Abstract
In Papua New Guinean (PNG) children with acute bacterial meningitis (ABM), all Haemophilus influenzae isolates were resistant to chloramphenicol. Although Streptococcus pneumoniae isolates had a median chloramphenicol MIC of 3 μg/ml, it was ≥4 μg/ml in 42.8%, and the likelihood of an area under the 24-hour concentration-time curve/MIC ratio of >100 h at a MIC of ≥4 μg/ml was approximately 50%. All isolates were ceftriaxone sensitive. These data support ceftriaxone rather than conventional chloramphenicol for all PNG children with suspected ABM.
TEXT
In Papua New Guinea (PNG), Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae are the major causes of acute bacterial meningitis (ABM) in children (6). Chloramphenicol has been used widely in PNG since the 1980s as empirical therapy for severe bacterial infections, including ABM, but increasing resistance of Hib isolates has prompted progressive changes to national treatment guidelines. The latest version recommends ceftriaxone for all children with suspected ABM (8). Hib vaccine was implemented as part of the PNG vaccine schedule in 2008. Its increasing coverage may see S. pneumoniae emerge as the dominant local pathogen in pediatric ABM, especially as pneumococcal vaccination is not currently part of the schedule.
The antibiotic susceptibility of S. pneumoniae in PNG is unknown, but if most strains are sensitive, the expected reduction in invasive Hib disease might allow chloramphenicol to be reconsidered as an inexpensive component of the ABM treatment algorithm. To assess this, we characterized the antimicrobial susceptibility of Hib and S. pneumoniae isolated from children age 2 months to 10 years who were admitted to Modilon Hospital, the sole referral hospital in Madang Province, between October 2006 and December 2009 with proven ABM (≥20 white cells/mm3 in cerebrospinal fluid [CSF] and positive culture from CSF and/or blood) (5).
Preliminary S. pneumoniae identification was through the presence of Gram-positive cocci forming flat, alpha-hemolytic colonies with a >14-mm inhibition zone around optochin discs. Small Gram-negative coccobacilli growing preferentially on chocolate agar were assumed to be H. influenzae. Invasive bacterial isolates were stored frozen in skim-milk broth until confirmatory identification, serotyping, and susceptibility testing were performed. Susceptibilities to antibiotics, including chloramphenicol and ceftriaxone, were determined using the Kirby-Bauer disc diffusion method (1). The MICs of Hib and S. pneumoniae to penicillin antibiotics, chloramphenicol, and tetracycline were determined using Etest strips (AB Biodisk, Solna, Sweden).
We estimated the likelihood of attaining a target chloramphenicol pharmacokinetic/pharmacodynamic (PK/PD) ratio at each S. pneumoniae MIC. After an initial intramuscular or intravenous dose of 25 mg/kg of body weight in Melanesian children, the mean ± standard deviation (SD) area under the concentration-time curve from 0 to 6 h (AUC0–6) was 101 ± 26 or 108 ± 51 μg · h/ml, respectively, and 134 ± 63 or 110 ± 41 μg · h/ml, respectively, after subsequent doses (11). The AUC0–24 was estimated assuming that children received four doses, one every six hours, of chloramphenicol. We considered an AUC0–24/MIC ratio of >100 h to be the target PK/PD parameter. In the absence of definitive data for chloramphenicol from animal and human studies, this cut point was derived from published time-kill curves, a rabbit model of pneumococcal meningitis, and clinical observational data for linezolid (2, 10), an antibiotic with a structure, pharmacodynamic properties, and a toxicity similar to those of chloramphenicol.
