score [17]). The APACHE II score was calculated using the patient's worst physiological score within 24 h of the vancomycin sample collection. Variables for which patient data were not available were considered to be in the normal range for calculation of the APACHE II score (32). Other variables extracted from the patients' medical records included the vancomycin dose and the duration of therapy prior to sample collection, creatinine clearance (CLCR) estimated by the Cockcroft-Gault formula (7), and laboratory results (white blood cells [WBC], serum creatinine [SCr], blood urea nitrogen [BUN], albumin, total protein, total bilirubin, and total and free vancomycin concentrations). All reported laboratory values were obtained on the day of sample collection. If a laboratory value was not measured on the same day as sample collection, the closest value within 3 days of sample collection was used when available.
Clinical vancomycin samples collected for therapeutic drug monitoring as part of patients' routine care were sent to the AMCH chemistry laboratory for processing. Total concentrations were quantified per standard of care, and free concentrations were determined for this analysis. Previous analyses have reported ultrafiltration to be adequate and reliable for assessing vancomycin's protein binding due to very minimal adsorption of the drug to the ultrafiltration device (1, 2, 23, 28, 29). Therefore, free vancomycin concentration (f[vanco]) determination was performed by ultrafiltration. The blood samples were centrifuged at 730 × g for 10 min, and the serum was harvested. The serum was split into 2 aliquots for determining the total [vanco] and f[vanco]. The serum (1 to 2 ml) designated for determining the f[vanco] was processed through a Centrifree ultrafiltration device (Tullagree, Carrigtwohill, County Cork, Ireland) with swinging-bucket centrifugation for 30 min at 1,300 × g prior to assay. Total [vanco] and f[vanco] were determined using the TDx/fluorescence polarization immunoassay (FPIA) method (Abbott Diagnostics, Abbott Park, IL). The lower limit of detection of the vancomycin assay was 0.7 mg/liter, and the intra- and interday coefficients of variation were 4.8% and 5.5%, respectively. All vancomycin concentrations (total and free) were determined within 4 h of sample collection.
Model validation.
The performance of the prediction model was assessed to determine the validity of the f[vanco] estimate. The study cohort for validation of the prediction tool consisted of 13 consecutive hospitalized adult patients with a vancomycin pharmacokinetic sample collected between 10 April 2011 and 13 April 2011. The methodology used for development of the prediction model, including data collection, sample collection, and f[vanco] determination, was also used for model validation. Goodness of fit was assessed by regression with an observed-predicted plot, and predictive performance evaluation was based on bias and precision. Mean bias was calculated using the following equation: (1/n) × ∑ {(predicted f[vanco] − observed f[vanco])/predicted f[vanco]}, where n is the number of predicted f[vanco]. Mean precision was calculated using the following equation: (1/n) × ∑ {|predicted f[vanco] − observed f[vanco]|)/predicted f[vanco]}, where n is the number of predicted f[vanco].
Statistical analysis.
To determine the extent that vancomycin was protein bound, descriptive statistics were utilized to compute the mean, median, and mode for total [vanco] and f[vanco]. The measure of central tendency that most closely approximated a normal distribution was used to compute the ratio of f[vanco] to total [vanco]. The quotient of f[vanco] and total [vanco] subtracted from 1 represented the extent that vancomycin was protein bound. To determine the clinical factors mediating vancomycin protein binding, linear regression was used to predict the f[vanco]. Prior to inclusion in the multivariable linear regression analysis, all variables were evaluated to ensure linearity, independence, existence, homoscedasticity, and Gaussian distribution. The classification and regression tree (CART) technique was used to identify significant breakpoints in continuous clinical features when f[vanco] was distinctly different between the resulting groups (34). Variables determined to be nonlinear in the univariate analysis were log transformed and reassessed. A clinical prediction tool was devised in a manner similar to that for a previously published prediction rule for methicillin-resistant Staphylococcus aureus (MRSA) (20). A backwards stepwise selection process was employed until the most parsimonious multivariable linear regression model was derived. Interaction and confounding were assessed in the multivariate linear regression analysis. All calculations were computed with SYSTAT for Windows (version 11.0) and SPSS version 12.0.1 (SPSS, Chicago, IL).
