Abstract
Twenty Klebsiella pneumoniae isolates producing OXA-48 were collected from April 2009 to September 2010. Strains were clonally related and coproduced a CTX-M-15 β-lactamase. A conjugative plasmid of circa 70 kb carrying blaOXA-48 was identified. Eleven isolates showed low-level resistance to carbapenems, whereas nine showed high-level resistance. Decreased expression of OmpK36 was related to high-level resistance to carbapenems. The isolates belonged to sequence type 101 (ST101). This is the first outbreak caused by an OXA-48-producing K. pneumoniae strain in Spain.
TEXT
Carbapenems currently represent the drugs of choice for treatment of serious infections caused by multidrug-resistant strains of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs). Recently, however, the emergence of carbapenem resistance has been increasingly reported among Enterobacteriaceae and is a matter of major clinical concern. The most common mechanism for carbapenem resistance in Klebsiella pneumoniae is the production of carbapenemases belonging to Ambler class A, B, or D (21). Acquired class D carbapenemases have previously been reported mainly in Acinetobacter spp. and occasionally in Enterobacteriaceae. The oxacillinase OXA-48 was first identified from a K. pneumoniae isolate in Istanbul, Turkey (24), and spread of OXA-48-producing Enterobacteriaceae throughout the Mediterranean area has been observed (3, 12, 16, 19). OXA-48-producing Enterobacteriaceae have also been reported in Senegal (22), Belgium (11), Argentina (8), and India (2), and several outbreaks have been described in Turkey (6), the United Kingdom (27), and more recently in France (13).
The blaOXA-48 gene is part of the Tn1999 composite transposon made of two copies of the insertion sequence IS1999 (1) and is located in a conjugative plasmid of circa 70 kb (7). Modification of outer membrane proteins (OMPs) has also been shown to play an additional role in increasing carbapenem resistance in K. pneumoniae producing KPC carbapenemase (18), as well as in strains bearing plasmid-mediated AmpC cephalosporinases and ESBLs showing resistance to ertapenem (5, 20).
In this study we describe the first detection as well as the first outbreak of a K. pneumoniae strain producing OXA-48 and CTX-M-15 in Spain. The role of the expression of outer membrane proteins in the increased carbapenem resistance phenotype of these isolates is also analyzed.
On 7 April 2009, a male (patient 2) was transferred to the neurosurgical intensive care unit (SICU) at the Hospital Clínic of Barcelona, Spain, from the ICU of a hospital in Marrakesh, Morocco, where he had been admitted after head trauma and had stayed for 24 days with several episodes of fever and pneumonia. Two days later the first K. pneumoniae isolate producing OXA-48 carbapenemase was found from a patient in the same SICU (patient 1), whereas an OXA-48-producing K. pneumoniae isolate from patient 2 (presumably the index case) was not detected until 14 April 2009. Active surveillance rectal cultures were collected from all patients from the SICU. Screening was performed using the chromogenic medium chromID ESBL (bioMérieux) and the Hodge test to confirm carbapenemase production. During the subsequent period of time (April 2009 to September 2010), 18 more OXA-48-producing K. pneumoniae isolates were recovered. Rectal colonization was found in each of all tested patients who were infected, and one additional patient was also colonized (patient 6). The implementation of barrier precautions as well as promotion of hand hygiene and reinforcement of room cleaning measures led to the successful control of the outbreak. Table 1 summarizes the characteristics of the patients and the origin of the isolates.
Table 1.
