Abstract
Src homology-2 (SH2) domain-containing protein tyrosine phosphatases (SHPs) have been identified as either positive or negative regulators of signaling events downstream of receptor protein tyrosine kinases (R-PTKs). We describe here our characterization of ptp-2, a Caenorhabditis elegans gene that encodes a 668-amino-acid SHP. We isolated a recessive ptp-2 loss-of-function allele, op194, that lacks the conserved protein tyrosine phosphatase catalytic domain by screening for transposon-mediated deletion mutations. Homozygous ptp-2(op194) hermaphrodites exhibit a completely penetrant zygotic semisterile/maternal effect lethal phenotype, characterized by the presence of abnormally large oocytes in the zygotic semisterile animals. These phenotypes indicate that PTP-2 activity is essential for proper oogenesis. Gain-of-function let-60 ras alleles rescued the defects associated with ptp-2(op194), suggesting that LET-60 Ras acts downstream of, or in parallel to, PTP-2 during oogenesis. Although ptp-2 function is not required for normal vulval development, ptp-2(op194) altered significantly the vulval phenotypes caused by mutations in several genes of the inductive signaling pathway. The penetrance of the multivulva phenotype caused by loss-of-function mutations in lin-15, and gain-of-function mutations in let-23 or let-60 ras, was reduced by ptp-2(op194). Moreover, ptp-2(op194) increased the penetrance of the vulvaless phenotype conferred by a weak loss-of-function sem-5 allele. Taken together, our genetic data positions PTP-2 activity downstream of LET-23 in the vulval induction signaling pathway. Although PTP-2 functions to transmit a requisite signal during oogenesis, PTP-2 function during C. elegans vulval cell differentiation appears to be directed at regulating the overall strength of the inductive signal, which may contribute to the quantitative differences in signaling required for the proper specification of the 1°, 2°, and 3° vulval cell fates.
Keywords: C. elegans, tyrosine phosphatase, signal transduction, germ-line development, vulval development
Signal transduction pathways initiated by growth factors and their specific receptor protein tyrosine kinases (R-PTKs) are essential for the proper regulation of cell proliferation and differentiation. Furthermore, genetic studies are now beginning to reveal the importance of R-PTK-initiated signaling pathways during many aspects of development. In Drosophila, loss-of-function mutations in the torso R-PTK alters proper determination of cell fates at the termini of the embryo, whereas the sevenless R-PTK is required for R7 photoreceptor cell differentiation during compound eye development (Baker and Rubin 1989; Simon et al. 1992). In Caenorhabditis elegans, genetic screens have led to the identification of two R-PTKs, LET-23 and EGL-15, that are required for multiple developmental events (Aroian et al. 1990; DeVore et al. 1995). LET-23, a homolog of the mammalian epidermal growth factor receptor (EGFR), has been demonstrated to participate in several aspects of development, including vulval induction, oocyte activation, and male tail development (Aroian et al. 1990; Aroian and Sternberg 1991; Lesa and Sternberg 1997). Analysis of mutations in egl-15, which encodes a fibroblast growth factor R-PTK (FGFR) homolog, revealed that EGL-15 signaling specifies proper migration and positioning of the sex muscle cells, as well as other developmental processes that are yet to be characterized (DeVore et al. 1995).
Specificity of R-PTK signaling is thought to be achieved, at least in part, by interaction of the activated R-PTK with multiple intracellular regulators of signal transduction. For example, in addition to interacting with the SH2/SH3 adaptor protein Grb2, mammalian R-PTKs have also been demonstrated to bind, in a ligand-dependent fashion, phospholipase C-γ (PLCγ), Ras GTPase-activating protein (RasGAP), Shc, the p85 subunit of phosphatidylinositol-3-OH kinase (PI3K), and the SH2 domain-containing protein tyrosine phosphatases SHP-1 and SHP-2 (for review, see Pawson 1995). The SHPs are a class of evolutionarily conserved signaling molecules that interact with and regulate signaling from activated R-PTKs (Neel and Tonks 1997). Structurally, these proteins contain two SH2 domains at their amino terminus, and a conserved protein tyrosine phosphatase catalytic domain at the carboxyl terminus. In addition, a predicted phosphotyrosine-containing motif has been noted in the extreme carboxy-terminal segment. To date, two mammalian SHPs, SHP-1 and SHP-2, have been cloned (Shen et al. 1991; Freeman et al. 1992; Plutzky et al. 1992; Ahmad et al. 1993). SHP-1 is expressed predominately in hematopoietic cells where it regulates negatively signaling from the receptor for erythropoietin (Epo), as well as the c-kit and colony stimulating factor 1 (CSF-1) R-PTKs (Klingmuller et al. 1995; Chen et al. 1996; Lorenz et al. 1996; Paulson et al. 1996). In contrast, SHP-2 is expressed ubiquitously and functions primarily as a positive mediator of R-PTK signaling. Biochemical analyses have revealed that SHP-2 binds through its SH2 domains to the tyrosine phosphorylated platelet-derived growth factor receptor (PDGFR), EGFR, and R-PTK substrates such as the insulin receptor substrate-1 (IRS-1) and SHP substrate-1 (SHPS-1) (Kuhne et al. 1993; Lechleider et al. 1993a; Fujioka et al. 1996). Overexpression or microinjection of a catalytically inactive, dominant-negative SHP-2 inhibited both EGF-induced and insulin-induced mitogen-activated protein kinase (MAPK) activation, demonstrating that protein tyrosine phosphatase (PTP) catalytic activity is required for positive regulation of R-PTK signaling (Milarski and Saltiel 1994; Yamauchi et al. 1995; Bennett et al. 1996).
Genetic and molecular analyses have clearly defined essential roles for SHPs in the regulation of signaling during development. The requirement of SHP-1 for hematopoiesis was illustrated by the finding that a spontaneous mutation of shp-1 causes multiple hematopoietic defects, resulting in the lethal motheaten phenotype (Shultz et al. 1993). Mice containing a targeted disruption of the SH2 domains of SHP-2 have severe gastrulation defects, which may be a consequence of the decreased activation of MAPK by the FGFR in cells derived from these mice (Saxton et al. 1997). Experiments where a catalytically inactive, dominant-negative form of the Xenopus SHP-2 homolog XSHP-2 was microinjected into Xenopus embryos yielded animals with severe tail truncations and also inhibited FGF and activin-mediated mesoderm induction in ectodermal explants (Tang et al. 1995). Loss-of-function mutations in the Drosophila SHP corkscrew have revealed an essential and positive role in transmitting signals initiated by both the torso and sevenless R-PTKs (Perkins et al. 1992; Allard et al. 1996).
These genetic and molecular studies indicate that SHPs are integral regulators of R-PTK-initiated signal transduction pathways. Therefore, to further our understanding of the developmental role of SHPs and their regulation of R-PTK-initiated signals, we have cloned and subsequently isolated a loss-of-function mutation in ptp-2, a C. elegans gene that encodes a SHP. Our genetic analyses indicate that PTP-2 function is required for proper oogenesis, where it functions upstream of, or in parallel to, Ras. Furthermore, PTP-2 also participates in the regulation of signal transduction during vulval development, in a manner that apparently modulates the intensity or strength of the inductive signal. Thus, a differential requirement for PTP-2 activity exists within distinct developmental programs in C. elegans.
Results
Identification and cloning of ptp-2
To identify novel PTPs in C. elegans, we designed degenerate PCR primers to conserved motifs present in all PTP catalytic domains and used them to amplify putative PTPs from wild-type (N2) C. elegans genomic DNA. The PCR-amplified products from this screen were subcloned and subjected to DNA sequence analysis. The predicted translated product of one PCR-amplified fragment, ce17, showed higher sequence similarity to the SH2 domain-containing PTPs (A.J. Flint and N.K. Tonks, unpubl.) and was pursued further.
To isolate the full-length cDNA, we generated a probe by designing PCR primers to amplify a 504 bp fragment that is predicted to encode the second more carboxy-terminal SH2 domain of this putative PTP (see Materials and Methods). This PCR-amplified product was then used to probe a C. elegans mixed stage cDNA library. After screening ∼300,000 plaques, we isolated 20 positive clones, 13 of which contained cDNA inserts of ∼2.8 kb. These clones were sequenced and found to be identical, and we will refer to this gene as ptp-2 (for protein tyrosine phosphatase homolog). One clone contained an additional stretch of 8 nucleotides at the 5′ end that are not encoded by the ptp-2 locus, but are identical to the SL1 trans-spliced leader (Fig. 1A) (Blumenthal and Steward 1997). The presence of an SL1 spliced leader sequence and the fact that the putative initiating methionine is directly downstream of a consensus translation initiation sequence strongly suggests that this cDNA corresponds to the full-length ptp-2 transcript.
Figure 1.

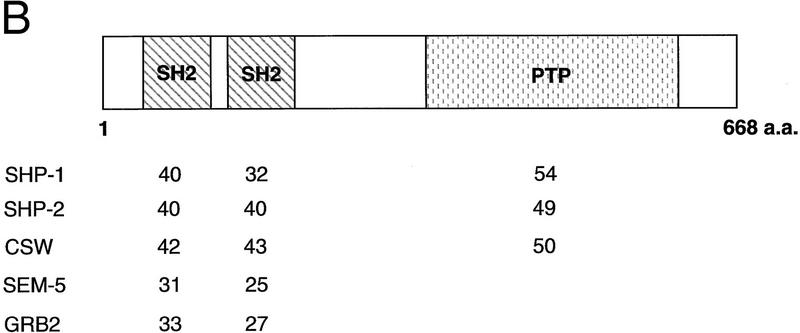
Nucleotide and predicted amino acid sequence of ptp-2. (A) The full-length ptp-2 cDNA sequence is shown on top and the predicted amino acid sequence is in single letter code below. The numbers at right indicate nucleotides or amino acids. The underlined nucleotides at the start of the ptp-2 cDNA indicate the putative SL1 trans-spliced leader sequence. The underlined amino acids are predicted to constitute the amino-terminal (amino acids 8–116) and carboxy-terminal (amino acids 134–229) SH2 domains. The boxed amino acids (amino acids 516–526) comprise the evolutionarily conserved protein tyrosine phosphatase catalytic core motif (I/V-H-C-X-X-G-X-X-R-S/T-G). The 5′ black arrow (nucleotide 882) and the 3′ black arrow (nucleotide 2260) encompass the boundaries of the ptp-2(op194) deletion in the cDNA, and the protein predicted to be encoded by this truncated cDNA. The actual 5′ breakpoint occurs within an intron and is not depicted here (see Fig. 2A). (B) Comparative amino acid sequence analysis. Schematic representation of the wild-type PTP-2 protein is shown on top. The numbers listed below represent the percent identity between the amino-terminal SH2 domain, the carboxy-terminal SH2 domain, and the PTP catalytic domain of PTP-2 and those domains of the human SHP-1 and SHP-2 and Drosophila corkscrew enzymes. The single SH2 domain of the adaptor proteins SEM-5 and human Grb2 were compared to both the amino- and carboxy-terminal SH2 domains of PTP-2. Sequence comparisons were performed using the GCG Bestfit program.