RESULTS
Vancomycin protein binding was evaluated in 50 patients. The mean age ± standard deviation (SD) was 52.9 ± 15.8 years (median, 54 years, and range, 18 to 89 years), and the majority (62%) of patients were female. The mean ± SD hospital length of stay (LOS) prior to sample collection was 6.3 ± 8.4 days (median, 3 days, and range, 1 to 38 days). Over half had received vancomycin for a skin and soft tissue infection (n = 24) or a lower respiratory tract infection (n = 7). Ten patients (20%) had received vancomycin for a documented MRSA infection, and 9 (18%) were in the ICU at the time of sample collection. Of the 50 patients, 27 had 1 or more comorbidities. The most common comorbid condition was diabetes (n = 18), followed by end-stage renal disease (n = 9). No colinearity was noted between diabetes and end-stage renal disease. There was a wide distribution in estimated CLCR among our patients. The mean ± SD was 61.3 ± 52.6 ml/min, and the median (interquartile range [IQR]) was 55.5 (23.1 to 94.6) ml/min. Nine patients were on dialysis.
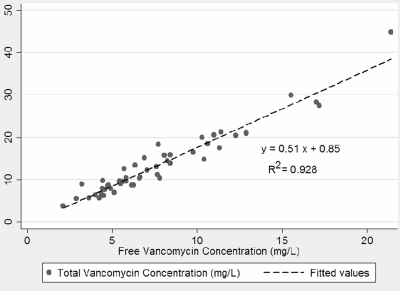
The mean total [vanco] ± SD was 13.6 ± 7.7 mg/liter (median, 10.9 mg/liter, and range, 3.8 to 44.8 mg/liter). The mean f[vanco] ± SD was 7.8 ± 4.0 mg/liter (median, 6.6 mg/liter, and range, 2.1 to 21.4 mg/liter). The mean ± SD percentage of bound vancomycin was 41.5% ± 8.6% (median, 41.0%, and range, 24.3 to 64.0%). A scatter plot comparing f[vanco] and total [vanco] is shown in Fig. 1. Linear regression of f[vanco] versus total [vanco] resulted in an excellent correlation, with a coefficient of determination (R2) of 0.93 (P < 0.05). Seventeen patients had ≥2 samples analyzed for total [vanco] and f[vanco]. A strong correlation between f[vanco] and total [vanco] was also demonstrated when multiple levels from patients were added to the analysis (R2 = 0.88).
Fig. 1.
Correlation between free vancomycin concentration and total vancomycin concentration (n = 50).
The relationship (β weights and standard errors) between baseline covariates and f[vanco] included in the bivariate linear regression analysis are shown in Table 1. Using CART analysis, significant breakpoints were identified for BUN, SCr, CLCR, total protein, total bilirubin, vancomycin dose, total [vanco], and APACHE II score. The variables found to be significantly associated with f[vanco] in the bivariate linear regression analysis were the APACHE II score, an APACHE II score of <13, a history of diabetes mellitus, dialysis, WBC, BUN, a BUN of ≥20 mg/dl, an SCr of ≥1.2 mg/dl, CLCR, a CLCR of <51.815 ml/min, total bilirubin, total bilirubin of ≥1.7 mg/dl, total protein of ≥6.7 g/dl, a vancomycin dose of ≥1,500 mg, total [vanco], and total [vanco] of ≥18.4 mg/liter. Effect modification was assessed, and interactions were observed between dialysis times total [vanco] and total protein of ≥6.7 g/dl times total [vanco].
Table 1.