Characteristics of 20 patients carrying K. pneumoniae isolates producing OXA-48 and CTX-M-15
| Patient no. | Hospital unit | Isolate no. | Date of isolation | Site of isolation | Underlying disease | Final antibiotic therapya | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | SICU | 5837 | 9 April 2009 | Bronchoaspirate | Brain trauma | AMK + MEM | Cure |
| 2 | SICU | 5836 | 14 April 2009 | Catheter blood culture | Head trauma | TGC + TMP-SXT + AMK | Cure |
| 3 | SICU | 5834 | 16 April 2009 | Urine | Subdural hemorrhage | TGC + AMK | Cure |
| 4 | SICU | 5839 | 16 April 2009 | Catheter blood culture | Subarachnoidal hemorrhage | AMK + CST | Cure |
| 5 | SICU | 5835 | 20 April 2009 | Urine | Subarachnoidal hemorrhage | TGC + AMK | Cure |
| 6 | SICU | 6082 | 3 June 2009 | Rectal swab | None | ||
| 7 | SICU | 6083 | 5 June 2009 | Urine | Vertebral fracture | TGC + AMK | Cure |
| 8 | SICU | 6168 | 25 June 2009 | Catheter blood culture | Subarachnoidal hemorrhage | None | Exitus |
| 9 | SICU | 6167 | 25 June 2009 | Bronchoaspirate | Subarachnoidal hemorrhage | TGC + AMK | Exitus |
| 10 | SICU | 6440 | 20 August 2009 | Urine | Subdural hematoma | TGC + FOF | Cure |
| 11 | SICU | 7310 | 16 February 2010 | Catheter | Severe pneumonia | None | Exitus |
| 12 | SICU | 7591 | 7 April 2010 | Urine | Subarachnoidal hemorrhage | TGC + FOF + AMK | Cure |
| 13 | Hepatology ward | 7619 | 19 April 2010 | Urine | Liver transplant | TGC + FOF | Cure |
| 14 | SICU | 7680 | 5 May 2010 | Catheter blood culture | Septic shock | TGC | Cure |
| 15 | Hepatology ward | 7745 | 18 May 2010 | Urine | Liver transplant | TGC + FOF | Cure |
| 16 | SICU | 7911 | 25 June 2010 | Blood culture | Nosocomial pneumonia | TGC + AMK + CST | Exitus |
| 17 | Hepatology ward | 7951 | 5 May 2010 | Catheter blood culture | Liver transplant | TGC + FOF | Cure |
| 18 | SICU | 8037 | 24 July 2010 | Urine | Liver cancer | TGC + FOF | Cure |
| 19 | Hepatology ward | 8064 | 26 July 2010 | Catheter blood culture | Liver transplant | TGC + FOF | Cure |
| 20 | Hepatology ward | 8268 | 20 September 2010 | Urine | Liver cirrhosis | TGC + FOF | Cure |
TMP-SXT, trimethoprim-sulfamethoxazole; AMK, amikacin; TGC, tigecycline; CST, colistin; MEM, meropenem; FOF, fosfomicin.
Antimicrobial susceptibility testing was performed by using the Phoenix system (Becton Dickinson, Franklin Lakes, NJ), and MICs of β-lactam antibiotics and tigecycline were also evaluated by Etest (AB bioMérieux, Solna, Sweden). Eleven strains collected from April 2009 to February 2010 were resistant to all the antibiotics tested, except cefoxitin, amikacin, fosfomycin, tigecycline, and colistin, and showed low-level resistance to carbapenems, with three being susceptible to imipenem and meropenem according to CLSI breakpoints updated in June 2010 (9). The remaining nine strains, collected from April 2010 to September 2010, showed the same resistance pattern except that they were resistant to cefoxitin and showed high-level resistance to carbapenems (Table 2).
Table 2.
In vitro susceptibilities of the low- and high-level carbapenem-resistant K. pneumoniae 5837 and K. pneumoniae 7680 isolates, respectively, and the E. coli transconjugant expressing OXA-48 carbapenemase
| β-Lactam(s) | MIC (μg/ml) |
|||
|---|---|---|---|---|
| K. pneumoniae 5837 (OXA-48, CTX-M-15, OmpK36+) | K. pneumoniae 7680 (OXA-48, CTX-M-15, OmpK36−) | E. coli J53 7680T (OXA-48) | E. coli J53 | |
| Amoxicillin | >256 | >256 | >256 | 4 |
| Amoxicillin + clavulanatea | >256 | >256 | 256 | 4 |
| Piperacillin + tazobactamb | >256 | >256 | 64 | 1 |
| Cefoxitin | 6 | 96 | 2 | 2 |
| Cefotaxime | >256 | >256 | 0.75 | 0.094 |
| Ceftazidime | 96 | >256 | 0.125 | 0.125 |
| Cefepime | 128 | >256 | 0.38 | 0.25 |
| Imipenem | 1 (0.75–4)c | >32 (12 to >32)d | 0.5 | 0.19 |
| Meropenem | 2 (0.75–4)c | >32 (12 to >32)d | 0.125 | 0.023 |
| Doripenem | 1.5 (1–2)c | >32 (8 to >32)d | 0.125 | 0.032 |
| Ertapenem | 24 (3 to >32)c | >32 (>32)d | 0.5 | 0.008 |
| Aztreonam | >256 | >256 | 0.064 | 0.064 |
Clavulanate was used at a fixed concentration of 2 μg/ml.