The predicted structure of PTP-2 displayed similarity to the previously characterized SHPs in that it contained two tandem SH2 domains at the amino terminus and a carboxy-terminal PTP catalytic domain. Interestingly, PTP-2 lacks the carboxy-terminal, phosphotyrosine-containing motif pY-X-N-X, a sequence that is conserved between the human, mouse, Xenopus, and Drosophila SHPs. PTP-2, like the vertebrate SHPs, does not contain the ∼150-amino-acid, serine- and cysteine-rich insert sequence that is observed in the PTP catalytic domain of corkscrew. Comparative amino acid analyses illustrated that the SH2 domains of PTP-2 are more closely related to SHP-1, SHP-2, and corkscrew, than those of the adaptor proteins SEM-5 and Grb2 (Fig. 1B). Overall, PTP-2 did not exhibit any preferential similarity to either SHP-1, SHP-2, or corkscrew. Therefore, we were unable to infer whether PTP-2 regulates PTK signaling positively or negatively simply from its structure.
Isolation of a ptp-2 loss-of-function allele
To investigate the physiological role of PTP-2, we undertook a reverse genetic approach in an attempt to isolate loss-of-function mutations in ptp-2. A PCR-based screen was applied to identify individual worms that carry an insertion of the endogenous transposon Tc1 in the ptp-2 locus (Zwaal et al. 1993; Plasterk 1995). ptp-2-specific and Tc1-specific primers were used together to screen a frozen worm library of the strain MT3126 (Collins et al. 1987), which has many active copies of Tc1 within its genome. We detected, and isolated subsequently, individual worms in which Tc1 inserted into the intron between exons 4 and 5 of ptp-2 and will refer to this insertion allele as op193::Tc1 (Fig. 2A). Homozygous ptp-2(op193::Tc1) hermaphrodites were phenotypically wild type, which is consistent with previous reports that the vast majority of Tc1 insertions occurring within introns are spliced-out during mRNA processing, leaving the mRNA intact.
Figure 2.
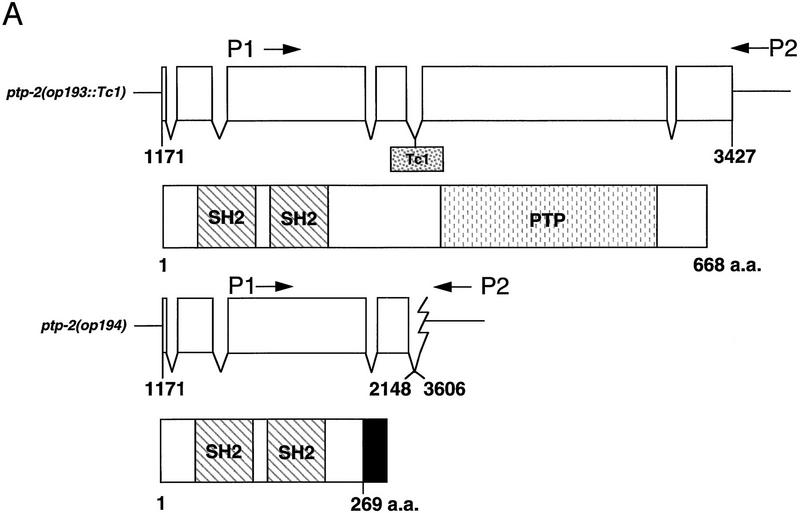
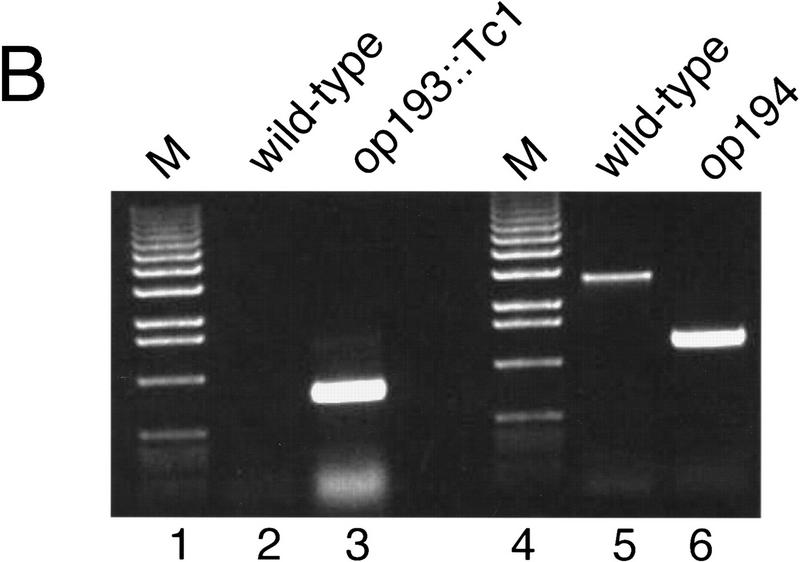
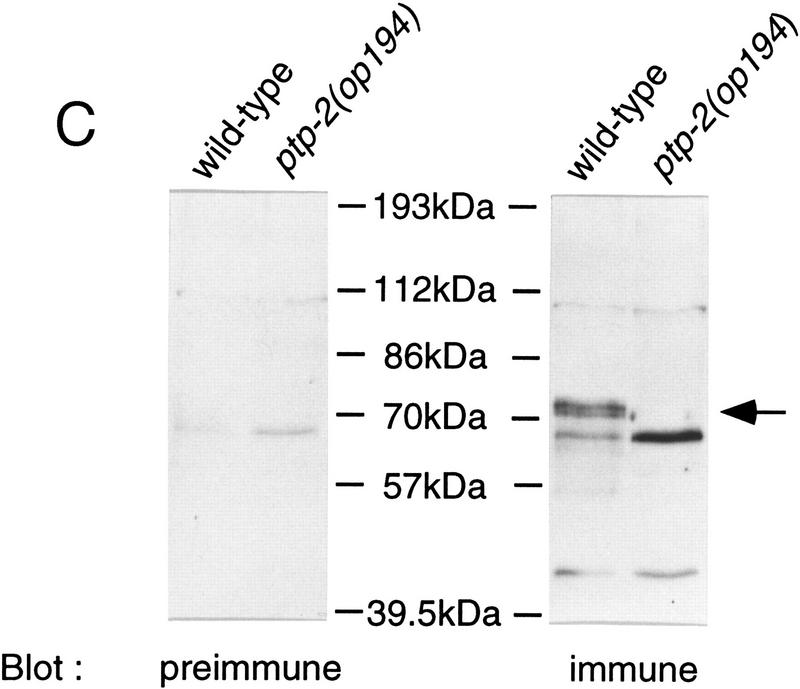
Genomic structure of the ptp-2(op193::Tc1) and ptp-2(op194) alleles and their encoded proteins. (A) Exons are indicated as boxes (top) and the nucleotide numbers underneath correspond to cosmid F28B12 and represent the start and end of the ptp-2 coding sequences. The approximate positions of the P1 and P2 primers used for the PCR-based screens are shown. The boxed Tc1 indicates the approximate location of the op193::Tc1 insertion. The proteins predicted to be encoded by the ptp-2(op193::Tc1) (wild-type PTP-2) and ptp-2(op194) (truncated PTP-2) alleles are depicted schematically below. The black region at the carboxyl terminus of the truncated PTP-2 protein depicts the possible addition of 28 amino acids from the fourth intron and 3′ UTR sequence, as the op194 deletion removes the fourth intron splice acceptor and normal in-frame stop codon. (B) PCR products demonstrating wild-type, op193::Tc1, and op194 alleles. Lanes 2 and 3 show the presence of the insertion allele op193::Tc1 using primer P2 and a transposon-specific primer, but its absence in the wild-type strain N2. Lanes 5 and 6 demonstrate the wild-type and op194 alleles using PCR primers P1 and P2, respectively. Lanes 1 and 4 are 1-kb ladder DNA markers (M). (C) Immunoblot analysis using anti-PTP-2 polyclonal serum. N2 or ptp-2(op194) protein lysates were blotted with preimmune (left) or immune serum (right). Molecular mass standards are indicated. Arrow denotes the presence of wild-type PTP-2 in the N2 lysate, but not in the ptp-2(op194) lysate when blotting with the immune serum. The ∼57-kD band present in wild-type but not the ptp-2(op194) lysate may result from the degradation of wild-type PTP-2.
Because the op193::Tc1 transposon was still mobile, we screened ptp-2(op193::Tc1) hermaphrodites by PCR with primers flanking the insertion in an attempt to detect deletion mutations that arose after imprecise transposon excision (Zwaal et al. 1993; Plasterk 1995). At a relatively low frequency, imprecise Tc1 excision occurs with 5′ and/or 3′ flanking genomic DNA being removed during the process. From a screen of ∼64,000 animals, we identified a single deletion allele, op194, and isolated individual worms carrying this mutation. PCR and sequence analysis of the deletion allele using ptp-2-specific primers indicated that the op194 mutation deletes 1458 nucleotides of the genomic ptp-2 locus, which corresponds to nucleotides 2149–3605 of cosmid F28B12 (Figs. 1A and 2A,B). This mutation deletes all sequences coding for the carboxy-terminal half of PTP-2 (amino acids 269–668), which includes the entire tyrosine phosphatase catalytic domain of PTP-2.