Bivariate linear regression analysis of clinical characteristics predictive of free vancomycin concentration
| Variablea | βb | SE | P |
|---|---|---|---|
| Age (yr) | 0.041 | 0.036 | 0.267 |
| Male (n = 19) | −2.262 | 1.146 | 0.054 |
| Wt (kg) | −0.006 | 0.019 | 0.770 |
| Length of stay prior to sample collection | 0.026 | 0.069 | 0.709 |
| ICU at sample collection | −1.577 | 1.488 | 0.295 |
| APACHE II score | 0.237 | 0.083 | 0.006 |
| APACHE II < 13 (n = 28) | −3.125 | 1.074 | 0.005 |
| History of | |||
| Diabetes mellitus (n = 18) | 2.624 | 1.144 | 0.026 |
| Heart failure (n = 3) | 0.468 | 2.434 | 0.848 |
| Dialysis (n = 9) | 3.334 | 1.426 | 0.024 |
| Epilepsy (n = 2) | −3.611 | 2.905 | 0.220 |
| Hepatic dysfunction (n = 3) | 1.056 | 2.431 | 0.666 |
| White blood cell count (n = 46) | 0.171 | 0.072 | 0.022 |
| Albumin (n = 47) | −0.986 | 0.885 | 0.271 |
| Blood urea nitrogen (n = 49) | 0.067 | 0.027 | 0.017 |
| Blood urea nitrogen ≥ 20 mg/dl (n = 17) | 4.346 | 1.065 | <0.001 |
| SCr | 0.322 | 0.162 | 0.053 |
| SCr ≥ 1.2 mg/dl (n = 25) | 3.156 | 1.063 | 0.005 |
| CLCR, estimated | −0.030 | 0.010 | 0.006 |
| CLCR < 51.815 (n = 25) | 3.476 | 1.042 | 0.002 |
| Total protein (n = 47) | −1.122 | 0.580 | 0.059 |
| Total protein ≥ 6.7 g/dl (n = 16) | −2.957 | 1.216 | 0.019 |
| Total bilirubin (n = 47) | 0.578 | 0.179 | 0.002 |
| Total bilirubin ≥ 1.7 mg/dl (n = 5) | 4.706 | 1.860 | 0.015 |
| Vancomycin dose | 0.002 | 0.001 | 0.111 |
| Vancomycin dose ≥ 1500 mg (n = 8) | 3.360 | 1.506 | 0.031 |
| DOT prior to sample collection (n = 49) | 0.117 | 0.218 | 0.595 |
| Time postdose (n = 47) | 0.214 | 0.694 | 0.759 |
| Total [vanco] | 0.510 | 0.020 | <0.001 |
| Total [vanco] ≥ 18.4 (n = 11) | 7.785 | 0.829 | <0.001 |
| % Protein binding | 0.027 | 0.068 | 0.694 |
n = 50 except where otherwise noted. DOT, duration of therapy.
β, β coefficient.
The results of the multivariate linear regression are shown in Table 2. The R2 of the final model was 0.959. Total [vanco] was the strongest predictor of f[vanco] (β = 0.560; standard error [SE] = 0.021; P ≪ 0.001). The positive β coefficient indicated that f[vanco] increased as a function of total [vanco]. Other variables found to be predictive of f[vanco] included dialysis times total [vanco] (β = −0.067; SE = 0.018; P = 0.001) and total protein of ≥6.7 g/dl times total [vanco] (β = −0.071; SE = 0.021; P = 0.002), where dialysis and total protein of ≥6.7 g/dl were indicator variables coded as 0 for no dialysis or total protein of <6.7 g/dl and 1 for dialysis or total protein of ≥6.7 g/dl. The negative β coefficient for both dialysis and total protein indicated that protein binding was more pronounced in these patients. Overall, percent protein binding varies among four different patient types: nondialysis and total protein of <6.7 g/dl, nondialysis and total protein of ≥6.7 g/dl, dialysis and total protein of <6.7 g/dl, and dialysis and total protein of ≥6.7 g/dl.
Table 2.
Prediction model estimating free vancomycin concentration by multivariate linear regression
| Variable | βa | SE | P |
|---|---|---|---|
| Total [vanco] | 0.560 | 0.021 | <0.001 |
| Dialysis × total [vanco] | −0.067 | 0.018 | 0.001 |
| Total protein ≥ 6.7 g/dl × total [vanco] | −0.071 | 0.021 | 0.002 |
| Coefficient | 0.643 |
β, β coefficient.
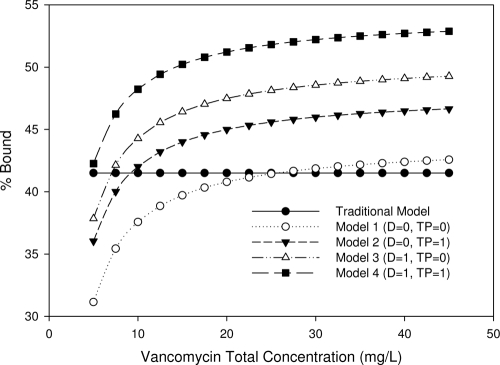
A graphic representation of the multivariate linear regression analysis is displayed in Fig. 2. The algebraic expression from the final model is as follows: f[vanco] = 0.643 + 0.560 × total[vanco] − (0.067 × total[vanco] × D) − (0.071 × total [vanco] × TP), where D is 1 if dialysis dependent or 0 if not dialysis dependent and TP is 1 if total protein is ≥6.7 g/dl or 0 if total protein is <6.7 g/dl. Separate protein binding curves were generated from our final prediction model for the four different patient types. A protein binding curve was also created using the mean protein binding estimate of 41.5%, and we labeled this the traditional model/method. The variability observed with percent protein binding as a function of total [vanco] can be illustrated using an example of patients on dialysis with a total protein of ≥6.7 g/dl and a total [vanco] of 18 mg/liter. Our final model predicted f[vanco] to be 8.2 mg/liter. In contrast, using the traditional method, f[vanco] was estimated to be 10.5 mg/liter.
