Abstract
Finafloxacin is a novel fluoroquinolone that exhibits enhanced antibacterial activity under acidic conditions. The aim of this study was to define the in vitro pH-activity relationship. Finafloxacin exhibited optimal antibacterial activity between pH 5.0 and 6.0 at which MICs were 4- to 8-fold lower than those determined at neutral pH. These observations were then confirmed against a larger collection of bacteria. These data suggest that finafloxacin could potentially offer a therapeutic advantage within acidic foci of infection.
TEXT
Fluoroquinolones are a widely utilized class of antibacterial agent. However, a number of attempts to develop new, more potent, members of this class have failed due to concerns over safety and tolerability that have resulted in a halt to development, withdrawal from the market, or restriction of the market (12). Finafloxacin is a new fluoroquinolone belonging to a novel 8-cyano subclass that exhibited a low potential for toxicity or tolerability issues in preclinical tests (14) and in later clinical trials (13). Finafloxacin was also highly effective when tested in in vivo infection models, perhaps more so than would have been predicted from its in vitro MIC (7, 8). This effect was attributed, at least in part, to the enhancement of finafloxacin activity at slightly acidic pH (5, 6, 9), which is a distinctive property of finafloxacin in contrast to other marketed fluoro-quinolones, which generally lose activity at pH below neutral (4, 15). The present study had two aims. First, the pH-antibacterial activity relationship for finafloxacin was investigated over a range of pH 4.8 to 7.4 in order to better define the optimal pH range for activity. Second, the activity of finafloxacin was investigated against strains from several bacterial collections, comprising 445 isolates belonging to 19 species, to determine the reproducibility of the pH effect across different species of pathogenic bacteria and to provide an initial description of the spectrum of finafloxacin activity.
(Part of the research reported in this paper was presented in a poster session at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy-Infectious Diseases Society of America 46th Annual Meeting, Washington, DC, 25 to 28 October 2008 [11]).
Finafloxacin (manufactured by MerLion Pharmaceuticals GmbH, Berlin, Germany, or by Bayer HealthCare AG [now Bayer-Schering AG], Elberfeld, Germany) and ciprofloxacin, levofloxacin, and moxifloxacin (Sigma-Aldrich, Republic of Singapore, or Bayer HealthCare AG, Leverkusen, Germany) were tested against strains from the culture collections of MerLion Pharmaceuticals and their research partners. Susceptibility testing was performed according to the CLSI protocol for broth microdilution (2). The pH of broth was adjusted by the addition of hydrochloric acid prior to autoclaving and was remeasured afterwards.
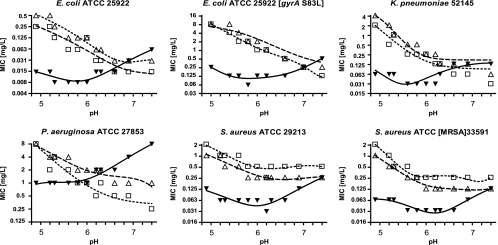
The MICs of finafloxacin, ciprofloxacin, and levofloxacin were determined against six reference strains (including an in vitro selected mutant of Escherichia coli ATCC 25922 with reduced susceptibility to finafloxacin) at pH values ranging from pH 4.8 to 7.4 (Fig. 1). Data were plotted in GraphPad Prism, version 4, software (La Jolla, CA), and trend lines were drawn with the nonlinear regression (polynomial) tool. Finafloxacin exhibited a 4- to 8-fold increase in activity (denoted by a 4- to 8-fold lowering of the MIC) at pH 6.0 compared to activity at pH 7.4. The pH range in which finafloxacin exhibited optimal activity was pH 5.0 to 6.0. Conversely, the activities of both ciprofloxacin and levofloxacin decreased at increasingly acidic pH. Both exhibited a 2- to 8-fold decrease in activity at pH 6.0, compared to activity at pH 7.4, and a further 8-fold decrease at pH 5.0 compared to activity at pH 6.0. These contrasting pH-dependent effects on the antibacterial activities of the different fluoroquinolones had the net result that finafloxacin exhibited MICs at pH 5.0 to 6.0 that were 8- to 16-fold lower than those of ciprofloxacin or levofloxacin against E. coli ATCC 25922, E. coli 25922 (gyrA S83L) (an in vitro selected mutant exhibiting reduced susceptibility to fluoroquinolones) and Klebsiella pneumoniae 52145 and 4- to 8-fold lower against Staphylococcus aureus ATCC 29213 and S. aureus ATCC 33591 (methicillin-resistant S. aureus [MRSA]). Finafloxacin MICs against Pseudomonas aeruginosa ATCC 27853 were 2- to 4-fold lower than those of ciprofloxacin or levofloxacin at pH 5.0 to 6.0.