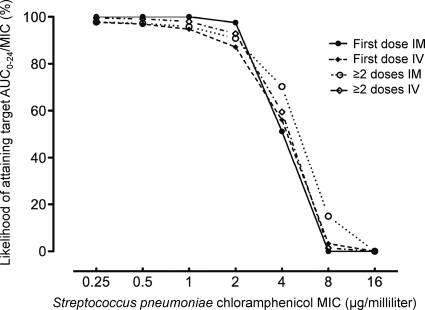
Hib and S. pneumoniae were isolated from 21 and 22 children, respectively. Difficulties keeping cultures viable until processing in a reference bacteriology laboratory limited susceptibility testing to 17 S. pneumoniae and 15 H. influenzae isolates. Thirteen of 15 H. influenzae isolates were Hib, and the remaining two were serotype A. The antibiotic susceptibilities of Hib and S. pneumoniae using disc diffusion and Etest methods are summarized in Table 1. All 14 H. influenzae isolates with available antimicrobial susceptibility data were resistant to chloramphenicol. Of the 14 S. pneumoniae isolates tested, the chloramphenicol MIC ranged from 0.75 to 24 μg/ml. The median chloramphenicol MIC was 3 μg/ml, and six (42.8%) had a MIC of ≥4 μg/ml. All S. pneumoniae isolates were fully susceptible to ceftriaxone. The likelihood of attaining an AUC0–24/MIC ratio of >100 h for S. pneumoniae isolates at different chloramphenicol MICs is shown in Fig. 1. This is close to 100% at low MICs (0.5, 1.0, and 2.0 μg/ml) but falls to approximately 50% and <20% at 4 μg/ml and ≥8 μg/ml, respectively.
Table 1.
Antibiotic susceptibility testing for Haemophilus influenzae and Streptococcus pneumoniae isolatesa
| Antibiotic |
Haemophilus influenzae (n = 15 isolates) |
Streptococcus pneumoniae (n = 17 isolates) |
||
|---|---|---|---|---|
| No. of isolates S/I/R by disc diffusion | Median (IQR) Etest MIC (μg/ml) | No. of isolates S/I/R by disc diffusion | Median (IQR) Etest MIC (μg/ml) | |
| Penicillin | 1/0/14 | 29 (12–≥256) | 11/2/2 | 0.032 (0.025–0.166) |
| Chloramphenicol | 0/0/14 | 16 (12–32) | 12/2/1 | 3 (2–4) |
| Sulfamethoxazole-trimethoprim | 0/0/15 | 6/7/3 | ||
| Tetracycline | 1/0/14 | >32 (>32–>32) | 11/4/1 | 0.38 (0.20–1.00) |
| Ceftriaxone | 10/0/0 | 15/0/0 | ||
Ampicillin disc diffusion and Etest were performed on H. influenzae isolates, while oxacillin and penicillin were used for disc diffusion and Etest, respectively, in S. pneumoniae isolates. S, sensitive; I, intermediate; R, resistant; IQR, interquartile range.
Fig. 1.
The likelihood of attaining an AUC/MIC ratio of >100 h by Streptococcus pneumoniae MIC. Individual plots by route and timing of chloramphenicol administration are shown.
Applying currently recommended breakpoints, S. pneumoniae isolates with chloramphenicol MICs of ≤4, 8, and ≥16 μg/ml are considered susceptible, intermediate, and resistant, respectively (1). There is, however, evidence that for an isolate with a MIC of 4 μg/ml there is a low likelihood of therapeutic success with standard-dose parenteral chloramphenicol. First, the present PK/PD model indicates that the chance of attaining the target AUC0–24/MIC ratio at this chloramphenicol MIC is 51 to 70%, depending on route and timing of chloramphenicol dosing. As nearly half our S. pneumoniae isolates had a MIC of ≥4 μg/ml, a substantial proportion of children with S. pneumoniae ABM treated with chloramphenicol may not attain the PK/PD target. Target concentrations could be further compromised by variability in protein binding and CSF penetration (7). Second, in a South African study of S. pneumoniae ABM (4), there was a substantially higher mortality rate among children with penicillin-resistant S. pneumoniae who were treated with chloramphenicol than among those with penicillin-sensitive organisms. All isolates were chloramphenicol susceptible on initial disc diffusion testing, but subsequent analysis showed that two-thirds had minimum bactericidal concentrations of ≥4 μg/ml. The authors hypothesized that excess mortality was due to diminished chloramphenicol susceptibility of penicillin-resistant S. pneumoniae isolates.