Fig. 2.
Predicted percent vancomycin protein bound by model and patient type. D, dialysis dependent; TP, total protein of ≥6.7 g/dl; 0, variable not present; 1, variable present. For the traditional model, f[vanco] was equal to 0.585 × total [vanco]. The final prediction model by patient type was as follows: model 1 (not on dialysis and total protein of <6.7 g/dl), f[vanco] = 0.643 + 0.560 × total [vanco]; model 2 (not on dialysis and total protein of ≥6.7 g/dl), f[vanco] = 0.643 + 0.560 × total [vanco] − 0.071 × total [vanco]; model 3 (dialysis dependent and total protein of <6.7 g/dl), f[vanco] = 0.643 + 0.560 × total [vanco] − 0.067 × total [vanco]; model 4 (dialysis dependent and total protein of ≥6.7 g/dl), f[vanco] = 0.643 + 0.560 × total [vanco] − 0.067 × total [vanco] − 0.071 × total [vanco].
Validation of our prediction model was evaluated in 13 patients. The mean total [vanco] ± SD was 13.8 ± 7.7 mg/liter (median, 13.1 mg/liter, and range, 4.1 to 29.4 mg/liter). The mean f[vanco] ± SD was 6.4 ± 2.9 mg/liter (median, 6.7 mg/liter, and range, 2.2 to 13.2 mg/liter). The mean ± SD percentage of bound vancomycin was 51.0% ± 7.1% (median, 49.5%, and range, 35.9% to 67.0%). There were one patient on chronic dialysis and three patients with a total protein of ≥6.7 g/dl. The observed f[vanco] versus predicted plots for the patients included in the validation model are displayed in Fig. 3. Overall, the observed versus predicted plot was highly acceptable. The R2 was 0.909, the mean bias was 0.18, and the mean precision was 0.18.
Fig. 3.
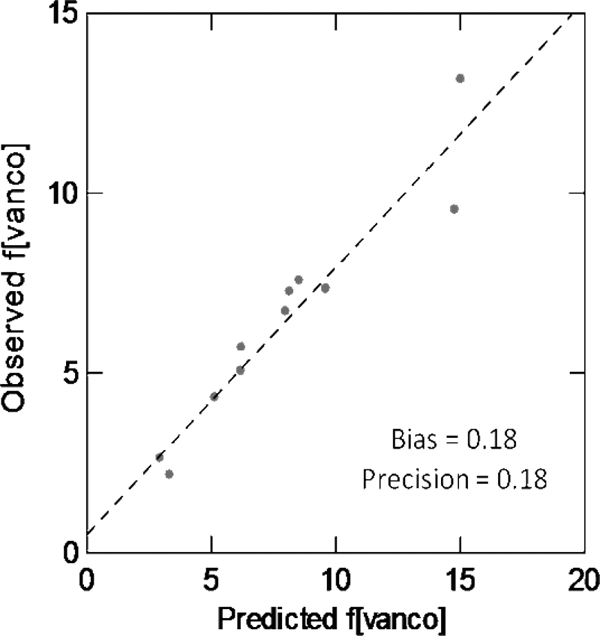
Correlation between observed free vancomycin concentration and predicted free vancomycin concentration (n = 13).
DISCUSSION
Our study refines the current understanding of the factors that modify the protein binding of vancomycin and improves the clinician's ability to predict the free concentration in a given individual. Even though Craig and Andes clearly demonstrated the importance of free vancomycin concentrations when modeling bacterial response (10), vancomycin pharmacodynamic evaluations have been carried out almost exclusively using total concentrations (22, 27). To exclusively examine the total concentration in exposure-response relationships, there ideally has to be a fixed unbound fraction of vancomycin that is constant across patients. Given that free vancomycin concentrations can be highly variable (12, 18, 30, 31), it is vital to have an understanding of the free or unbound concentration in a given individual when making quantitative exposure-response assessments. While it would be optimal to determine free concentrations, prediction models, such as the one derived in this paper, can be employed as a practical alternative to measuring f[vanco].
