Abstract
We investigated the cellular mechanisms responsible for the occurrence of miconazole-tolerant persisters in Candida albicans biofilms. Miconazole induced about 30% killing of sessile C. albicans cells at 75 μM. The fraction of miconazole-tolerant persisters, i.e., cells that can survive high doses of miconazole (0.6 to 2.4 mM), in these biofilms was 1 to 2%. Since miconazole induces reactive oxygen species (ROS) in sessile C. albicans cells, we focused on a role for superoxide dismutases (Sods) in persistence and found the expression of Sod-encoding genes in sessile C. albicans cells induced by miconazole compared to the expression levels in untreated sessile C. albicans cells. Moreover, addition of the superoxide dismutase inhibitor N,N′-diethyldithiocarbamate (DDC) to C. albicans biofilms resulted in an 18-fold reduction of the miconazole-tolerant persister fraction and in increased endogenous ROS levels in these cells. Treatment of biofilms of C. albicans clinical isolates with DDC resulted in an 18-fold to more than 200-fold reduction of their miconazole-tolerant persister fraction. To further confirm the important role for Sods in C. albicans biofilm persistence, we used a Δsod4 Δsod5 mutant lacking Sods 4 and 5. Biofilms of the Δsod4 Δsod5 mutant contained at least 3-fold less of the miconazole-tolerant persisters and had increased ROS levels compared to biofilms of the isogenic wild type (WT). In conclusion, the occurrence of miconazole-tolerant persisters in C. albicans biofilms is linked to the ROS-detoxifying activity of Sods. Moreover, Sod inhibitors can be used to potentiate the activity of miconazole against C. albicans biofilms.
INTRODUCTION
The increasing number of immunocompromised patients, combined with advances in medical technology, has led to an increase in fungal infectious diseases, with Candida albicans as the major fungal pathogen. Candida spp. are known to form biofilms on various surfaces, and these biofilms are responsible for medical device-associated infections. Such infections are difficult to treat, since C. albicans biofilms are resistant to most antifungal drugs (15). The basis of this drug resistance is not clear and involves different mechanisms, including the presence of a small number of persisters, which are cells that survive high doses of an antimicrobial agent. Persisters are not genetic mutants but rather are phenotypic variants of the wild type (WT). Unlike bacterial persisters, C. albicans persisters have so far been observed only in biofilms and not in planktonic populations (8). Recent data suggest that persisters may be the main culprit responsible for the recalcitrance of chronic infectious diseases against antimicrobial therapy (10). Identification of important cellular components that are responsible for the occurrence of persisters in fungal biofilms could open the way to the rational design of antibiofilm agents. For example, the combination of a conventional antibiotic with a compound inhibiting persister formation or survival may result in an effective therapy. However, till now, the molecular basis of persistence in C. albicans biofilms has not been unraveled (10).
Drug-tolerant persisters in C. albicans biofilms were previously reported to occur following treatment with high doses of amphotericin B (AmB) or chlorhexidine. In this respect, killing of C. albicans biofilms by these antifungals followed a biphasic pattern: while the majority of cells were killed by concentrations close to the MIC, a small fraction of biofilm cells survived treatment even with the highest concentration of AmB (100 μg/ml) or chlorhexidine (100 μg/ml) (8). In the present study, we aimed at unraveling the cellular mechanisms responsible for the occurrence of miconazole-tolerant persisters in C. albicans biofilms grown in the wells of microtiter plates. Miconazole is a fungicidal azole, and besides inhibiting ergosterol biosynthesis, it also induces reactive oxygen species (ROS) accumulation in planktonic and sessile C. albicans cells (3, 4, 19). The miconazole-induced ROS accumulation in C. albicans is probably caused by the inhibition of the enzymes implicated in the breakdown of peroxide radicals and hydrogen peroxide by miconazole (4). This is in contrast to the fungistatic agent fluconazole, which we previously reported to lack ROS-inducing capacity in yeast (4) and which shows no fungicidal activity against C. albicans biofilms (9, 19). In order to elucidate the molecular mechanism(s) responsible for the occurrence of miconazole-tolerant persisters in C. albicans biofilms, we focused on the role of superoxide dismutases (Sods) in this process. C. albicans contains 6 different Sods, which are involved in the detoxification of ROS by converting O2− into molecular oxygen and hydrogen peroxide. These Sods include cytoplasmic Sod1 and Sod3, mitochondrial Sod2, and the cell surface GPI-anchored Sod4, Sod5, and Sod6 (5, 12).