Fig. 1.
MICs of finafloxacin, ciprofloxacin, and levofloxacin at pH values of 7.4 and below. Key: ▾, finafloxacin; □, ciprofloxacin; ▵, levofloxacin.
The activity of finafloxacin was also determined against a panel of 19 bacterial species under both the standard susceptibility testing conditions (pH 7.2 to 7.4) (2) and slightly acidic pH (pH 5.8 to 6.2) (Table 1). This acidic pH range was chosen for this study to represent a slightly acidic pH that would be found in a range of indications including respiratory, intra-abdominal, urinary tract, and skin and soft tissue infection (1). Finafloxacin activity was increased at the slightly acidic pH, compared to activity at pH 7.2 to 7.4, with the magnitude of this increase differing between species but consistent among strains of the same species. Conversely, at pH 5.8 to 6.2, ciprofloxacin, levofloxacin, and moxifloxacin MICs were generally 2- to 8-fold higher than those determined at pH 7.2 to 7.4.
Table 1.
In vitro susceptibility to finafloxacin and other fluoroquinolones under standard testing conditions and at slightly acidic pH
| Organism | Susceptibility to ciprofloxacina | No. of isolates tested | Antibiotic | MIC (mg/liter) at:b |
|||||
|---|---|---|---|---|---|---|---|---|---|
| pH 7.2–7.4 |
pH 5.8–6.2 |
||||||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | ||||
| Community-associated MRSA | Susceptible | 33 | Finafloxacin | 0.125–0.25 | 0.125 | 0.25 | 0.06–0.125 | 0.06 | 0.125 |
| Ciprofloxacin | 0.25–1 | 0.5 | 1 | 1–4 | 1 | 4 | |||
| Moxifloxacin | 0.03–0.125 | 0.06 | 0.125 | 0.25–0.5 | 0.25 | 0.5 | |||
| Staphylococcus aureus | Resistant | 30 | Finafloxacin | 0.25–32 | 2 | 16 | 0.25–32 | 1 | 4 |
| Ciprofloxacin | 4–>64 | 32 | >64 | 16–>64 | 32 | >64 | |||
| Levofloxacin | 4–>64 | 2 | 32 | 4–>64 | 16 | >64 | |||
| Moxifloxacin | 2–32 | 2 | 32 | 0.5–64 | 8 | 32 | |||
| Coagulase-negative staphylococcus | Susceptible | 26 | Finafloxacin | 0.015–0.5 | 0.25 | 0.5 | 0.008–1 | 0.06 | 0.125 |
| Ciprofloxacin | 0.06–1 | 0.25 | 0.5 | 0.125–4 | 0.5 | 1 | |||
| Resistant | 16 | Finafloxacin | 0.06–>16 | 8 | 16 | 0.125–16 | 1 | 16 | |
| Ciprofloxacin | 2–>16 | >16 | >16 | 8–>16 | >16 | >16 | |||
| Streptococcus pneumoniae | Mixed | 21 | Finafloxacin | 0.5–4 | 1 | 2 | ND | ND | ND |
| Ciprofloxacin | 1–4 | 2 | 4 | ND | ND | ND | |||
| Levofloxacin | 0.5–2 | 1 | 2 | ND | ND | ND | |||
| Moxifloxacin | 0.125–0.5 | 0.5 | 1 | ND | ND | ND | |||
| Streptococcus pyogenes | Susceptible | 22 | Finafloxacin | 0.25–1 | 0.5 | 0.5 | 0.125–0.5 | 0.25 | 0.25 |
| Ciprofloxacin | 0.125–1 | 0.5 | 1 | 0.25–2 | 1 | 2 | |||