Tazobactam was used at a fixed concentration of 4 μg/ml.
Carbapenem MIC range for isolates collected from April 2009 to February 2010 (low-level resistance to carbapenems).
Carbapenem MIC range for isolates collected from April 2010 to September 2010 (high-level resistance to carbapenems).
PCR and sequence analysis for carbapenemases, ESBLs, and plasmid-mediated AmpC cephalosporinase-encoding genes were performed (4, 23–25). All isolates were positive for blaOXA-48 (carbapenemase), blaCTX-M-15 (ESBL), and blaSHV-1, the latter being the chromosomally encoded β-lactamase usually found in this microorganism.
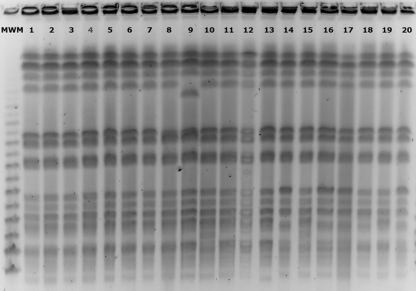
The analysis of the isolates by pulsed-field gel electrophoresis (PFGE) profiles of XbaI-digested genomic DNA (New England BioLabs, Beverly, MA) revealed that all isolates were clonally related (Fig. 1).
Fig. 1.
XbaI PFGE patterns of OXA-48-producing K. pneumoniae isolates. Lane 1, isolate 5837; lane 2, isolate 5836; lane 3, isolate 5834; lane 4, isolate 5839; lane 5, isolate 5835; lane 6, isolate 6083; lane 7, isolate 6168; lane 8, isolate 6167; lane 9, isolate 6440; lane 10, isolate 7310; lane 11, isolate 6082; lane 12, isolate 7591; lane 13, isolate 7619; lane 14, isolate 7680; lane 15, isolate 7745; lane 16, isolate 7951; lane17, isolate 8037; lane 18, isolate 8064, lane 19; isolate 8268; lane 20, isolate 7911. MWM, molecular weight marker.
Transferability of the blaOXA-48 gene was studied by conjugation experiments between the K. pneumoniae 7680 isolate and the recipient strain, Escherichia coli J53 AziR, in broth medium. Transconjugants were selected on MacConkey agar plates supplemented with 1 μg/ml of imipenem and 100 μg/ml of sodium azide (Sigma Chemical Co., St. Louis, MO) and were screened for blaOXA-48 and blaCTX-M-15. PCR analysis was only positive for the blaOXA-48 gene. E. coli J53 7680T producing OXA-48 was susceptible to all the β-lactam antibiotics except ampicillin, amoxicillin-clavulanate, and piperacillin-tazobactam and showed higher MICs of carbapenems than the original recipient strain (Table 2).
Extraction of plasmid DNA from K. pneumoniae strain 7680 and its transconjugant, E. coli J53 7680T, was performed by the method of Kado and Liu (17) and showed the presence of a plasmid of circa 70 kb in both strains (data not shown). Plasmids of similar sizes have already been described in previous OXA-48 carbapenemase producers (7).
The genetic environment of the blaOXA-48 gene was determined by PCR using primers matching the insertion sequence IS1999 as well as primers matching either the upstream or the downstream region of the blaOXA-48 gene to confirm the presence of the Tn1999 transposon (1). The presence of an IS1R element truncating the IS1999 insertion sequence upstream from blaOXA-48 allowed identification of a Tn1999.2-type transposon as previously described for Turkish isolates (6).