MATERIALS AND METHODS
Materials, yeast strains, plasmids, and growth media.
A Candida albicans homozygous double deletion mutant in SOD4 and SOD5 (Δsod4 Δsod5 mutant), the corresponding isogenic wild-type strain, CA-IF100 (5), and C. albicans clinical isolates (2CA, 10CA, and 15CA) that were isolated from the voice prosthesis of different laryngectomized patients were used in this study. The growth medium used was YPD (1% yeast extract, 2% peptone, and 2% glucose). N,N′-Diethyldithiocarbamate (DDC) (stock = 1 M in water), ascorbic acid (stock = 1 M in water), and miconazole or fluconazole (stock = 120 mM in dimethyl sulfoxide [DMSO]) were purchased from Sigma (St. Louis, MO). Phosphate-buffered saline (PBS) was prepared by combining 8 g/liter NaCl, 0.2 g/liter KCl, 1.44 g/liter Na2HPO4, and 0.24 g/liter KH2PO4 (pH 7.4). RPMI 1640 medium with l-glutamine, without bicarbonate, was purchased from Sigma.
Antifungal activity assay.
The activity of miconazole or fluconazole in PBS or RPMI 1640 medium (final DMSO concentration = 2%) in the absence or presence of ascorbic acid (20 mM) or DDC (15 or 20 mM) against 16-h-old C. albicans biofilms (the actual number of cells in the biofilm was approximately 2 × 106 cells) was assessed as described previously (18). DMSO (2%) in PBS was used as a control treatment. After incubation for 24 h, biofilms were washed and resuspended in PBS by vigorous vortexing. The fraction of persisters was determined by counting the colonies and calculating the number of CFU, as described previously (1).
ROS accumulation assay with biofilm and planktonic cells.
Accumulation of ROS was quantified using 2′,7′-dichlorodihydrofluorescein diacetate (DCFHDA) staining. To this end, 16-h-old C. albicans biofilms were incubated for 24 h with miconazole in PBS in the absence or presence of 20 mM ascorbic acid or 15 or 20 mM DDC. After washing the biofilms with PBS, a sample was taken for colony counting, whereafter 10 μM DCFHDA was added for 1 h during shaking at 37°C. Fluorescence was measured using a fluorescence spectrometer as described previously (3, 4), and values were normalized to the number of CFU.
Expression analysis of SOD genes in biofilm cells.
Following treatment of 16-h-old C. albicans biofilms with 0.3 mM miconazole for 24 h at 37°C, biofilm cells were collected and washed with PBS. The use of higher miconazole concentrations did not allow subsequent expression analysis of the biofilm cells due to the excessive killing (Fig. 1). Untreated C. albicans sessile cells served as a control. Cell disruption, RNA purification, and DNase treatment were performed according to the manufacturer's instructions (RiboPure-Yeast kit; Applied Biosystems). The isolated RNA was concentrated with an Amicon Ultra filter (Millipore), and 5 μl was diluted with diethyl pyrocarbonate (DEPC)-treated water to a final volume of 15 μl. The iScript cDNA synthesis kit (Bio-Rad) was used for the reverse transcriptase (RT) reaction. To this end, 1 μl RT and 4 μl reaction mix were added to each tube, and the mixture was incubated for 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. Real-time PCR (CFX96 real time system; Bio-Rad) was carried out using a Mesa Green qPCR kit (Eurogentec); the primers are listed in Table 1. The expression levels of the SOD genes were normalized using two reference genes (PMA1 and LSC2) (13). Experiments were carried out as three independent biological repeats, each consisting of six technical repeats.