Overall, we found percent protein binding to be rather heterogeneous, with an average percent protein binding of 41.5% and a range from 24.3 to 64.0%. Of note, we found a largely linear relationship between total [vanco] and f[vanco]. In particular, the free concentration varied as a function of total [vanco] and was modified by receipt of dialysis and total protein of ≥6.7 g/dl in the multivariate linear regression analysis. Linear regression was selected to model the data because it provided the best linear combination of predictors that maximized the likelihood of obtaining the observed f[vanco]. The major advantage of linear regression, however, is the utility of the final model. The final model is a parsimonious mathematical equation that can be used to predict f[vanco] on the basis of the combination of clinical characteristics present in a given individual being treated with vancomycin. Understanding the factors that predict f[vanco] enhances the ability of clinicians to make more informed treatment decisions and potentially to maximize clinical outcomes by increasing the likelihood of achieving an optimal vancomycin concentration-time profile, particularly when an f[vanco] target is identified.
To test the clinical relevance of our model, we estimated f[vanco] with our prediction model and a traditional model using a mean value, where protein binding was estimated to be 41.5%. As shown in Fig. 2, percent protein binding can vary considerably as a function of total [vanco]. The data points representing f[vanco] shift if the interacting terms, dialysis and/or total protein of ≥6.7 g/dl, are present. Using the traditional method (with an assumption of 41.5% protein binding in all patients), predicted f[vanco] could diverge by up to 22% from our prediction model's estimate. The clinical relevance of this tool is most clearly illustrated by considering those patients with higher total protein or on dialysis, because the free fraction of vancomycin is reduced among this population. Thus, these patients need a higher dose to achieve the same free vancomycin exposure as nondialysis patients or those with lower total protein. Conversely, patients with lower total protein or who are not on dialysis may benefit from a lower dose to minimize their risk of toxicity due to increased exposure to free drug.
Some of our findings diverge from past attempts to quantify this agent's protein binding profile. In contrast to findings presented by Zokufa et al. (35), we found that total protein was more predictive of f[vanco] than albumin. While the patients in our study had similar albumin concentrations, our sample size was considerably larger (50 versus 12 patients) and more heterogeneous. Most importantly, they restricted their analysis to burn patients. Given that total protein measurements are included as a part of standard chemistry panels, evaluation of this parameter should not be any more cumbersome than determining albumin values.
Previous analyses have found that dialysis patients usually have lower protein binding and a higher free fraction (31). The mechanism leading to lower protein binding in this population has been theorized to be reduced albumin binding affinity and competition with endogenous substrates, such as uremic toxins that accumulate due to reduced renal clearance (13). Our findings were in contrast to these studies. Various proteins have been implicated in affecting protein binding, and it is possible that proteins unaffected by renal function played a role in the free fraction of vancomycin in our study population. It is also possible that dialysis itself may have an impact on the free concentration by removal of uremic toxins that compete for protein binding sites. To shed light on these considerations, an additional stratified analysis was performed to evaluate whether dialysis was a modifying factor of free concentration as a result of higher total concentrations. Upon stratification, it was clear that relationships involving protein binding did not differ among those patients with total concentrations of 10 to 15, 15 to 20, and >20 mg/liter (data not shown). In addition, the observed relationship between dialysis and protein binding was not a function of dialysis patients having higher total protein levels (≥6.7 g/dl). When stratified by dialysis, there was no difference in the number of patients who had total protein of ≥6.7 g/dl (33.3% in dialysis patients versus 37.5% in nondialysis patients).
Finally, our results are not congruent with a recent paper published by Berthoin and colleagues (4), which demonstrated a weaker correlation between free and total concentrations. The coefficient of determination of free[vanco] versus total [vanco] was 0.55 compared to the 0.93 observed in our study. Key differences between the study design of the previous investigation and ours include (i) smaller sample size (n = 16 versus 50), (ii) use of multiple measurements from the same subjects compared to single measurements per subject, and (iii) freezing and thawing of serum samples prior to batch assay as opposed to real-time measurements in our study. We did not include repeated samples in our linear regression model because the use of repeated measures from a subject requires inclusion of an intrasubject correlation term. Given the robustness of the single sample per subject approach in our study, further refinement of the model through inclusion of an intrasubject correlation term was deemed unnecessary.