| Moxifloxacin | 0.125–0.5 | 0.25 | 0.25 | 0.25–1 | 0.5 | 0.5 | |||
| Streptococcus agalactiae | Mixed | 11 | Finafloxacin | 0.5–4 | 1 | 2 | 0.125–4 | 0.25 | 0.5 |
| Ciprofloxacin | 0.5–4 | 1 | 2 | 0.5–4 | 1 | 2 | |||
| Enterococcus faecalis | Mixed | 10 | Finafloxacin | 0.5–32 | 1 | 32 | 0.25–16 | 0.5 | 16 |
| Ciprofloxacin | 0.5–128 | 1 | 64 | 2–>256 | 4 | >256 | |||
| Enterococcus faecium | Mixed | 9 | Finafloxacin | 1–128 | NC | NC | 0.5–32 | NC | NC |
| Ciprofloxacin | 1–256 | NC | NC | 2–>256 | NC | NC | |||
| Escherichia coli | Resistant | 75 | Finafloxacin | 16–>256 | 128 | 256 | 2–64 | 8 | 32 |
| Ciprofloxacin | 8–>256 | 128 | >256 | >256 | >256 | >256 | |||
| Levofloxacin | 8–128 | 32 | 64 | 32–>256 | 256 | >256 | |||
| Susceptible | 12 | Finafloxacin | 0.03–1 | 0.125 | 0.25 | ≤0.008–0.125 | 0.016 | 0.03 | |
| Ciprofloxacin | ≤0.008–0.125 | 0.016 | 0.03 | 0.06–2 | 0.125 | 0.25 | |||
| Klebsiella spp. | Susceptible | 16 | Finafloxacin | 0.06–4 | 0.25 | 2 | 0.008–1 | 0.06 | 0.5 |
| Ciprofloxacin | 0.016–1 | 0.03 | 0.5 | 0.125–8 | 0.5 | 8 | |||
| Resistant | 7 | Finafloxacin | 2–>32 | NC | NC | 0.5–>32 | NC | NC | |
| Ciprofloxacin | 2–>16 | NC | NC | 8–>16 | NC | NC | |||
| Salmonella spp. | Mixed | 8 | Finafloxacin | 0.5–16 | NC | NC | 0.06–4 | NC | NC |
| Ciprofloxacin | 0.125–32 | NC | NC | 1–>32 | NC | NC | |||
| Levofloxacin | 0.25–16 | NC | NC | 0.5–>32 | NC | NC | |||
| Moxifloxacin | 0.25–16 | NC | NC | 1–>32 | NC | NC | |||
| Proteus mirabilis | Mixed | 10 | Finafloxacin | 0.5–>32 | 1 | 16 | 0.06–8 | 0.25 | 4 |
| Ciprofloxacin | ≤0.008–1 | 0.016 | 1 | 0.06–>16 | 0.125 | 8 | |||
| Providencia spp. | Mixed | 11 | Finafloxacin | 0.06–16 | 8 | 16 | ≤0.03–8 | 1 | 8 |
| Ciprofloxacin | ≤0.03–16 | 1 | 4 | 0.125–>16 | 16 | >16 | |||
| Levofloxacin | ≤0.03–16 | 1 | 2 | 0.06–>16 | 8 | >16 | |||
| Moxifloxacin | ≤0.03–16 | 0.5 | 2 | 0.125–>16 | 8 | >16 | |||
| Enterobacter spp. | Susceptible | 10 | Finafloxacin | 0.06–0.5 | 0.125 | 0.125 | ≤0.03–0.125 | ≤0.03 | ≤0.03 |
| Ciprofloxacin | ≤0.03 | ≤0.03 | ≤0.03 | 0.06–0.5 | 0.125 | 0.25 | |||
| Levofloxacin | ≤0.03–0.06 | ≤0.03 | 0.06 | 0.125–0.5 | 0.25 | 0.5 | |||
| Moxifloxacin | ≤0.03–0.25 | ≤0.03 | 0.06 | 0.125–2 | 0.25 | 0.5 | |||
| Morganella morganii | Mixed | 11 | Finafloxacin | 0.125–16 | 1 | 16 | 0.03–16 | 0.25 | 4 |
| Ciprofloxacin | ≤0.008–>16 | 0.008 | 4 | 0.016–>16 | 0.06 | >16 | |||
| Serratia marcescens | Susceptible | 12 | Finafloxacin | 0.125–8 | 1 | 8 | 0.06–4 | 0.25 | 2 |
| Ciprofloxacin | ≤0.03–1 | ≤0.03 | 1 | 0.125–>16 | 1 | 16 | |||
| Levofloxacin | ≤0.03–4 | 0.125 | 1 | 0.25–>16 | 1 | 16 | |||