To confirm that the op194 mutation inhibits the production of wild-type PTP-2, we performed an immunoblot analysis on lysates prepared from either wild-type (N2) or ptp-2(op194) mutant worms (Fig. 2C). Anti-PTP-2 rabbit polyclonal sera raised against a PTP-2 carboxy-terminal peptide (amino acids 650–667), but not preimmune sera, recognized a protein of ∼72 kD in lysates from N2, but not ptp-2(op194) worms. This immunoreactive protein corresponds closely to the predicted size of PTP-2 (73.5 kD) and confirms the PCR analysis of the ptp-2(op194) deletion allele (Fig. 2B). Our anti-PTP-2 polyclonal antibodies could not detect the presence of a truncated form of PTP-2 that consists of only the SH2 domains. However, if such a protein is present, it is unlikely to act as a dominant interfering mutant, because ptp-2(op194)/+ heterozygous hermaphrodites are phenotypically wild-type, and ptp-2(op194)/Df transheterozygotes are similar in phenotype to ptp-2(op194) homozygotes (see below).
ptp-2 is required for hermaphrodite fertility
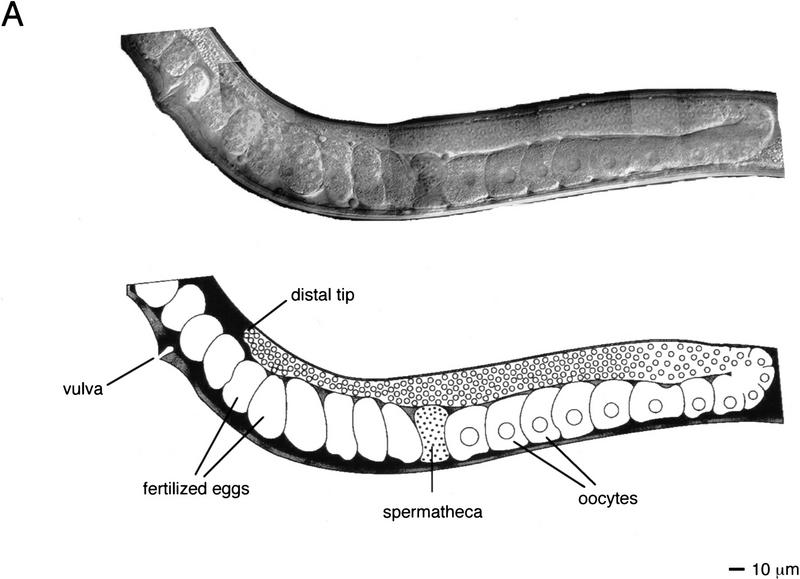
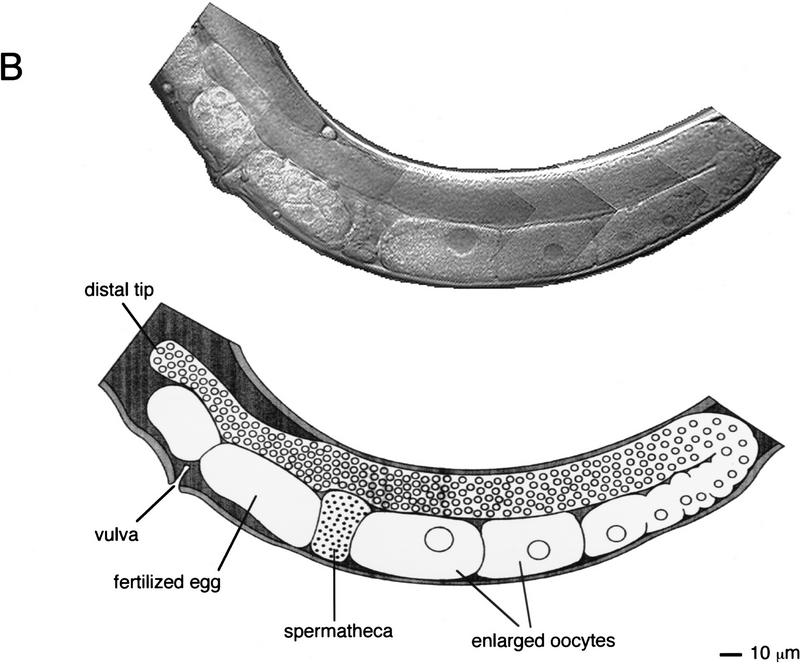
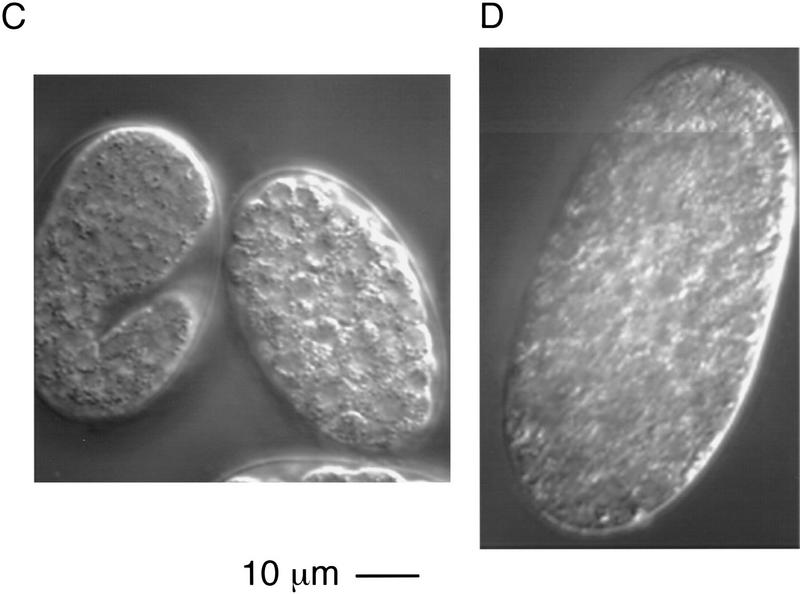
The C. elegans hermaphrodite gonad consists of two U-shaped arms that enclose the germ cells during their development. The most immature germ cells are at the distal tip, and as these cells move proximally they progress through the meiotic cell cycle, resulting in the generation of mature oocytes that await entry into the proximal spermatheca where fertilization occurs (Schedl 1997; Fig. 3A). ptp-2(op194) homozygous hermaphrodites born of heterozygous mothers appear normal at hatching and readily grow to adulthood, with all somatic tissues phenotypically wild type. However, such adult animals contain uncharacteristically large oocytes in the germ line (about two to threefold larger than wild type; Fig. 3), and show a dramatically reduced brood size, on average ∼10 eggs compared to ∼200 from wild-type hermaphrodites (Table 1). The few progeny produced by ptp-2(op194) homozygous mothers all die during embryogenesis or early larval development. Embryonic developmental arrest occurs after the initiation of gastrulation but before any morphogenesis of the embryo (Fig. 3D). We have observed occasional escapers of this embryonic lethality. These escapers are thin, uncoordinated, and invariably arrest as young larvae (data not shown). However, they do not exhibit the classic “rod-like” larval lethal phenotype observed in let-60 ras, sem-5, and lin-45 mutants (data not shown). From these observations we conclude that the loss of ptp-2 function results in the generation of large oocytes, a zygotic semisterile phenotype, as well as a fully penetrant maternal effect lethality. All three phenotypes could be the result of defective oogenesis. Alternatively, the embryonic or larval lethality might reflect the involvement of PTP-2 in distinct events during embryogenesis and larval development.
Figure 3.
Photomicrographs of wild-type (N2) and ptp-2(op194). Composite Nomarski images of (A) wild-type (N2) or (B) homozygous ptp-2(op194) germ lines in adult hermaphrodites. For clarity, an artistic illustration is shown below each Nomarski composite. The predominant phenotype of the ptp-2(op194) mutants are enlarged oocytes and fertilized eggs in the proximal germ line. (C,D) Nomarski images of a representative (C) wild-type (N2) embryo and (D) a developmentally arrested ptp-2(op194) embryo. Note the complete lack of morphogenesis in the ptp-2(op194) mutant.
Table 1.
Genetic analysis of ptp-2(op194): penetrance of sterile phenotype and brood size
| Genotype
|
Percent fertility (no.)
|
Brood size (no.)
|
|---|---|---|
| Wild type (N2) | 100 (>100) | 192 (4) |
| ptp (op194) II | 0 (100) | 10 (16) |
| maDf4/dpy-10(e128) | ||
| unc-104(e1265) IIa | 100 (50) | 187 (5) |
| maDf4/ptp-2(op194) IIb | 0 (22) | 3 (22) |
| let-60(n1046) IV | 100 (60) | 155 (4) |
| ptp-2(op194) II; let-60(n1046) IV | 96 (80) | 163 (4) |
| let-60(n1700) IV | 100 (40) | 75 (6) |
| ptp-2(op194)II; let-60(n1700) IV | 100 (40) | 164 (3) |
(no.) Number of hermaphrodites examined.
The strain VT454 contains maDf4/dpy-10(e128) unc-104(e1265) II. The maDf4 deficiency deletes a region of Chr. II that includes ptp-2.
maDf4/ptp-2(op194) II hermaphrodites were generated as described in Materials and Methods.
To examine whether ptp-2(op194) behaves like a genetic null mutation, we placed ptp-2(op194) in trans to maDf4 II, a deficiency (Df) that deletes the entire ptp-2 locus. We observed that maDf4/ptp-2(op194) hermaphrodites were qualitatively similar to op194/op194 hermaphrodites in that they generated only a few fertilized eggs and no viable progeny. Some escapers of this embryonic lethality were noted; however, they arrested during larval development, which is similar to the F1 escapers from ptp-2(op194) homozygotes (Table 1; data not shown). Moreover, after the mating of ptp-2(op194) unc-4(e120)/clr-1(e1745) dpy-10(e128) II males to maDf4/dpy-10(e128) unc-104(e1265) II hermaphrodites, we isolated equal numbers of ptp-2(op194) unc-4(e120)/maDf4 and ptp-2(op194) unc-4(e120)/dpy-10(e128) unc-104(e1265) II cross-progeny hermaphrodites (see Materials and Methods), indicating that ptp-2(op194) in trans to maDf4 does not result in an increase in zygotic lethality. Because ptp-2(op194)/maDf4 hermaphrodites show the same range of defects as the op194/op194 homozygotes, this result is consistent with the hypothesis that the op194 mutation eliminates PTP-2 function completely.