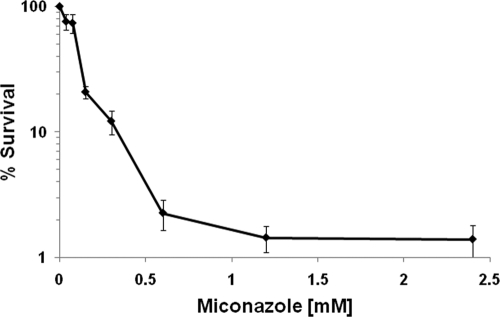
Fig. 1.
Killing of C. albicans WT biofilms by miconazole. Sixteen-hour-old biofilms of the C. albicans WT (CA-IF100) were treated with 0.04 to 2.4 mM miconazole in PBS. After incubation for 24 h, biofilms were washed and resuspended in PBS. The fraction of surviving cells was calculated by colony counting. Data represent the means and SEM for six independent experiments, each consisting of at least duplicate measurements.
Table 1.
Primer details
| Gene and primer description | Sequence (5′–3′) |
|---|---|
| SOD1 | |
| Forward | TCCGAATCCGCTCCAACCACA |
| Reverse | AAATGAGGACCAGCAGAAGTACAACCA |
| SOD2 | |
| Forward | TCAATTGAACAAGCCGTTGAAGCCAAA |
| Reverse | ACCACCTTGAGAGACAGGAGCCA |
| SOD3 | |
| Forward | CAATGCCGCTATTGACGCACTTGA |
| Reverse | TCCAGAACAAACTGTGGTTGGTGTGT |
| SOD4 | |
| Forward | TGACTCCAAAGGCAAGGCACCA |
| Reverse | TGGGCCAACACCTGAAGGCAAT |
| SOD5 | |
| Forward | ACGAGGGACACGGCAATGCT |
| Reverse | GCGCCATTACCTTGAGGAGCAGTA |
| SOD6 | |
| Forward | GACCCCGACCCACCTCAACAA |
| Reverse | GGGTAGCAAGGAGTGCCGGT |
| LSC2 | |
| Forward | CGTCAACATCTTTGGTGGTATTGT |
| Reverse | TTGGTGGCAGCAATTAAACCT |
| PMA1 | |
| Forward | TTGCTTATGATAATGCTCCATACGA |
| Reverse | TACCCCACAATCTTGGCAAGT |
RESULTS
Miconazole-tolerant persisters were detected in C. albicans biofilms.
To quantify the fraction of miconazole-tolerant persister cells in C. albicans CA-IF100 (WT) biofilms, we incubated 16-h-old biofilms with various concentrations of miconazole for 24 h and determined the percentage of surviving biofilm cells after treatment by plate counts. Killing of WT biofilms by miconazole followed a biphasic pattern. The number of miconazole-tolerant persisters in WT biofilms treated with 0.6 to 2.4 mM miconazole ranged from 2.3% to 1.3% (Fig. 1). In agreement with previous data (9, 19), we found that fluconazole had no effect on C. albicans biofilms, even when added in a final concentration of 3 mM (data not shown).
SOD expression levels in sessile C. albicans cells were induced by miconazole.
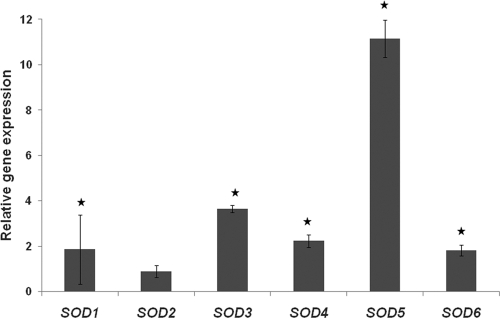
Since miconazole induces ROS in sessile C. albicans cells (19), we investigated the putative role of Sods in the occurrence of miconazole-tolerant persisters in C. albicans biofilms. To this end, we first assessed the effect of miconazole on the expression of SOD genes in C. albicans WT biofilms using real-time quantitative PCR. All SOD genes, except SOD2 (encoding mitochondrial Sod), were significantly (P < 0.05) upregulated in miconazole-treated biofilm cells compared to findings for untreated cells (Fig. 2). While this upregulation generally ranged between 1.8-fold (SOD6) and 3.7-fold (SOD3), it was most pronounced for SOD5 (11.2-fold). These data point to a putative involvement of Sods in governing tolerance of C. albicans biofilms for miconazole.