Taking cefoxitin resistance and the level of resistance to carbapenems into account, two resistance patterns were defined, as previously mentioned. In order to investigate the differences between the two groups, the outer membrane proteins of one representative isolate from each group (K. pneumoniae 5837 and K. pneumoniae 7680, respectively) (Table 1) were extracted and analyzed by SDS-PAGE together with protein extracts from known control strains (15) (Fig. 2). SDS-PAGE analysis indicated that the high-level carbapenem- and cefoxitin-resistant isolate presented decreased expression of the protein band corresponding to OmpK36 in the control strains. Matrix-assisted laser desorption ionization–time of flight–time of flight mass spectrometry of the gel-purified band correctly identified this protein as OmpK36, thereby confirming that cefoxitin-susceptible isolates produced OmpK36, whereas cefoxitin-resistant isolates showed decreased expression of this protein (Fig. 2). The sequences of the ompK36 gene and its promoter were also analyzed in both isolates, and no differences were found.
Fig. 2.
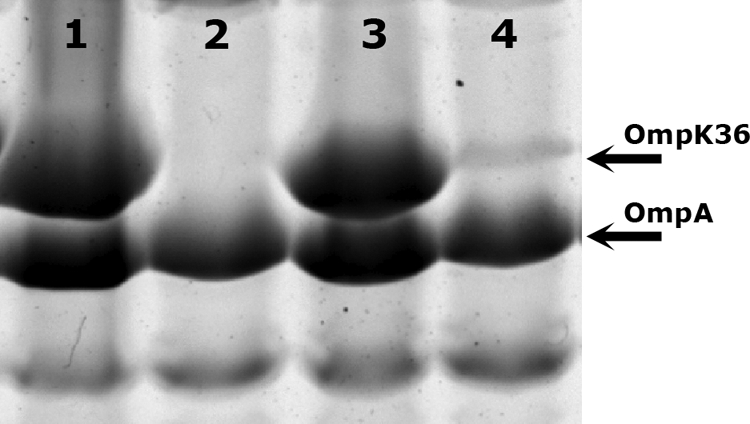
SDS-PAGE of OMPs from K. pneumoniae isolates. Lane 1, CSUB 10S control strain expressing OmpK36; lane 2, CSUB 10R control strain not expressing OmpK36; lane 3, K. pneumoniae 5837; lane 4, K. pneumoniae 7680. Only the relevant parts of the gel are shown.
Until the isolation of the present K. pneumoniae isolate harboring OXA-48 and CTX-M-15, carbapenem resistance in K. pneumoniae associated with carbapenemases in Spain was exclusively attributed to VIM-1 and KPC-3 (10, 26). This is the first Enterobacteriaceae strain identified in Spain that carries a carbapenem-hydrolyzing oxacillinase. Multilocus sequence typing was performed as previously described (14) and revealed that the K. pneumoniae isolate belonged to sequence type 101 (ST101), which has previously been found in an OXA-48-producing K. pneumoniae isolate from Tunisia (13).
In this report we describe the first outbreak in Spain involving 20 patients at our hospital that was caused by a single clone of K. pneumoniae carrying OXA-48 and CTX-M-15. The outbreak was divided into two periods of time in which the level of resistance to carbapenems increased, apparently due to a decrease in the expression of OmpK36. The facts that these strains contained a plasmid of similar size to that of previously identified OXA-48-producing K. pneumoniae isolates (6) and that all were clonally related and belonged to the same sequence type (ST101) as OXA-48-producing K. pneumoniae isolates from Tunisia (even though the presumable index case of this outbreak, patient 2, had returned from Morocco) suggest their spread among Mediterranean countries.
It is worth mentioning that three of the isolates were susceptible to imipenem and meropenem according to CLSI guidelines, further complicating the detection of OXA-48-producing Enterobacteriaceae isolates, which, in turn, may delay the administration of appropriate antimicrobial therapy as well as the enforcement of infection control measures.
Acknowledgments
This study was supported by grant 2009 SGR 1256 from the Departament de Universitats, Recerca i Societat de la Informació de la Generalitat d'Catalunya, and by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Spanish Network for the Research in Infectious Disease (REIPI 06/0008). This work was also supported by funding from the European Community (TROCAR contract HEALTH-F3-2008-223031).