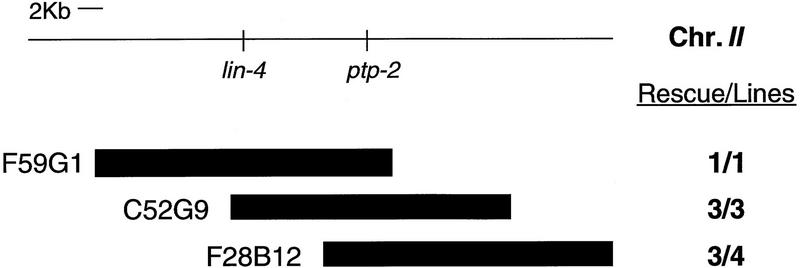
To rule out the possibility that a mutation closely linked to ptp-2(op194) contributes to the observed mutant phenotypes, we attempted to rescue these defects through germ-line transformation (Mello and Fire 1995). To this end, we microinjected hermaphrodites heterozygous for ptp-2(op194) [genotype: ptp-2(op194) unc-4(e120)/dpy-10(e128) unc-104(e1265) II] with cosmids containing wild-type genomic ptp-2 and the plasmid pRF4 (Kramer et al. 1990). Rescue was defined by the ability of an individual cosmid to allow the establishment of a fertile obligate-transgenic line of the genotype ptp-2(op194) unc-4(e120) II; Ex[ptp-2(+); pRF4]. We were able to generate stable lines of this genotype with all three ptp-2-containing cosmids tested, F59G1, C52G9, and F28B12, and found that each rescued all of the ptp-2(op194) conferred phenotypes (Fig. 4). As a control we also microinjected the cosmid F38A3 that contains the gene ptp-1, which encodes a receptor-like PTP (M. Gutch, N. Tonks, and M. Hengartner, unpubl.). However, F38A3 failed to rescue any of the mutant defects (data not shown). Because ptp-2 is the only complete gene within the ∼4.2 kb of sequence common to F59G1, C52G9, and F28B12, the phenotypes we observed can likely be attributed to a loss of PTP-2 function and not a closely linked mutation.
Figure 4.
Rescue of the ptp-2(op194) phenotypes by germ-line transformation. YAC filter hybridization and sequence analysis by the C. elegans genome sequencing project placed ptp-2 ∼10 kb to the right of lin-4 (top). Cosmids containing wild-type genomic ptp-2 and the plasmid pRF4, which contains the marker mutation rol-6(su1006), were microinjected into ptp-2(op194) unc-4(e120)/dpy-10(e128) unc-104(e1265) II adult hermaphrodites in an attempt to establish fertile obligate-transgenic lines (see Materials and Methods). Numbers to the right indicate the number of rescued lines/number of stable transgenic lines established. Of the cosmids microinjected, the relative boundaries of the F59G1 3′ end and F28B12 5′ end were confirmed by PCR; however, the exact boundaries of C52G9, the 5′ end of F59G1, and 3′ end of F28B12 are approximated.
Genetic interaction between PTP-2 and LET-60 Ras during oogenesis
Molecular studies on the EGFR signaling pathway demonstrated that mammalian SHP-2 functions upstream of, or in parallel to, Ras (Bennett et al. 1996). These results are consistent with genetic studies on torso R-PTK signaling, where activated p21v-ras rescued corkscrew null mutations (Lu et al. 1993). However, corkscrew may also function downstream of or in parallel to Ras, as signaling by the sevenless R-PTK requires corkscrew function even in the presence of activated Ras or activated Raf (Allard et al. 1996).
To determine whether a relationship exists between ptp-2 and let-60 ras during C. elegans oogenesis, we generated double mutants with gain-of-function let-60 ras alleles and assessed their brood sizes and ability to generate viable offspring (Table 1). The let-60(gf) ras alleles n1046 and n1700 both encode a constitutively active Ras protein caused by a point mutation converting the amino acid glycine to glutamic acid at position 13 (Beitel et al. 1990). We observed almost complete rescue of the zygotic semisterile and maternal effect lethal phenotypes of ptp-2(op194) using either the let-60 (n1046) ras or let-60 (n1700) ras alleles. Loss-of-function mutations in let-60 ras can result in sterility because germ cells fail to exit from the pachytene stage of meiosis I, inhibiting the generation of mature oocytes (Church et al. 1995). To investigate whether PTP-2 and LET-60 Ras may cooperate to promote exit from pachytene, we generated the double mutant with ptp-2(op194) and the weak loss-of-function allele let-60(n2021), which alone causes no germ-line phenotype. Interestingly, all ptp-2(op194) II; let-60(n2021) IV double mutant hermaphrodites arrested developmentally as rod-like larval animals (data not shown), mimicking the phenotype normally observed in strong loss-of-function let-60 ras mutants. The ability of the gain-of-function LET-60 Ras to rescue the ptp-2(op194) sterility is consistent with the hypothesis that LET-60 Ras acts downstream of PTP-2 during C. elegans oogenesis, possibly relaying a signal from PTP-2, which allows exit from pachytene. However, we cannot exclude the possibility that LET-60 Ras may function in a parallel pathway, which when hyperactivated, can compensate for a loss of PTP-2 function.
Involvement of ptp-2 in signaling during vulval development
During C. elegans vulval induction, the LET-23 R-PTK, SEM-5/Grb2, LET-60 Ras, LIN-45 Raf, MEK-2/LET-537 MEK, and MPK-1/SUR-1 MAPK transmit positively the LIN-3 EGF inductive signal in the vulval precursor cells (VPCs), resulting in an invariant pattern of cell divisions and morphogenesis (for review, see Kornfeld 1997). In wild-type hermaphrodites, this signaling pathway induces the VPCs P5.p, P6.p, and P7.p to undergo three rounds of cell division, producing 22 cells that form the vulva. The VPCs P3.p, P4.p, and P8.p undergo only a single division, with one of the daughter cells eventually fusing with the ventral hypodermal syncytium. Loss-of-function mutations in genes that mediate the inductive signaling pathway can result in a vulvaless (Vul) phenotype because of insufficient induction of P5.p, P6.p, and P7.p. In contrast, mutations that enhance inductive signaling can yield a multivulva (Muv) phenotype, which results from aberrant hyperinduction of P3.p, P4.p, and P8.p. Genetic analyses of let-23 have demonstrated that this R-PTK transmits the LIN-3 inductive signal during vulval development (Aroian and Sternberg 1991; Aroian et al. 1994). One negative regulator of vulval induction is lin-15, a gene that functions genetically upstream of let-23 (Clark et al. 1994; Huang et al. 1994). Mosaic analysis demonstrated that LIN-15 does not function in the VPCs, but in the neighboring hypodermal syncytial cell hyp7, which makes extensive contacts with the VPCs (Herman and Hedgecock 1990). Hermaphrodites carrying loss-of-function mutations in lin-15 are Muv, possibly attributable to the inappropriate activation of LET-23 in the six VPCs. However, the precise mechanism of negative regulation of vulval induction by LIN-15 remains unclear.
To test the possibility that PTP-2 might also regulate LET-23 signaling during vulval development, we generated double mutants between ptp-2(op194) and various mutations affecting vulval induction. We observed that a loss of PTP-2 activity reduced the penetrance of the Muv phenotype conferred by either of two lin-15 loss-of-function (lf) alleles (Fig. 5A,B and Table 2). Between 30% and 40% of the ptp-2(op194) II; lin-15(lf) X double mutant hermaphrodites exhibited complete suppression of the Muv phenotype. The remaining 60–70% showed a significant reduction in the size of the protruding pseudovulvae (data not shown). We also examined the effect of the ptp-2(op194) mutation on the Muv phenotype conferred by the gain-of-function mutations let-23(sa62) and let-60(n1046) or let-60(n1700) (Table 2). The loss-of-function mutation in PTP-2 was also able to reduce significantly the penetrance of the Muv phenotype caused by an activating mutation in LET-60 Ras (Fig. 5C,D and Table 2). A point mutation in the extracellular domain of LET-23, which converts amino acid cysteine 359 to tyrosine, results in a constitutively active form of this R-PTK and a semidominant Muv phenotype (Katz et al. 1996). Hermaphrodites homozygous for ptp-2(op194) let-23(sa62) unc-4(e120) II had a slight, but statistically significant, reduction in the Muv phenotype (96% vs. 79%), when compared to let-23(sa62) unc-4(e120) II homozygous hermaphrodites. Interestingly, the protruding pseudovulvae in the Muv ptp-2(op194) let-23(sa62) unc-4(e120) II homozygotes were also greatly reduced in size, similar to the small pseudovulvae observed in ptp-2(op194) II; lin-15(lf) X homozygotes (data not shown). Equally important, we did not detect any suppression of the Muv phenotype induced by a loss-of-function mutation in another negative regulator of vulval development, lin-1, which encodes a putative ETS DNA-binding domain transcription factor that acts downstream of LET-60 Ras (Beitel et al. 1995). The sterility and low brood size conferred by ptp-2(op194) was not altered when placed in the background of the lin-15(lf), let-23(gf), or lin-1(lf) mutations (data not shown).
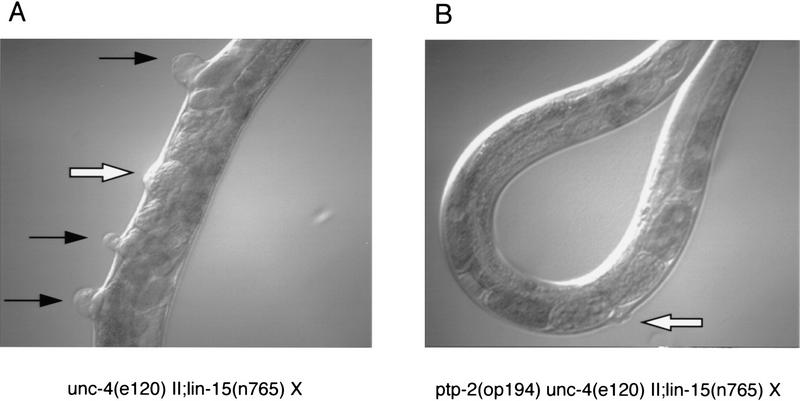
Figure 5.
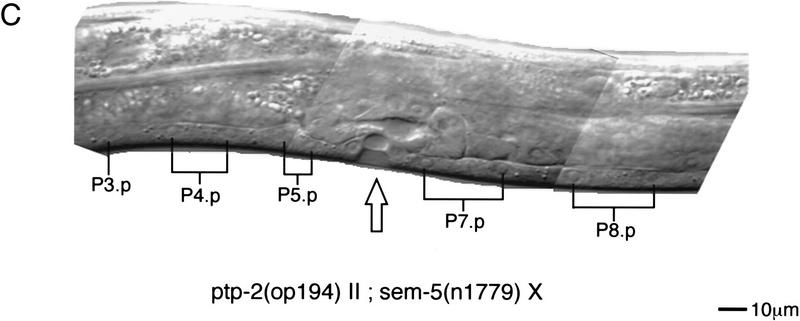
Suppression of vulval induction by ptp-2(op194). (A,B) Nomarski images of unc-4(e120) II; lin-15(n765) X (top left) and ptp-2(op194) unc-4(e120) II; lin-15(n765) X (top right) adult hermaphrodites. Open arrows denote the position of the functional vulva derived from the VPCs P5.p, P6.p, and P7.p. Black arrows indicate ectopic pseudovulvae that arise from aberrant induction of P3.p, P4.p, and P8.p. Note the complete suppression of P3.p, P4.p, and P8.p induction in B. Magnification, 200×. (C) Nomarski composite of ptp-2(op194) II; sem-5(n1779) X L4 hermaphrodite. The open arrow indicates the partial induction of P6.p. The remaining uninduced Pn.p cells are indicated. Anterior is to the left. The scale bar is for C only.