Since the process of freeze-thaw has the potential to alter protein binding, we conducted a post hoc analysis to describe the extent of vancomycin protein binding after one freeze-thaw cycle. Serum samples were obtained from two healthy volunteers. Vancomycin was obtained from Sigma-Aldrich, Inc. (St. Louis, MO) and was reconstituted with sterile water to make a stock solution of 2,000 μg/ml. Doubling concentrations of vancomycin were then prepared and added to a sample of each subject's serum for final concentrations ranging from 2.5 to 80 mg/liter. The mean percent binding was 54.0% (±18.4%) before the samples were frozen and 40.2% (±16.4%) after being thawed and retested approximately 30 days later (P = 0.008). This demonstrates that the freeze-thaw process alters the estimate of the f[vanco].
Limitations to our study also exist and should be noted. First, previous studies had determined that vancomycin is predominantly bound not only by albumin, but also by immunoglobulin A (IgA) (6, 30, 35). We were unable to specifically assay IgA given that our study was noninterventional, but none of our patients had evidence of myeloma or other disorders involving IgA. Second, since this study was restricted to adult patients, our results are not generalizable to pediatric patients. In addition, there were no infective endocarditis cases and a limited number of patients with bacteremia (n = 13) or osteomyelitis (n = 4). This may further limit the generalizability of our model to these patient populations. However, it is unlikely that these infection types would result in altered vancomycin protein binding. Furthermore, the highly favorable predictive performance of the prospective model validation suggests that this model is suitable for use in practice.
In conclusion, our model refines our understanding of factors that modify vancomycin protein binding. Given the variability surrounding protein binding of vancomycin, it is not advisable to assume a fixed free fraction of vancomycin. More importantly, our findings highlight the importance of considering free vancomycin concentrations in future exposure-effect studies. In particular, future pharmacodynamic evaluations should assess whether free or total drug concentrations are more predictive of response. Determining the pharmacodynamic target of interest as it relates to free versus total concentrations will be essential for proper therapeutic drug monitoring. If free levels are found to be better correlated with response, free levels should be obtained in practice, and this could potentially represent a paradigm shift in vancomycin monitoring. While it would be optimal to determine free concentrations, our prediction model can be employed as a practical alternative to measuring f[vanco], since most institutions do not routinely monitor free levels. Our prediction tool is simple and can easily be applied in the clinical setting. Similar to other tools developed, external validity should be determined with our model in other settings before the prediction tool is routinely applied in clinical practice.
ACKNOWLEDGMENT
The manuscript greatly benefited from the thoughtful editing of Allison Krug.
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Ackerman B. H., Taylor E. H., Olsen K. M., Abdel-Malak W., Pappas A. A. 1988. Vancomycin serum protein binding determination by ultrafiltration. Drug Intell. Clin. Pharm. 22:300–303 [DOI] [PubMed] [Google Scholar]
- 2. Albrecht L. M., Rybak M. J., Warbasse L. H., Edwards D. J. 1991. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP 25:713–715 [DOI] [PubMed] [Google Scholar]
- 3. Bailey E. M., Rybak M. J., Kaatz G. W. 1991. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob. Agents Chemother. 35:1089–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berthoin K., Ampe E., Tulkens P. M., Carryn S. 2009. Correlation between free and total vancomycin serum concentrations in patients treated for Gram-positive infections. Int. J. Antimicrob. Agents 34:555–560 [DOI] [PubMed] [Google Scholar]
- 5. Bilello J. A., et al. 1996. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:1491–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cantú T. G., Dick J. D., Elliott D. E., Humphrey R. L., Kornhauser D. M. 1990. Protein binding of vancomycin in a patient with immunoglobulin A myeloma. Antimicrob. Agents Chemother. 34:1459–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cockcroft D. W., Gault M. H. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 8. Craig W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501 [DOI] [PubMed] [Google Scholar]
- 9. Craig W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 10.Craig W. A., Andes D. R.Abstr. 46th Annu. Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-644.2006. [Google Scholar]