The median S. pneumoniae MIC of 3 μg/ml is 2-fold higher than that reported in studies of invasive S. pneumoniae isolates conducted in the PNG highlands from 1996 to 2000 (D. Lehmann, personal communication). This rise in MICs and the complete resistance of Hib to chloramphenicol in the present study suggest that all ABM infections should be treated with an expanded-spectrum cephalosporin, consistent with PNG national recommendations (8). It is likely that other countries in Oceania and beyond where chloramphenicol has been used widely and vaccination programs do not cover S. pneumoniae will be in a similar epidemiologic situation. The strategy of empirical ceftriaxone therapy for children with ABM due to Hib that is resistant to chloramphenicol and reversion to chloramphenicol if the results of in vitro testing demonstrate susceptibility has been assessed in PNG and reduces mortality as well as costs associated with the use of expanded-spectrum cephalosporins (3). However, this policy will be applicable only to the few PNG centers with bacteriology laboratories.
In addition to cost, widespread use of expanded-spectrum cephalosporins promotes emergence of vancomycin-resistant enterococci and extended-spectrum beta-lactamase-producing organisms (9). Because of this, simple algorithms guiding antibiotic therapy could prove valuable in the absence of even basic bacteriology. These algorithms could be based partly on exclusion of ABM through initial observation following a single febrile seizure, absence of neck stiffness or a bulging fontanel in a child with normal consciousness, and/or a positive thick blood film for malaria in a child without signs of meningism (5).
Acknowledgments
We gratefully acknowledge the assistance of the staff at the Pediatric Ward at Modilon Hospital, the Papua New Guinea Institute of Medical Research staff at Modilon Hospital and the Yagaum and Goroka campuses, and the patients and their families for their participation.
This study was funded by a National Health and Medical Research Council (NHMRC) grant (number 513782). We also acknowledge support from the MalariaGen Genomic Epidemiology Network. L.M. was supported by a Basser scholarship from the Royal Australasian College of Physicians and an NHMRC scholarship, M.L. by a Fogarty Foundation scholarship, and T.M.E.D. by an NHMRC Practitioner Fellowship.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 2. Cottagnoud P., et al. 2000. Linezolid against penicillin-sensitive and -resistant pneumococci in the rabbit meningitis model. J. Antimicrob. Chemother. 46:981–985 [DOI] [PubMed] [Google Scholar]
- 3. Duke T., Michael A., Mokela D., Wal T., Reeder J. 2003. Chloramphenicol or ceftriaxone, or both, as treatment for meningitis in developing countries? Arch. Dis. Child. 88:536–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedland I. R., et al. 1993. Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 37:1630–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laman M., et al. 2010. Lumbar puncture in children from an area of malaria endemicity who present with a febrile seizure. Clin. Infect. Dis. 51:534–540 [DOI] [PubMed] [Google Scholar]
- 6. Lehmann D., et al. 1999. Aetiology and clinical signs of bacterial meningitis in children admitted to Goroka Base Hospital, Papua New Guinea, 1989-1992. Ann. Trop. Paediatr. 19:21–32 [DOI] [PubMed] [Google Scholar]
- 7. Nau R., Sorgel F., Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 23:858–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paediatrics Society of Papua New Guinea 2011. Standard management for common illness of children in Papua New Guinea, 8th ed Papua New Guinea Department of Health, National Capital District, Papua New Guinea [Google Scholar]
- 9. Patterson J. E. 2001. Antibiotic utilization: is there an effect on antimicrobial resistance? Chest 119:426S–430S [DOI] [PubMed] [Google Scholar]
- 10. Rayner C. R., Forrest A., Meagher A. K., Birmingham M. C., Schentag J. J. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 42:1411–1423 [DOI] [PubMed] [Google Scholar]
- 11. Shann F., et al. 1985. Absorption of chloramphenicol sodium succinate after intramuscular administration in children. N. Engl. J. Med. 313:410–414 [DOI] [PubMed] [Google Scholar]