Table 2.
Genetic interactions of ptp-2(op194) and regulators of vulval development: penetrance of multivulva phenotype and VPC induction
| Genotype
|
Percent Muv (no.)a
|
VPC induction (no.)b
|
|---|---|---|
| Wild type (N2) | 0 (>200) | 3.0 (23) |
| ptp-2(op194) II | 0 (>200) | 3.0 (32) |
| unc-4(e120) II;lin-15(n765) X | 97 (156) | 5.0 (33) |
| ptp-2(op194) unc-4(e120) II; lin-15(n765) X | 59 (150) | 3.7 (53) |
| unc-4(e120) II;lin-15(n309) X | 100 (150) | 5.8 (22) |
| ptp-2(op194) unc-4(e120) II; lin-15(n309) X | 69 (195) | 4.5 (42) |
| let-23(sa62) unc-4(e120) II | 96 (294) | 4.5 (38) |
| ptp-2(op194) let-23(sa62) unc-4(e120) II | 79 (191) | 3.9 (18) |
| let-60(n1046) IV | 55 (606) | 4.0 (53) |
| ptp-2(op194) II;let-60(n1046) IV | 17 (517) | 3.4 (64) |
| let-60(n1700) IV | 68 (671) | 3.7 (72) |
| ptp-2(op194) II;let-60(n1700) IV | 13 (548) | 3.8 (62) |
| unc-4(e120) II;lin-1(e1275) IVc | 100 (40) | N.D. |
| ptp-2(op194) unc-4(e120) II; lin-1(e1275) IVc | 100 (50) | N.D. |
(no.) Number of hermaphrodites examined.
Muv was defined as visible pseudovulvae derived from either P3.p, P4.p or P8.p using a dissecting microscope at 20°C.
VPC induction was determined by examining L3/L4 larval hermaphrodites individually using Nomarski optics. An animal with all VPCs induced (P3.p–P8.p) would receive a score of six, a wild-type animal where only the VPCs p5.p, P6.p and P7.p are induced, would receive a score of three, and a hermaphrodite with no vulval induction would receive a score of zero. The results presented are an average from the number of worms scored.
(N.D.) Not determined.
Homozygous unc-4(e120) II;lin-1(e1275) IV and ptp-2(op194) unc-4(e120) II; lin-1(e1275) IV are subviable strains due to the rupture of the pseudovulvae prior to the generation of self-progeny in the majority of the animals. The Muv phenotype was inferred from the observance of ruptured pseudovulvae in these animals.
We also examined L3/L4 larval animals to assess the extent of VPC induction in these mutant backgrounds (Table 2). Animals containing either of the lin-15(lf) mutations showed a strong induction (5.0–5.8 VPCs per hermaphrodite), whereas let-23(sa62) and let-60(n1046) or let-60(n1700) were observed to induce the VPCs to a lesser extent (3.7–4.5 VPCs per hermaphrodite). In general, ptp-2(op194) was able to reduce the number of VPCs induced per animal by the lin-15(lf), let-23(gf), and let-60(gf) mutations, which is consistent with partial suppression of the Muv phenotype by ptp-2(op194).
Finally, we investigated whether the loss-of-function mutation in PTP-2 would affect the extent of vulval induction when combined with a weak loss-of-function mutation in SEM-5. SEM-5 is a SH3-SH2-SH3 domain-containing adaptor protein that is essential for proper transmission of the LET-23 inductive signal in the VPCs, as strong loss-of-function mutations in SEM-5 are almost completely Vul (Clark et al. 1992). The weak sem-5(n1779) allele, which contains a point mutation converting Glu to Lys in the SH2 domain, results in 11% Vul hermaphrodites. Significantly, we observed that the penetrance of the Vul phenotype conferred by sem-5(n1779) was enhanced from 11% to 74% when placed in the background of the ptp-2(op194) mutation (Fig. 5C; Table 3). Therefore, our interpretation of these results is that PTP-2 functions as a positive mediator of LET-23 R-PTK signaling during vulval development.
Table 3.
Genetic interaction of ptp-2(op194) and sem-5(n1779): penetrance of vulvaless phenotype
| Genotype
|
Percent vulvaless (no.)
|
|---|---|
| Wild type (N2) | 0 (>200) |
| ptp-2(op194) II | 0 (>200) |
| sem-5(n1779) X | 11a |
| ptp-2(op194) II; sem-5(n1779) X | 74 (103) |
(no.) Number of hermaphrodites examined.
sem-5(n1779) X single mutant data were taken from Clark et al. (1992). Wild-type (N2) and ptp-2(op194) adult hermaphrodites were examined by dissecting microscope for retention of eggs. The ptp-2(op194) II; sem-5(n1779) X double mutant hermaphrodites generated very few fertilized eggs; therefore, vulval induction was scored in early adult hermaphrodites by Nomarski microscopy.
Effect of ptp-2 on vulval cell fates
The nematode vulva develops from six VPCs, P3.p–P8.p. In wild-type hermaphrodites, P6.p invariantly adopts the 1° fate, P5.p and P7.p adopt a 2° fate, and P3.p, P4.p, and P8.p adopt the nonvulval or 3° fate (Sternberg and Horvitz 1989). Morphologically, the 1° fate consists of eight cells that form a symmetrical arch and detach from the ventral hypoderm. The 2° lineage comprises seven cells and an asymmetrical appearance, with the three cells proximal to P6.p detaching from the ventral hypoderm and the four cells distal to P6.p attached ventrally. The 3° fate is where the VPC divides only once, with one of the daughter cells eventually fusing with the ventral hypoderm. In hermaphrodites with mutations in vulval development genes, two nonclassic lineages have been described (Katz et al. 1995). The intermediate lineage contains a variable number of cells and exhibits characteristics of both the 1° and 2° fates. A half-vulval lineage occurs when one VPC daughter cell fails to divide, adopting the 3° fate, whereas the other daughter cell divides twice and displays some morphogenesis.
We examined L3 larval hermaphrodites to determine the effect of ptp-2(op194) on the vulval cell lineages (Table 4). In a wild-type background, ptp-2(op194) did not alter the invariant 3°-3°-2°-1°-2°-3° pattern of vulval cell lineages. lin-15(lf) or let-60(gf) mutations, which result in Muv phenotypes, convert the 3° lineage adopted normally by P3.p, P4.p, and P8.p to 1°, 2°, half-vulval, or intermediate lineages. In ptp-2(op194); lin-15(lf) and ptp-2(op194); let-60(gf) double mutants, the loss-of-function ptp-2(op194) mutation predominately reverted the induction of P3.p, P4.p, and P8.p to the 3° fate, and less frequently to the half-vulval cell fate. Moreover, adoption of the 3° fate by P5.p, P6.p, and P7.p was also observed in ptp-2(op194); sem-5(n1779) double mutants. We conclude that the ability of ptp-2(op194) to suppress the Muv phenotype of lin-15(lf) or let-60(gf) mutations, and enhance the Vul phenotype of sem-5(n1779), results primarily from an abrogation of VPC induction that is likely a consequence of a reduced level of inductive signal transmitted within the VPCs, and not from altered morphogenesis after VPC induction, which prevents formation of ectopic pseudovulvae or a normal vulva.
Table 4.
VPC lineage analysis
| Genotype
|
P3.p
|
P4.p
|
P5.p
|
P6.p
|
P7.p
|
P8.p
|
|---|---|---|---|---|---|---|
VPC divisions and adherence to ventral hypodermis were examined at the mid-L3 larval stage, L3 molt, and mid-L4 larval stage. VPC fates from representative hermaphrodites were classified as outlined in the text and as described in Sternberg and Horvitz (1989) and Katz et al. (1995). Abbreviations represent VPC division axes and include L (lateral), T (transverse), O (oblique), N (no division), and D (did not observe). Adherence to the ventral hypodermis is indicated by boldface type (i.e., L). A solid box (unshaded) denotes a primary (1°) fate, a dashed box (unshaded) denotes a secondary (2°) fate, S (syncytial) indicates the tertiary (3°) or nonvulval cell fate, a dashed and shaded box denotes an intermediate fate, and a solid and shaded box denotes a half-vulval fate. Each line represents the vulval lineage from a single hermaphrodite.
Discussion
Studies of signaling pathways in humans, mice, Xenopus, and Drosophila have revealed that SHPs are essential for the proper transmission of R-PTK-initiated signals. In this report we have described the cloning and initial characterization of ptp-2, a C. elegans gene that encodes a SH2 domain-containing PTP. Generation of a ptp-2 loss-of-function allele, op194, revealed an essential role for PTP-2 activity during oogenesis. Functionally, PTP-2 is likely to act as a positive mediator of tyrosine kinase-initiated signals during this aspect of development, as an activated let-60 ras suppressed all defects associated with a loss of ptp-2 function. Furthermore, genetic analyses indicated that ptp-2(op194) reduced the penetrance of the Muv phenotype and VPC induction conferred by lin-15 (lf), let-23(gf), and let-60(gf) mutations, and enhanced the Vul phenotype of a weak sem-5(lf) allele, indicating that PTP-2 also participates in the regulation of signal transduction during vulval development.
ptp-2 function during germ-line development
Oogenesis in C. elegans proceeds as the germ cells migrate proximally from the distal tip of the gonad (Schedl 1997). As these cells move proximally, they progress through the meiotic cell cycle, which continues until the pachytene stage of meiosis I, at which point the germ cells arrest until they reach the flexure (bottom of the U) of each gonadal arm. Exit from pachytene (when bivalent, homologous chromosomes are closely aligned) to diakinesis (when chromosomes recondense) requires the activity of the genes let-60 ras, mek-2/let-537, and mpk-1/sur-1 (Church et al. 1995). We have shown here that ptp-2 is required for proper C. elegans oogenesis. Hermaphrodites homozygous for ptp-2(op194) display abnormally large oocytes, about two to three times the size of mature wild-type oocytes. Moreover, on average ptp-2(op194) hermaphrodites only produce ∼10 abnormally large fertilized embryos, of which the majority do not hatch. These phenotypes can be attributed solely to a reduction in PTP-2 function, as they were absent in animals transgenic for any one of three overlapping cosmids, which contained only ∼4.2 kb of genomic sequence in common, where the only complete gene in this region is ptp-2. Thus, PTP-2 is an essential component of a signaling cascade that regulates normal oogenesis.