Fig. 2.
Relative gene expression in miconazole-treated sessile cells compared to that in untreated sessile cells. Sixteen-hour-old biofilms of C. albicans WT (CA-IF100) were treated with 0.3 mM miconazole, and expression of SODs was assessed using real-time PCR. Data represent the means and SEM for three independent experiments. *, P < 0.05.
Miconazole-tolerant persisters were decreased in C. albicans biofilms by DDC.
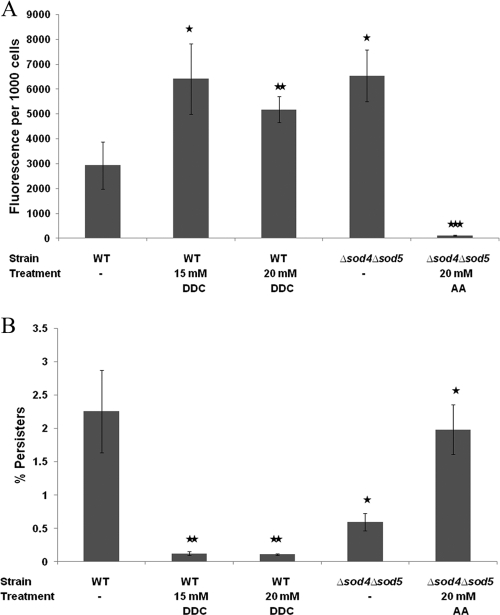
Sod1, Sod4, Sod5, and Sod6 of C. albicans are Cu,Zn-containing superoxide dismutases (12) that can be inhibited using the Cu,Zn-Sod inhibitor DDC (11). It was previously demonstrated that treatment of yeast with DDC caused a dose-dependent inhibition of Sod activity in vivo, with 75% inhibition occurring at 10 mM DDC (11). We found that coincubation of WT biofilms with 0.6 mM miconazole and 15 or 20 mM DDC resulted in increased endogenous ROS levels in sessile C. albicans cells compared to results with miconazole treatment alone (Fig. 3A), confirming the inhibitory activity of DDC on C. albicans Sods. Moreover, coincubation of the biofilms with miconazole and DDC in PBS resulted in an at least 18-fold reduction in the fraction of miconazole-tolerant persisters compared to results with miconazole treatment alone (Fig. 3B): treatment of WT biofilms with 0.6 mM miconazole and 20 mM DDC resulted in only 0.1% ± 0.01% miconazole-tolerant persisters (P < 0.01). Treatment of the WT biofilms with 20 mM DDC alone resulted in a 1.15-fold reduction in the biofilm cells (data not shown), pointing to a synergy between miconazole and DDC with regard to miconazole-tolerant persisters in C. albicans biofilms. Note that similar data were obtained when incubating the biofilms with DDC and miconazole in RPMI medium: treatment of WT biofilms with 0.6 mM miconazole and 20 mM DDC in RPMI resulted in 0.08% ± 0.02% miconazole-tolerant persisters, whereas 0.6 mM miconazole in RPMI alone resulted in 12.46% ± 1.96% miconazole-tolerant persisters (P < 0.05). Treatment of the WT biofilms with 20 mM DDC alone in RPMI resulted in a 1.62-fold reduction in biofilm cells. Hence, the effect of DDC on miconazole-tolerant persisters in C. albicans biofilms is independent of the incubation medium.
Fig. 3.