| Moxifloxacin | 0.06–4 | 0.125 | 2 | 1–>16 | 4 | >16 | |||
| Stenotrophomonas maltophilia | Mixed | 19 | Finafloxacin | 0.5–32 | 2 | 4 | 0.125–16 | 0.5 | 1 |
| Ciprofloxacin | 0.5–64 | 4 | 8 | 2–256 | 8 | 64 | |||
| Pseudomonas aeruginosa | Susceptible | 22 | Finafloxacin | 1–32 | 4 | 16 | 0.25–8 | 0.5 | 2 |
| Ciprofloxacin | 0.03–1 | 0.25 | 0.5 | 0.125–2 | 0.5 | 1 | |||
| Resistant | 9 | Finafloxacin | >32 | NC | NC | 16–>32 | NC | NC | |
| Ciprofloxacin | 8–32 | NC | NC | 8–>32 | NC | NC | |||
| Haemophilus influenzae | Susceptible | 35 | Finafloxacin | ≤0.004–0.06 | 0.008 | 0.03 | ND | ND | ND |
| Ciprofloxacin | 0.008–0.03 | 0.008 | 0.016 | ND | ND | ND | |||
| Levofloxacin | 0.008–0.03 | 0.016 | 0.03 | ND | ND | ND | |||
| Moxifloxacin | ≤0.004–0.125 | 0.016 | 0.06 | ND | ND | ND | |||
| Neisseria gonorrhoeae | Mixed | 10 | Finafloxacin | ≤0.03–0.25 | 0.06 | 0.125 | ≤0.03–0.125 | 0.06 | 0.06 |
| Ciprofloxacin | ≤0.03–0.125 | 0.06 | 0.06 | ND | ND | ND | |||
| Levofloxacin | 0.06–0.25 | 0.06 | 0.25 | 0.06–0.125 | 0.06 | 0.125 | |||
| Moxifloxacin | ≤0.03–0.125 | 0.06 | 0.06 | ND | ND | ND | |||
According to the CLSI susceptibility breakpoint for ciprofloxacin (3). Mixed, susceptible and resistant.
ND; not determined (usually strains grew poorly at low pH); NC, MIC50 and MIC90 were not calculated for groups of less than 10 strains.
These preliminary in vitro findings suggest that finafloxacin may have an advantage over other fluoroquinolones in terms of potency within acidic foci of infection. Bacteria infect body sites, including those which are typically at pH values below neutral, e.g., the urinary tract, skin, or respiratory epithelia (1). The environmental conditions and pH experienced by the invading bacteria could be further diversified if the bacteria were localized within, e.g., host phagocytotic cells or inflammatory compartments such as abscesses. The host immune response may also play a role in lowering the pH, e.g., during infections that result in chronic inflammation as experienced during airway infections of cystic fibrosis (CF) or chronic obstructive pulmonary disease (COPD) patients (10, 16). The relevance of this pH-dependent activity needs to be further explored using in vivo infection models to determine the pharmacokinetic/pharmacodynamic drivers of activity and the contribution of infection site pH to these. Furthermore, clinical trials to demonstrate the pharmacokinetics and efficacy of finafloxacin during treatment of bacterial infections, especially those within acidic foci, will be required in order to understand the clinical relevance of this effect.