Several observations suggest that the ptp-2(op194) germ-line defects likely reside within the presumptive oocytes. First, that ptp-2(op194) hermaphrodites can generate self-progeny indicates that these animals do contain functional sperm. Second, inspection of homozygous ptp-2(op194) hermaphrodites and males by DAPI staining and Nomarski microscopy indicated wild-type morphology, numbers, and appropriate location of sperm (data not shown). Third, the inability of wild-type males to increase the generation of embryos when mated to ptp-2(op194) hermaphrodites is consistent with defects in oogenesis (data not shown). Finally, defects in sperm that reduce fertilization efficiency would likely lead to the laying of unfertilized oocytes, which we do not observe. Our interpretation of these observations is that defects in oogenesis are the primary cause of the limited generation of embryos by ptp-2(op194) hermaphrodites. However, we did notice that ptp-2(op194) homozygous males fail to sire any cross-progeny when mated to appropriately marked hermaphrodites. Because both germ line and sperm appear normal in ptp-2(op194) males (as detected by Nomarski microscopy; data not shown), this sterility may indicate a requirement for PTP-2 function for the generation of male-specific structures needed for mating, or for proper male mating behavior.
Currently, we are not sure why ptp-2(op194) hermaphrodites produce such limited numbers of embryos, given that the distal germ line appears wild type. DAPI staining of ptp-2(op194) hermaphrodites did reveal some minor germ-line irregularities, such as occasional clumping of pachytene nuclei (data not shown), which is somewhat reminiscent of the germ lines of animals that contain mutations that block pachytene exit (Church et al. 1995). Thus, one possibility is that loss of PTP-2 activity may result in an incomplete block of pachytene exit, and the few abnormally sized oocytes that develop may be occasional escapers of this block. Such dysregulated or delayed exit from the meiotic pachytene stage could reduce brood size, and may also affect the size of the presumptive oocytes in the ptp-2(op194) mutants. The hypothesis that PTP-2 participates in a cascade that signals to exit from pachytene arrest is consistent with the ability of hyperactivated Ras to rescue the oogenesis defects caused by loss of PTP-2 function. However, it is equally possible that PTP-2 and LET-60 Ras also function in distinct signaling pathways during oogenesis, which contributes to the generation and size of mature oocytes.
ptp-2 participates in signaling during vulval development
Genetic screens for mutations that confer a Vul phenotype identified loss-of-function mutations in lin-3, a gene that encodes a transmembrane EGF motif containing protein expressed by the anchor cell during the time of vulval induction (Hill and Sternberg 1992). Interaction of LIN-3 with the LET-23 R-PTK on the surface of the P6.p VPC activates subsequently a signaling pathway, which is transmitted through SEM-5, LET-60, LIN-45, MEK-2/LET-537, and MPK-1/SUR-1 (for review, see Kornfeld 1997). This inductive signaling pathway ultimately affects the activity of the proteins LIN-1 (Beitel et al. 1995), LIN-25 (Tuck and Greenwald 1995), LIN-31 (Miller et al. 1993), and SUR-2 (Singh and Han 1995), resulting in changes in gene expression and the proper specification of vulval and nonvulval cell fates.
Hermaphrodites homozygous for ptp-2(op194) do not exhibit any overt vulval defects, indicating that the LIN-3-induced, SEM-5-mediated signal transduction pathway is functional in the absence of PTP-2. Furthermore, lineage analysis indicated that ptp-2(op194) alone also does not alter lateral signaling between the VPCs, as the 3°-3°-2°-1°-2°-3° pattern was always observed. However, ptp-2(op194) did increase the penetrance of the Vul phenotype of a weak sem-5(lf) allele and in addition reduced the penetrance of the Muv phenotype conferred by lin-15(lf), let-23(gf), and let-60 ras(gf) mutations. These results imply that PTP-2 does participate in transmitting at least some signaling downstream from LET-23. Although the exact contribution of this PTP-2-regulated signal to vulval development remains to be established, our data seem to favor a role for PTP-2 in regulating the signals that lead to induction of the VPCs. Examination of VPC induction and lineage analysis of the ptp-2(op194) double mutants indicated that enhancement of the Vul phenotype, as well as the suppression of the Muv phenotype, was primarily through conversion of induced (1° and 2°) lineages to the 3° or uninduced vulval cell fate. Such reduced VPC induction, and not an alteration of induced VPC morphogenesis, in the ptp-2(op194) double mutants is consistent with a reduction in signaling that normally initiates this developmental program. To our knowledge, ptp-2(op194) is the first described mutation that is epistatic to lin-15(lf) mutations, but does not exhibit vulval defects on its own; previously described suppressors of the lin-15(lf) conferred Muv, including loss-of-function mutations in let-23, sem-5, and let-60 all yield Vul phenotypes in an otherwise wild-type background (Beitel et al. 1990; Aroian and Sternberg 1991; Clark et al. 1992).
Models for ptp-2 signaling
Our genetic analyses suggest that PTP-2 participates in regulating cell signaling during several aspects of C. elegans development. The most visible role of ptp-2 appears to be during oogenesis, as ptp-2(op194) mutants are almost completely sterile. Mechanistically, how might PTP-2 function to promote oogenesis? The let-23(sy10) hypomorphic allele also results in sterile hermaphrodites. In these animals, ovulation defects caused by a lack of a functional spermatheca results in an endomitotic oocyte (Emo) phenotype (Aroian et al. 1994). Because ptp-2 mutants do not exhibit any Emo defect, we favor a model where PTP-2 functions downstream of a yet uncharacterized R-PTK or PTK in the germ line (Fig. 6A). Moreover, the inability of the hypermorphic let-23(sa62) allele to rescue the sterility and low brood size of ptp-2(op194) also favors a model where an uncharacterized R-PTK/PTK functions to promote oogenesis. A possible candidate may be a homolog of the murine c-kit R-PTK, which has been shown to be expressed in developing oocytes during the diplotene stage (Manova et al. 1993). Because the cell lineages requiring ptp-2 expression have not yet been determined, we cannot rule out the possibility that PTP-2 contributes to oocyte development by functioning downstream of a R-PTK or PTK in cells other than the germ line, such as the somatic gonad.
Figure 6.
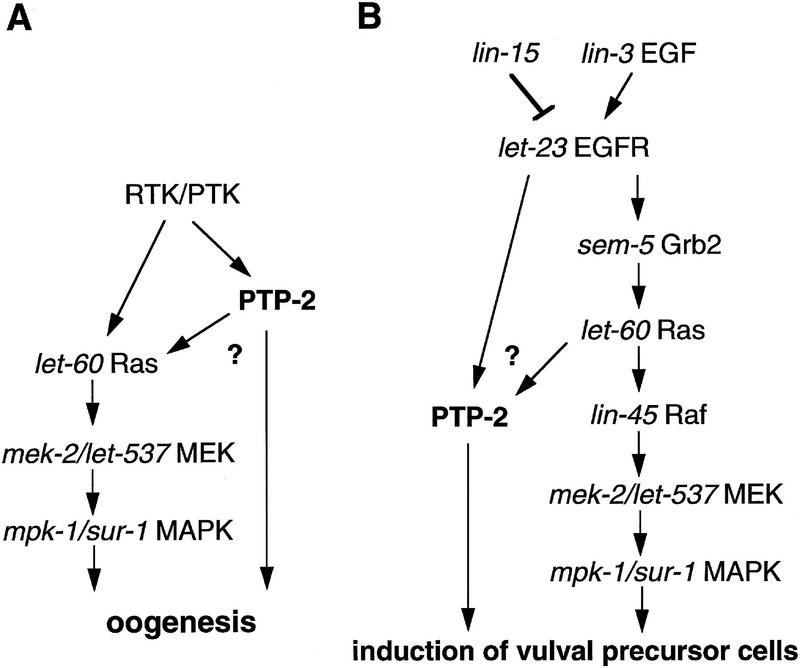
Genetic models for ptp-2 signaling during (A) oogenesis and (B) vulval development. C. elegans genes are in italics, and their mammalian homologs are listed to the right. During oogenesis, PTP-2 likely functions to regulate positively signaling downstream from a yet uncharacterized PTK or R-PTK. PTP-2 may lead directly to the activation of the Ras, MEK, and MAPK cascade, or to the activation of an uncharacterized signaling pathway that is required for proper oogenesis. During vulval development, PTP-2 appears to be acting as a positive regulator of LET-23 signaling, functioning downstream of or in parallel to LET-60 Ras, and in a pathway that cooperates with SEM-5. All genes known to participate in the regulation of vulval induction are not shown, and this model does not intend to imply that ptp-2 does not interact with these genes, but rather that such putative relationships are yet to be defined.
Our genetic analyses with let-60 ras(gf) alleles demonstrated that hyperactivated Ras can rescue completely the zygotic semisterile/maternal effect lethal phenotype of ptp-2(op194). Church et al. (1995) have shown previously that let-60 ras, mek-2/let-537, and mpk-1/sur-1 are required for hermaphrodite fertility, specifically for exit from pachytene arrest in meiosis I. Although ptp-2 is also essential for fertility, the germ-line phenotype that results from ptp-2(op194) differs somewhat from the classic pachytene arrest phenotype, which could indicate that these genes are contributing to the regulation of distinct signaling pathways during oogenesis. Alternatively, as alluded to above, ptp-2(op194) may yield an incomplete pachytene arrest phenotype. Thus, PTP-2 may regulate signals downstream from a R-PTK or PTK, which leads to the activation of LET-60 Ras, MEK-2/LET-537, and MPK-1/SUR-1 and release from pachytene arrest to diakinesis.