Effect of N,N′-diethyldithiocarbamate (DDC) and ascorbic acid (AA) on the fraction of miconazole-tolerant persisters and ROS levels in C. albicans biofilms. Sixteen-hour-old C. albicans WT (CA-IF100) and Δsod4 Δsod5 biofilms were treated with 0.6 mM miconazole in the absence (−) or presence of 15 or 20 mM DDC or 20 mM AA in PBS. After the biofilms were washed with PBS, a sample was taken for CFU determination, whereafter 10 μM DCFHDA was added. (A) The fluorescence was normalized to the number of CFU after treatment with miconazole. (B) The miconazole persister fraction was determined relative to DMSO treatment. Data represent the means and SEM for one representative experiment out of two, each consisting of triplicate measurements. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
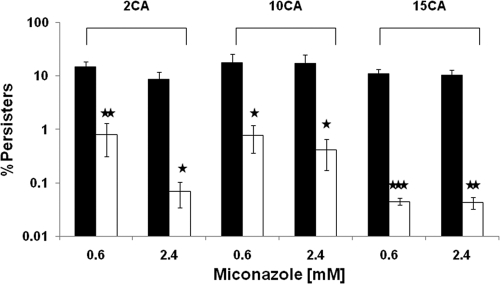
We also included C. albicans clinical isolates that form biofilms with a high fraction of miconazole-tolerant persisters. To this end, we treated biofilms of three C. albicans clinical isolates (2CA, 10CA, and 15CA) with 0.6 to 2.4 mM miconazole in the presence or absence of 20 mM DDC and determined the fraction of surviving miconazole-tolerant persisters (Fig. 4). The fraction of persisters upon miconazole treatment ranged from 8 to 17%, which is at least 5-fold higher than the fraction in WT CA-IF100 biofilms. Addition of 20 mM DDC to biofilms of C. albicans 2CA, 10CA, and 15CA resulted in an 18-fold to more than 200-fold reduction in miconazole-tolerant persisters compared to results with miconazole treatment alone (Fig. 4). Treatment of biofilms of the clinical isolates with 20 mM DDC alone resulted in only a 2.5-fold reduction in biofilm cells (data not shown).
Fig. 4.
N,N′-diethyldithiocarbamate (DDC) decreases the fraction of miconazole-tolerant persisters in biofilms of three C. albicans clinical isolates. Sixteen-hour-old biofilms of C. albicans isolates 2CA, 10CA, and 15CA were treated with 0.6 to 2.4 mM miconazole in the absence (black bars) or presence (white bars) of 20 mM DDC in PBS for 24 h. After the biofilms were washed with PBS, the surviving persister cells were calculated by colony counting. Data represent the means and SEM for three independent experiments, each consisting of duplicate measurements. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further validate the above data, we used the available C. albicans homozygous Δsod4 Δsod5 double deletion mutant and determined miconazole-tolerant persisters and endogenous ROS levels in biofilms of this mutant relative to those for its isogenic WT. We found that biofilms of the Δsod4 Δsod5 deletion mutant contained significantly fewer persisters than WT biofilms following treatment with miconazole: only 0.6% ± 0.1% (P < 0.05) of the Δsod4 Δsod5 biofilm cells survived treatment with 0.6 mM miconazole (Fig. 3B). Next, we determined miconazole-induced ROS levels in Δsod4 Δsod5 and WT biofilm cells after treatment with various concentrations of miconazole and found that ROS levels were increased in Δsod4 Δsod5 biofilm cells compared to those in WT biofilms following treatment with 0.6 mM miconazole (Fig. 3A).
To link the observed reduction in miconazole-tolerant persisters in Δsod4 Δsod5 biofilms to the increased endogenous ROS levels, we used the antioxidant ascorbic acid. Addition of ascorbic acid (final concentration, 20 mM) to Δsod4 Δsod5 biofilms reduced the accumulation of endogenous ROS in the presence of miconazole and concomitantly increased the fraction of persisters (Fig. 3) compared to results with miconazole treatment alone, indicating that the lower number of miconazole-tolerant persisters in Δsod4 Δsod5 biofilms is associated with the increased accumulation of ROS in this mutant.