- 11. Craig W. A., Kunin C. M. 1976. Significance of serum protein and tissue binding of antimicrobial agents. Annu. Rev. Med. 27:287–300 [DOI] [PubMed] [Google Scholar]
- 12. Cutler N. R., Narang P. K., Lesko L. J., Ninos M., Power M. 1984. Vancomycin disposition: the importance of age. Clin. Pharmacol. Ther. 36:803–810 [DOI] [PubMed] [Google Scholar]
- 13. Dreisbach A. W., Lertora J. J. 2008. The effect of chronic renal failure on drug metabolism and transport. Expert Opin. Drug Metab. Toxicol. 4:1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drusano G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289–300 [DOI] [PubMed] [Google Scholar]
- 15. Dykhuizen R. S., Harvey G., Stephenson N., Nathwani D., Gould I. M. 1995. Protein binding and serum bactericidal activities of vancomycin and teicoplanin. Antimicrob. Agents Chemother. 39:1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henricks J. N., Schumacher G. E. 1980. Using pharmacokinetics in drug therapy. VIII. Pharmacokinetic evaluation of antibiotic dosage regimens. Am. J. Hosp. Pharm. 37:1356–1366 [PubMed] [Google Scholar]
- 17. Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818–829 [PubMed] [Google Scholar]
- 18. Krogstad D. J., Moellering R. C., Jr., Greenblatt D. J. 1980. Single-dose kinetics of intravenous vancomycin. J. Clin. Pharmacol. 20:197–201 [DOI] [PubMed] [Google Scholar]
- 19. Lam Y. W., Duroux M. H., Gambertoglio J. G., Barriere S. L., Guglielmo B. J. 1988. Effect of protein binding on serum bactericidal activities of ceftazidime and cefoperazone in healthy volunteers. Antimicrob. Agents Chemother. 32:298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lodise T. P., Jr., McKinnon P. S., Rybak M. 2003. Prediction model to identify patients with Staphylococcus aureus bacteremia at risk for methicillin resistance. Infect. Control Hosp. Epidemiol. 24:655–661 [DOI] [PubMed] [Google Scholar]
- 21. Merrikin D. J., Briant J., Rolinson G. N. 1983. Effect of protein binding on antibiotic activity in vivo. J. Antimicrob. Chemother. 11:233–238 [DOI] [PubMed] [Google Scholar]
- 22. Moise-Broder P. A., Forrest A., Birmingham M. C., Schentag J. J. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925–942 [DOI] [PubMed] [Google Scholar]
- 23. Rodvold K. A., et al. 1988. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob. Agents Chemother. 32:848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rolinson G. N., Sutherland R. 1965. The binding of antibiotics to serum proteins. Br. J. Pharmacol. Chemother. 25:638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rybak M., et al. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82–98 [DOI] [PubMed] [Google Scholar]
- 26. Rybak M. J. 2006. Pharmacodynamics: relation to antimicrobial resistance. Am. J. Med. 119:S37–S44; discussion, S62–S70 [DOI] [PubMed] [Google Scholar]
- 27. Rybak M. J. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl. 1):S35–S39 [DOI] [PubMed] [Google Scholar]
- 28. Rybak M. J., Albrecht L. M., Berman J. R., Warbasse L. H., Svensson C. K. 1990. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob. Agents Chemother. 34:792–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sophianopoulos J. A., Durham S. J., Sophianopoulos A. J., Ragsdale H. L., Cropper W. P., Jr 1978. Ultrafiltration is theoretically equivalent to equilibrium dialysis but much simpler to carry out. Arch. Biochem. Biophys. 187:132–137 [DOI] [PubMed] [Google Scholar]
- 30. Sun H., Maderazo E. G., Krusell A. R. 1993. Serum protein-binding characteristics of vancomycin. Antimicrob. Agents Chemother. 37:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan C. C., Lee H. S., Ti T. Y., Lee E. J. 1990. Pharmacokinetics of intravenous vancomycin in patients with end-stage renal failure. Ther. Drug Monit. 12:29–34 [DOI] [PubMed] [Google Scholar]
- 32. Thom K. A., et al. 2008. Controlling for severity of illness in outcome studies involving infectious diseases: impact of measurement at different time points. Infect. Control Hosp. Epidemiol. 29:1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tompsett R., Shultz S., McDermott W. 1947. The relation of protein binding to the pharmacology and antibacterial activity of penicillins X, G, dihydro F, and K. J. Bacteriol. 53:581–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H., Singer B. 1999. Recursive partitioning in the health sciences. Springer, New York, NY [Google Scholar]
- 35. Zokufa H. Z., et al. 1989. The influence of serum albumin and alpha 1-acid glycoprotein on vancomycin protein binding in patients with burn injuries. J. Burn Care Rehabil. 10:425–428 [DOI] [PubMed] [Google Scholar]