With respect to PTP-2 function during vulval development, we have shown that ptp-2(op194) is epistatic to lin-15(lf), let-23(gf), and let-60(gf) mutations. Furthermore, the weak Vul phenotype of sem-5(n1779) was enhanced significantly by ptp-2(op194). On the basis of these results, we propose that PTP-2 functions downstream of LET-23 in the inductive signaling pathway as a positive regulator of signal transduction (Fig. 6B). PTP-2 may act through a direct association with a putative phosphotyrosine-containing motif, pY-T-A-V, in the cytoplasmic tail of LET-23, which is identical to the motif present in the PDGF-β R-PTK that is bound by SHP-2 (Kazlauskas et al. 1993; Lechleider et al. 1993b). Mutagenesis studies have indicated that the pY-T-A-V motif is not essential for normal vulval development (Lesa and Sternberg 1997), which would be consistent with PTP-2 acting through this site, contributing to the “strength” or “intensity” of the inductive signal. It is important to note that the relationship between PTP-2 and LET-60 Ras differs between oogenesis and vulval development. During oogenesis, activated LET-60 Ras rescues the defects arising from the loss of PTP-2, indicating PTP-2 functions upstream of, or in parallel to, LET-60 Ras. However, during vulval development our results indicated that PTP-2 functions in a pathway that likely functions downstream of, or in parallel to, LET-60 Ras.
It is intriguing that PTP-2 functions as an essential signaling molecule during oogenesis, and only as a supporting component during vulval development. This differential requirement for ptp-2 activity may reflect mechanistic differences in PTP-2 function that are governed by the precise upstream PTK or R-PTK, the complement of intracellular phosphotyrosine-containing signaling molecules, or both. Alternatively, this differential requirement may indicate the presence of a redundant signaling pathway within the VPCs, which is not found in the developing oocytes. The identification of PTP-2 substrates should increase our understanding of SH2 domain-containing PTPs and their contributions to aspects of cell signaling, differentiation, and development.
Materials and methods
General methods and strains
All nematode strains were maintained and manipulated using standard procedures at 20°C (Brenner 1974). Wild-type C. elegans corresponds to Bristol strain N2. All alleles not described here are described in Riddle et al. (1997). The following alleles were used in this study: (LGI) mut-2(r459); (LGII) mC6 (generous gift of M. Edgley and D.L. Riddle, University of Missouri, Columbia), maDf4, clr-1(e1745), ptp-2(op193::Tc1) (this study), ptp-2(op194) (this study), dpy-10(e128), unc-104(e1265), let-23(sa62) (generously provided by P.W. Sternberg, Caltech, Pasadena, CA), unc-4(e120); (LGIII) dpy-19(n1347); (LGIV) lin-1(e1275), let-60(n1046, n1700, n2021); (LGV) him-5(e1490); (LGX) sem-5(n1779), lin-15(n765, n309).
Identification and cloning of ptp-2
To isolate C. elegans PTPs, degenerate PCR primers were designed based on the KCAQYWP and HCSAGIG motifs present in all PTP catalytic domains. The primers [nucleotides in parentheses represent degeneracy at that position (I = inosine)] 5′-ggatccAA(GA)TG(TC)GC(GATC)CA(GA)TA(TC)TGGCC and 5′-gaattcCC(GTA)ATICC(GATC)GC(AG)CT(AG)CA(AG)TG were used in a PCR reaction with 300 ng of wild-type (N2) genomic DNA. Fifty-two PCR-amplified inserts were subcloned and sequenced using standard techniques with one clone, ce17, pursued further (Sambrook et al. 1989). Independently, the C. elegans genome sequencing project identified a putative SH2 domain-containing PTP on cosmid F59G1 (Waterston et al. 1993), which was confirmed to contain the sequence of the PCR-amplified fragment ce17. To isolate the fulllength ptp-2 cDNA, the oligonucleotides 5′-GAATTGCTCAAAGAGTATGGCG and 5′-GCGGAAATTACGAGAGCTCCTGG were used to amplify by PCR a 504-bp fragment from the cosmid F59G1. The PCR-amplified product was used to screen a C. elegans λZAP mixed-stage cDNA library (gift of R. Barstead, Oklahoma Medical Research Foundation, Oklahoma City) using standard techniques (Sambrook et al. 1989). Approximately 300,000 plaques were screened and 20 positively hybridizing plaques were picked. pBluescript SK phagemid vectors containing the cDNA inserts were isolated subsequently (Stratagene). The insert ends of 13 of these clones, which appeared to contain identical insertions of 2.8 kb after an EcoRI restriction digest, were sequenced using T3 and T7 primers on an ABI373A sequencer, as suggested by the manufacturer. Complete sequencing of potential full-length clones was subsequently performed with a variety of synthesized oligonucleotide primers.
Identification and isolation of a ptp-2 loss-of-function allele
To generate a loss-of-function mutation in ptp-2, we used the Tc1 transposon technique developed by Zwaal et al. (1993), with minor modifications. Briefly, a frozen library containing 2880 independent cultures of the strain MT3126 was established, and DNA was prepared and pooled from all samples. To identify a Tc1 insertion in ptp-2, we synthesized the nested ptp-2-specific genomic oligonucleotide primer pairs ce17-1/P1:5′-GCAAGTCAACACATACCAGG, ce17-2:5′-CAAGATCCAAGTACTGGAAG, and ce17-3:5′-GAAACACGCGAGTATTGC, ce17-4/P2:5′-CGGTGGCATTCTACCTG. These primers were used in conjunction with the Tc1-specific nested PCR primer pairs to screen the pooled DNA samples from the frozen MT3126 library. After identification of a putative Tc1 insertion in ptp-2 after the third dimension screen, individual worms harboring this insertion were isolated as described by Zwaal et al (1993). After the identification of a single worm that is positive for the Tc1 insertion, homozygosity was determined by selecting 24 self-progeny siblings for single worm PCR, where 24 positives allows for the assumption that the insertion is homozygous.
Because hermaphrodites homozygous for op193::Tc1 were phenotypically wild type, we undertook a second PCR-based screen to identify deletions that arose after transposon excision, as described by Zwaal et al. (1993). The PCR oligonucleotide primers ce17-1/P1 and ce17-4/P2 were used to screen DNA lysates prepared from 64 10-cm plates of homozygous op193::Tc1 hermaphrodites. One lysate contained a strong PCR-amplified signal of ∼1.4 kb, in contrast to the 2.7-kb between 17-1/P1 and 17-4/P2 for wild-type ptp-2 or the 4.2-kb for ptp-2(op193::Tc1). Individual worms carrying the putative deletion (referred to as op194) were isolated as described by Zwaal et al (1993). To eliminate the mut-2 allele on Chr. 1 and additional Tc1 mutations from this strain, hermaphrodites carrying the op194 allele were backcrossed to N2 ten times. The exact boundaries of the transposon-mediated deletion was determined by sequence analysis. The ptp-2(op194) deletion allele contained a 1458-bp deletion, corresponding to nucleotides 2149–3605 of the cosmid sequence F28B12.
Generation of anti-ptp-2 rabbit polyclonal antibodies and Western blot analysis
Two NZW rabbits were immunized with a peptide corresponding to amino acids 650–667 of PTP-2. Before injection, the peptide was coupled to Keyhole Limpet Hemocyanin (Pierce) with SPDP (Pharmacia), and injected subsequently at an approximate peptide concentration of 200 μg per injection per rabbit with complete Freund’s adjuvant to improve the immune response (Harlow and Lane 1988). The immunized rabbits were boosted every 3 weeks with an approximate peptide concentration of 100 μg per injection per rabbit in incomplete Freund’s adjuvant. Before all injections, 10 ml of serum was obtained from each rabbit for preimmune controls.
Protein lysate for immunoblot analysis was prepared by picking 100 N2 or ptp-2(op194) adult hermaphrodites into 100 μl of M9 buffer in a 500-μl eppendorf tube. Worms were washed three times with M9 buffer, with the worms being allowed to settle between each wash. After the last wash, the M9 supernatant was aspirated and 50 μl of 2× SDS/PAGE sample buffer containing 5% β-mercaptoethanol was added to the washed worms and placed at 95°C for 5–10 min. Samples were centrifuged and soluble protein lysates were subjected to 10% SDS-PAGE. The resolved protein lysates were transferred to PVDF (Immobilon) and the blots stained with Ponceau S to confirm equal loading of lysate and efficient transfer to the membrane. Protein blots were blocked with 5% milk/1× TTBS [20 mm Tris (pH 8.0), 150 mm NaCl, 0.2% Tween 20] for 1 hr at room temperature, followed by the addition of preimmune or immune serum to the blots at a dilution of 1:100. The blots were incubated for 4 hrs at room temperature, washed with 1× TTBS, and incubated with a goat anti-rabbit HRP-conjugated monoclonal antibody (Cappel) at a dilution of 1:4000 for 30 min at room temperature. Immunoreactive proteins were detected using ECL (Amersham) followed by exposure to film (Kodak Bio-Max).
Germ-line transformation
Germ-line transformation experiments were performed essentially as described by Mello and Fire (1995). A mixture of a cosmid and the plasmid pRF4, which contains the dominant transformation marker mutation rol-6(su1006) (Kramer et al. 1990), were injected into early-adult hermaphrodites of the phenotype ptp-2(op194) unc-4(e120)/dpy-10(e128) unc-104(e1265) II. The cosmids F59G1, C52G9, and F38A3 were injected at a concentration of 10 ng/μl, F28B12 at 2 ng/μl, and pRF4 at 50 ng/μl. F2 Rol animals containing an array of genomic ptp-2 and pRF4 were designated Ex[ptp-2(+); rol-6(su1006)], and rescue of the maternal effect semisterile/lethal phenotype was defined by the establishment of a fertile line of the genotype ptp-2(op194) unc-4 (e120); Ex[ptp-2(+); rol-6(su1006)].
Genetic analyses
Because the ptp-2(op194) mutation cannot be maintained as a homozygous strain, the ptp-2(op194) mutation was placed in trans to mC6, which balances the left arm of Chr. II. mC6 is a putative intrachromosomal rearrangement, marked with dpy-10(e128). Because mC6 [dpy-10(e128)] II is homozygous viable, ptp-2(op194)/mC6 [dpy-10(e128)] II heterozygous hermaphrodites are maintained by selecting wild-type animals that yield both sterile and Dpy F1 self-progeny.
Double mutants between ptp-2(op194) and other mutations were generated using standard genetic methods. When necessary, the presence of the op194 allele was confirmed by single worm PCR analysis.