DISCUSSION
In this study, we investigated the molecular basis of the occurrence of miconazole-tolerant persisters in Candida albicans biofilms. It was previously reported that miconazole induces the accumulation of endogenous ROS in C. albicans biofilms (19). Treatment of WT Candida biofilms with a high concentration of miconazole resulted in the occurrence of miconazole-tolerant persisters, typically about 1 to 2% of the total cell population of the biofilm, as was previously also reported for amphotericin B and chlorhexidine (8). It should be noted that the miconazole concentrations used in our in vitro biofilm setup (0 to 2.4 mM) to observe the phenomenon of miconazole-tolerant persisters are higher than the commonly used therapeutic miconazole concentrations. However, such high concentrations are achievable during antifungal lock therapy (2, 16). In the present study, we demonstrated an important role for superoxide dismutases in the occurrence of miconazole-tolerant persisters in C. albicans biofilms by using the Cu,Zn-Sod inhibitor N,N′-diethyldithiocarbamate (DDC). Addition of DDC resulted in an at least 18-fold reduction in miconazole-tolerant persisters in WT biofilms and in increased ROS levels. Treatment of biofilms of three C. albicans clinical isolates with DDC resulted in 18- to 200-fold fewer miconazole-tolerant persisters, pointing to an increased efficacy of miconazole against C. albicans biofilms using a Sod inhibitor. To further validate these data, we phenocopied the DDC effect using a Δsod4 Δsod5 C. albicans deletion mutant and found that Δsod4 Δsod5 biofilm cells contained significantly fewer persisters and increased ROS levels compared to findings for WT biofilms following treatment with miconazole. All these data indicate that Sods play an important role in the generation of miconazole-tolerant persisters in C. albicans biofilms via detoxification of endogenous ROS induced by miconazole. These findings are in line with earlier reports, demonstrating a role for Sod4 and Sod5 in ROS detoxification in C. albicans (5, 12). For example, it was shown that the expression of SOD5 in planktonic C. albicans cultures is induced by treatment with agents that induce osmotic or oxidative stress, such as menadione or riboflavin (12). Moreover, Frohner et al. showed that Sod4 and Sod5 are involved in detoxification of ROS induced in C. albicans cultures cocultivated with macrophages (5). To our knowledge, detoxification of ROS has never been linked to the occurrence of persisters in fungal biofilms following treatment with ROS-inducing antimicrobials. However, there is a single report on the upregulation of various antioxidant proteins (including alkyl hydroperoxide reductase, thioredoxin peroxidase, and thioredoxin) in sessile C. albicans cells compared to levels in planktonic cells, suggesting that these antioxidant proteins may contribute to the higher resistance to ROS-inducing antifungals of Candida biofilms (17).
There are several reports documenting the possibility of enhancing the fungicidal activity of ROS-inducing antimicrobials in planktonic cultures by interfering with the oxidative stress response. For example, increased activity of amphotericin B against Aspergillus fumigatus could be achieved by coincubation with thymol, the latter acting on the osmotic/oxidative stress mitogen-activated protein kinase (MAPK) pathway via SakA (6). Additionally, increased activity of fludioxonil, strobilurin, and antimycin A (antifungals targeting the oxidative and osmotic stress response systems) against various yeasts and filamentous fungi was obtained using certain benzaldehydes and benzoic acids (7). In Saccharomyces cerevisiae, tolerance to 2,3-dihydroxybenzaldehyde or 2,3-dihydroxybenzoic acid was found to rely upon mitochondrial superoxide dismutase (SOD2) or glutathione reductase (GLR1), respectively (7). However, so far there are no indications that such compounds would have an effect on the occurrence of antimicrobial-tolerant persisters in microbial biofilms. The present study points to an important role for Sods in protecting C. albicans biofilms against high doses of miconazole and to the ability of DDC to increase the efficacy of miconazole against C. albicans biofilms, resulting in reduced persister levels in miconazole-treated biofilms. Hence, this is the first report on the identification of specific enzymes involved in governing persistence in fungal biofilms.
In summary, oxidative stress response pathways, aimed at the detoxification of ROS induced by miconazole, could be efficient molecular targets to control persister formation in fungal biofilms. Indeed, using the general Sod inhibitor DDC, we could phenocopy the effect of SOD deletions on the occurrence of miconazole-tolerant persisters in biofilms of C. albicans laboratory strains and clinical isolates. Since dithiocarbamates have been associated with neurotoxicity (20), this compound is, as such, not suitable for combination therapy with miconazole. However, the design of specific inhibitors of Sods might lead to a novel antibiofilm therapy, consisting of the combination of a ROS-inducing antifungal with such an inhibitor.