Acknowledgments
This paper is dedicated to Harald Labischinski, who died on 24 August 2010. Harald was a valued colleague and advisor on the finafloxacin project and is missed by all who worked with him.
W.S., P.L., F.G., and G.C.Y. are all current or previous employees of MerLion Pharmaceuticals GmbH or MerLion Pharmaceuticals Pte Ltd.
Part of this work was supported by an unrestricted grant from MerLion Pharmaceuticals GmbH to Antiinfectives Intelligence GmbH in 2007.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Byerly T. C. 1953. Handbook of biological data. Science 118:313076170 [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed Approved standard M07–A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. Document M100–S17. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Dalhoff A., Schubert S., Ullmann U. 2005. Effect of pH on the in vitro activity of and propensity for emergence of resistance to fluoroquinolones, macrolides, and a ketolide. Infection 33(Suppl. 2):36–43 [DOI] [PubMed] [Google Scholar]
- 5. Dalhoff A., Stubbings W., Schubert S. 2011. Comparative in vitro activities of the novel antibacterial finafloxacin against selected Gram-positive and Gram-negative bacteria tested in Mueller-Hinton broth and synthetic urine. Antimicrob. Agents Chemother. 55:1814–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emrich N. C., Heisig A., Stubbings W., Labischinski H., Heisig P. 2010. Antibacterial activity of finafloxacin under different pH conditions against isogenic strains of Escherichia coli expressing combinations of defined mechanisms of fluoroquinolone resistance. J. Antimicrob. Chemother. 65:2530–2533 [DOI] [PubMed] [Google Scholar]
- 7. Endermann R., Ladel C., Stubbings W., Labischinski H. 2008. Pharmacokinetics (PK) and in vivo efficacy of oral finafloxacin (FIN) and comparators in rodent models of systemic infection, poster F1-2044. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 8. Endermann R., Ladel C., Stubbings W., Labischinski H. 2008. In vivo efficacy of finafloxacin in difficult to treat animal models of infection, poster F1-2045. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 9. Higgins P. G., Stubbings W., Wisplinghoff H., Seifert H. 2010. Activity of the investigational fluoroquinolone finafloxacin against ciprofloxacin-sensitive and -resistant Acinetobacter baumannii isolates. Antimicrob. Agents Chemother. 54:1613–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacquot J., Tabary O., Clement A. 2008. Hyperinflammation in the airways of cystic fibrosis patients: what's new? Expert Rev. Mol. Diagn. 8:359–363 [DOI] [PubMed] [Google Scholar]
- 11. Kresken M., Körber-Irrgang B., Labischinski H., Stubbings W. 2008. Effect of pH on the in vitro activity of finafloxacin against gram-negative and gram-positive bacteria, poster F1-2037. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 12. Owens R. C., Jr., Ambrose P. G. 2005. Antimicrobial safety: focus on fluoroquinolones. Clin. Infect. Dis. 41:S144–157 [DOI] [PubMed] [Google Scholar]
- 13. Patel H., et al. 2008. A phase I study to determine safety, tolerability and pharmacokinetics (PK) of finafloxacin (FIN) in healthy subjects, poster F1-2048. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 14. Schmuck G., Wasinska-Kempka G., Vente A., Labischinski H. 2008. In vitro toxicological profiling of finafloxacin, poster F1-2047. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 15. Smith S. M., Eng R. H. K., Cherubin C. E. 1988. Conditions affecting the results of susceptibility testing for the quinolone compounds. Chemotherapy 34:308–314 [DOI] [PubMed] [Google Scholar]
- 16. Yoon S. S., et al. 2006. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J. Clin. Invest. 116:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]