We thank Sebastià Albertí for providing isolates of Klebsiella pneumoniae controls with known OMP profiles to perform OMP analysis.
Footnotes
Published ahead of print on 11 July 2011.
REFERENCES
- 1. Aubert D., Naas T., Heritier C., Poirel L., Nordmann P. 2006. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 188:6506–6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell J. M., Mathai D., Jones R. N., Turnidge J. D. 2010. Emergence of OXA-48 carbapenemases among Klebsiella spp. from India, abstr. C2-650. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA American Society for Microbiology, Washington, DC [Google Scholar]
- 3. Benouda A., Touzani O., Khairallah M. T., Araj G. F., Matar G. M. 2010. First detection of oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Morocco. Ann. Trop. Med. Parasitol. 104:327–330 [DOI] [PubMed] [Google Scholar]
- 4. Bradford P. A., et al. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55–60 [DOI] [PubMed] [Google Scholar]
- 5. Bradford P. A., et al. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrer A., et al. 2008. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 52:2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrer A., et al. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob. Agents Chemother. 54:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castanheira M. A., et al. 2010. Emergence of blaOXA-48 among Enterobacter cloacae in Argentina and its prevalence among carbapenem non-susceptible Enterobacteriaceae, C2-647. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA American Society for Microbiology, Washington, DC [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement, M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Curiao T., et al. 2010. Emergence of bla KPC-3-Tn4401a associated with a pKPN3/4-like plasmid within ST384 and ST388 Klebsiella pneumoniae clones in Spain. J. Antimicrob. Chemother. 65:1608–1614 [DOI] [PubMed] [Google Scholar]
- 11. Cuzon G., et al. 2008. Plasmid-encoded carbapenem-hydrolyzing β-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob. Agents Chemother. 52:3463–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuzon G., Naas T., Lesenne A., Benhamou M., Nordmann P. 2010. Plasmid-mediated carbapenem-hydrolysing OXA-48 β-lactamase in Klebsiella pneumoniae from Tunisia. Int. J. Antimicrob. Agents 36:91–93 [DOI] [PubMed] [Google Scholar]
- 13. Cuzon G., Ouanich J., Gondret R., Naas T., Nordmann P. 2011. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob. Agents Chemother. 55:2420–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diancourt L., Passet V., Verhoef J., Grimont P. A., Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doménech-Sánchez A., et al. 2003. Role of Klebsiella pneumoniae OmpK35 porin in antimicrobial resistance. Antimicrob. Agents Chemother. 47:3332–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goren M. G., Chmelnitsky I., Carmeli Y., Navon-Venezia S. 2011. Plasmid-encoded OXA-48 carbapenemase in Escherichia coli from Israel. J. Antimicrob. Chemother. 66:672–673 [DOI] [PubMed] [Google Scholar]
- 17. Kado C. I., Liu S. T. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitchel B., et al. 2010. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4201–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matar G. M., et al. 2008. Oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Lebanon. Clin. Microbiol. Infect. 14:887–888 [DOI] [PubMed] [Google Scholar]
- 20. Mena A., et al. 2006. Characterization of a large outbreak by CTX-M-1-producing Klebsiella pneumoniae and mechanisms leading to in vivo carbapenem resistance development. J. Clin. Microbiol. 44:2831–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miriagou V., et al. 2010. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin. Microbiol. Infect. 16:112–122 [DOI] [PubMed] [Google Scholar]
- 22. Moquet O., et al. 2011. Class D OXA-48 carbapenemase in multidrug-resistant enterobacteria, Senegal. Emerg. Infect. Dis. 17:143–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez-Pérez F. J., Hanson N. D. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poirel L., Heritier C., Tolun V., Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poirel L., et al. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tato M., et al. 2007. Complex clonal and plasmid epidemiology in the first outbreak of Enterobacteriaceae infection involving VIM-1 metallo-β-lactamase in Spain: toward endemicity? Clin. Infect. Dis. 45:1171–1178 [DOI] [PubMed] [Google Scholar]
- 27. Thomas C. P., et al. 2009. Hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the United Kingdom, abstr. C2-647. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA American Society for Microbiology, Washington, DC [Google Scholar]