ACKNOWLEDGMENTS
This work was supported by a grant from FWO-Vlaanderen (to T.C., H.N., B.P.A.C., and K.T.). K.T. and A.B. acknowledge the receipt of a postdoctoral fellowship from the Industrial Research Fund (K.U.Leuven) and a predoctoral fellowship from FWO-Vlaanderen, respectively.
We thank K. Kuchler for kindly providing C. albicans mutants in superoxide dismutases.
Footnotes
Published ahead of print on 11 July 2011.
REFERENCES
- 1. Aerts A. M., et al. 2009. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 583:2513–2516 [DOI] [PubMed] [Google Scholar]
- 2. Buckler B. S., et al. 2008. Treatment of central venous catheter fungal infection using liposomal amphotericin B lock therapy. Pediatr. Infect. Dis. J. 27:762–764 [DOI] [PubMed] [Google Scholar]
- 3. François I. E., et al. 2009. Membrane rafts are involved in intracellular miconazole accumulation in yeast cells. J. Biol. Chem. 284:32680–32685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. François I. E., et al. 2006. Azoles: mode of antifungal action and resistance development. Effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti-Infect. Agents Med. Chem. 5:3–13 [Google Scholar]
- 5. Frohner I. E., Bourgeois C., Yatsyk K., Majer O., Kuchler K. 2009. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 71:240–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim J., et al. 2008. Chemosensitization prevents tolerance of Aspergillus fumigatus to antimycotic drugs. Biochem. Biophys. Res. Commun. 372:266–271 [DOI] [PubMed] [Google Scholar]
- 7. Kim J. H., et al. 2008. Chemosensitization of fungal pathogens to antimicrobial agents using benzo analogs. FEMS Microbiol. Lett. 281:64–72 [DOI] [PubMed] [Google Scholar]
- 8. LaFleur M., Kumamoto C. A., Lewis K. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamfon H., Porter S. R., McCullough M., Pratten J. 2004. Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole and miconazole: a longitudinal study. J. Antimicrob. Chemother. 53:383–385 [DOI] [PubMed] [Google Scholar]
- 10. Lewis K. 2010. Persister cells. Annu. Rev. Microbiol. 64:357–372 [DOI] [PubMed] [Google Scholar]
- 11. Lushchak V., Semchyshyn H., Lushchak O., Mandryk S. 2005. Diethyldithiocarbamate inhibits in vivo Cu,Zn-superoxide dismutase and perturbs free radical processes in the yeast Saccharomyces cerevisiae cells. Biochem. Biophys. Res. Commun. 338:1739–1744 [DOI] [PubMed] [Google Scholar]
- 12. Martchenko M., Alarco A. M., Harcus D., Whiteway M. 2004. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol. Biol. Cell 15:456–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nailis H., Coenye T., Van Nieuwerburgh F., Deforce D., Nelis H. J. 2006. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol. Biol. 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reference deleted.
- 15. Ramage G., Mowat E., Jones B., Williams C., Lopez-Ribot J. 2009. Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 35:340–355 [DOI] [PubMed] [Google Scholar]
- 16. Schinabeck M. K., et al. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48:1727–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seneviratne C. J., Wang Y., Jin L., Abiko Y., Samaranayake L. P. 2008. Candida albicans biofilm formation is associated with increased anti-oxidative capacities. Proteomics 8:2936–2947 [DOI] [PubMed] [Google Scholar]
- 18. Thevissen K., Marchand A., Chaltin P., Meert E. M., Cammue B. P. 2009. Antifungal carbazoles. Curr. Med. Chem. 16:2205–2211 [DOI] [PubMed] [Google Scholar]
- 19. Vandenbosch D., Braeckmans K., Nelis H. J., Coenye T. 2010. Fungicidal activity of miconazole against Candida spp. biofilms. J. Antimicrob. Chemother. 65:694–700 [DOI] [PubMed] [Google Scholar]
- 20. Viquez O. M., Valentine H. L., Amarnath K., Milatovic D., Valentine W. M. 2008. Copper accumulation and lipid oxidation precede inflammation and myelin lesions in N,N-diethyldithiocarbamate peripheral myelinopathy. Toxicol. Appl. Pharmacol. 229:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]