To generate ptp-2(op194) unc-4(e120) II; sem-5(n1779) X mutants, ptp-2(op194) unc-4(e120)/mC6 II; him-5(e1490) V males were crossed to clr-1(e1745) II; sem-5(n1779) X hermaphrodites, with wild-type F1 hermaphrodites selected to individual plates. Cross progeny F1 hermaphrodites that generated wild type, Clr, and Ste–Unc were identified, with wild-type F2 hermaphrodites selected to individual plates. F2 hermaphrodites that generated wild-type, Vul, Ste–Unc, but no Clr progeny [sem-5(n1779) suppresses clr-1(e1745)], were selected for analysis and maintained by picking Vul hermaphrodites that generate Ste–Unc self-progeny. The presence of ptp-2(op194) was confirmed by PCR.
To generate the ptp-2(op194) let-23(sa62) unc-4(e120) II recombinant, ptp-2(op194)/let-23(sa62) unc-4(e120) II hermaphrodites (phenotypically wild type) were generated and 100 Muv–Unc self-progeny selected to individual plates. Three Muv–Unc F1 hermaphrodites were identified that generated Muv–Unc, Ste–Muv–Unc, and Ste-Unc F2 self-progeny. Because let-23(sa62) confers a semidominant Muv, we confirmed that ptp-2(op194) recombined with let-23(sa62) unc-4(e120) and not with unc-4(e120), by balancing the recombinant chromosome II over mC6, and being unable to separate a Muv–Unc phenotype [from let-23(sa62) unc-4(e120) II] and a Ste–Unc phenotype [from ptp-2(op194) unc-4(e120) II]. The presence of ptp-2(op194) was confirmed by PCR.
Fertility and brood analysis
To quantitate fertility, individual hermaphrodites were placed onto single plates and observed for the generation of viable self-progeny. If no viable progeny were observed over a 7-day period, the hermaphrodite was classified as sterile. Brood size was determined by placing individual hermaphrodites on single plates, with replating to a new plate every 12–18 hr, followed by counting the eggs and larvae (viable and nonviable) that remained. For let-60(n1700), under our growth conditions the pseudovulvae frequently ruptured lowering the average brood, hence this reduction should not be attributed to abnormal germ lines in these animals. To generate maDf4/ptp-2(op194) unc-4(e120) II hermaphrodites, ptp-2(op194) unc-4(e120)/clr-1(e1745) dpy-10(e128) II males were mated to maDf4/dpy-10(e128) unc-104(e1265) II hermaphrodites, and all wild-type hermaphrodites cloned to individual plates. From two independent crosses, 22 maDf4/ptp-2(op194) unc-4(e120) II and 22 ptp-2(op194) unc-4(e120)/dpy-10(e128) unc-104(e1265) II cross-progeny hermaphrodites were identified, indicating no enhanced zygotic lethality is associated with ptp-2(op194) in trans to maDf4.
Analysis of vulval development
To assess effects of the ptp-2(op194) mutation on the Muv phenotype, eggs and L1 larvae from the selected strains were transferred individually to new plates (∼10/plate), and 48–72 hr later examined by dissecting microscope. For the VPC induction analysis, L3/L4 hermaphrodites were placed onto 2% agarose pads (∼10/pad) in a small amount of 10 mm sodium azide to anesthetize the animals. Induction of the VPCs was examined using Nomarski optics on a Zeiss axioplan microscope at 1000× magnification. Hermaphrodites selected for VPC lineage analysis were prepared as described in Sulston and Horvitz (1977) except that nail hardener was used to seal the coverslip to prevent dehydration. Hermaphrodites in the third larval stage were selected for analysis, and animals with VPCs at the four-cell stage or slightly beyond (some divisions in progress) lineaged using Nomarski optics on a Zeiss axioplan microscope at 1000× magnification. VPC fates were classified according to Sternberg and Horvitz (1989) and Katz et al. (1995).
Acknowledgments
We thank M. Daddario for sequence analysis, J. Duffy for artwork, R. Barstead for the cDNA library, A. Coulson and colleagues at the Sanger Center for cosmids and sequence information regarding unfinished regions of Chr. II, M. Edgley and D.L. Riddle for sending the mC6 balancer for chromosome II. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. We also thank T. Tiganis and A.M. Bennett for critical reading of the manuscript, as well as P.W. Sternberg and S.G. Clark for interesting discussions on unpublished work. This work was supported by grants to M.O.H. (NIH GM-54520 and generous support of the Donaldson Charitable Trust) and to N.K.T. (NIH CA53840 and GM-55989). M.J.G. was supported by a National Cancer Institute (NCI) post-doctoral training grant. M.O.H. is a Rita Allen Foundation Scholar.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Note added in proof
The nucleotide sequence data reported in this paper have been deposited in the GenBank data library under accession number AF015882.
Footnotes
E-MAIL tonks@cshl.org; FAX (516) 367-6812.
References
- Ahmad S, Banville D, Zhao Z, Fischer EH, Shen SH. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci. 1993;90:2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JD, Chang HC, Herbst R, McNeill H, Simon MA. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- Aroian RV, Sternberg PW. Multiple functions of let-23, a Caenorhabditis elegans receptor tyrosine kinase gene required for vulval induction. Genetics. 1991;128:251–267. doi: 10.1093/genetics/128.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroian RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- Aroian RV, Lesa GM, Sternberg PW. Mutations in the Caenorhabditis elegans let-23 EGFR-like gene define elements important for cell-type specificity and function. EMBO J. 1994;13:360–366. doi: 10.1002/j.1460-2075.1994.tb06269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Rubin GM. Effect on eye development of dominant mutations in Drosophila homolog of the EGF receptor. Nature. 1989;340:150–153. doi: 10.1038/340150a0. [DOI] [PubMed] [Google Scholar]
- Beitel GJ, Clark SG, Horvitz HR. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature. 1990;348:503–509. doi: 10.1038/348503a0. [DOI] [PubMed] [Google Scholar]
- Beitel GJ, Tuck S, Greenwald I, Horvitz HR. The Caenorhabditis elegans gene lin-1 encodes an ETS-domain protein and defines a branch of the vulval induction pathway. Genes & Dev. 1995;9:3149–3162. doi: 10.1101/gad.9.24.3149. [DOI] [PubMed] [Google Scholar]
- Bennett AM, Hausdorff SF, Oreilly AM, Freeman RM, Neel BG. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T, Steward K. RNA processing and gene structure. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 117–145. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HE, Chang S, Trub T, Neel BG. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996;16:3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Clark SG, Lu X, Horvitz HR. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics. 1994;137:987–997. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J, Saari B, Anderson P. Activation of a transposable element in the germ line but not the soma of Caenorhabditis elegans. Nature. 1987;328:726–728. doi: 10.1038/328726a0. [DOI] [PubMed] [Google Scholar]
- DeVore DL, Horvitz HR, Stern MJ. An FGF receptor signaling pathway is required for the normal cell migrations of the sex myoblasts in C. elegans hermaphrodites. Cell. 1995;83:611–620. doi: 10.1016/0092-8674(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Freeman RM, Jr, Plutzky J, Neel BG. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Herman RK, Hedgecock EM. Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature. 1990;348:169–171. doi: 10.1038/348169a0. [DOI] [PubMed] [Google Scholar]
- Hill RJ, Sternberg PW. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992;358:470–476. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- Huang LS, Tzou P, Sternberg PW. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol Biol Cell. 1994;5:395–411. doi: 10.1091/mbc.5.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz WS, Hill RJ, Clandinin TR, Sternberg PW. Different levels of the C. elegans growth factor LIN-3 promote distinct vulval precursor fates. Cell. 1995;82:297–307. doi: 10.1016/0092-8674(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Katz WS, Lesa GM, Yannoukakos D, Clandinin TR, Schlessinger J, Sternberg PW. A point mutation in the extracellular domain activates LET-23, the Caenorhabditis elegans epidermal growth factor receptor homolog. Mol Cell Biol. 1996;16:529–537. doi: 10.1128/mcb.16.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A, Feng GS, Pawson T, Valius M. The 64-kDa protein that associates with the platelet-derived growth factor receptor beta subunit via Tyr-1009 is the SH2-containing phosphotyrosine phosphatase Syp. Proc Natl Acad Sci. 1993;90:6939–6943. doi: 10.1073/pnas.90.15.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld K. Vulval development in Caenorhabditis elegans. Trends Genet. 1997;13:55–61. doi: 10.1016/s0168-9525(97)01005-6. [DOI] [PubMed] [Google Scholar]
- Kramer JM, French RP, Park EC, Johnson JJ. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990;10:2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne MR, Pawson T, Lienhard GE, Feng GS. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- Lechleider RJ, Freeman RM, Jr, Neel BG. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homolog, SH-PTP2. J Biol Chem. 1993a;268:13434–13438. [PubMed] [Google Scholar]
- Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE, Walsh CT, Neel BG. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J Biol Chem. 1993b;268:21478–21481. [PubMed] [Google Scholar]
- Lesa GM, Sternberg PW. Positive and negative tissue-specific signaling by a nematode epidermal growth factor receptor. Mol Biol Cell. 1997;8:779–793. doi: 10.1091/mbc.8.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz U, Bergemann AD, Steinberg HN, Flanagan JG, Li X, Galli SJ, Neel BG. Genetic analysis reveals cell type-specific regulation of receptor tyrosine kinase c-Kit by the protein tyrosine phosphatase SHP1. J Exp Med. 1996;184:1111–1126. doi: 10.1084/jem.184.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chou TB, Williams NG, Roberts T, Perrimon N. Control of cell fate determination by p21ras/Ras1, an essential component of torso signaling in Drosophila. Genes & Dev. 1993;7:621–632. doi: 10.1101/gad.7.4.621. [DOI] [PubMed] [Google Scholar]
- Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, Besmer P, Bachvarova RF. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol. 1993;157:85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Milarski KL, Saltiel AR. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- Miller LM, Gallegos ME, Morisseau BA, Kim SK. lin-31, a Caenorhabditis elegans HNF-3/fork head transcription factor homolog, specifies three alternative cell fates in vulval development. Genes & Dev. 1993;7:933–947. doi: 10.1101/gad.7.6.933. [DOI] [PubMed] [Google Scholar]
- Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- Paulson RF, Vesely S, Siminovitch KA, Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase Shp1. Nature Genet. 1996;13:309–315. doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Larsen I, Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Plasterk RH. Reverse genetics: From gene sequence to mutant worm. Methods Cell Biol. 1995;48:59–80. doi: 10.1016/s0091-679x(08)61383-7. [DOI] [PubMed] [Google Scholar]
- Plutzky J, Neel BG, Rosenberg RD. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng G-S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T. Developmental genetics of the germ line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 241–270. [PubMed] [Google Scholar]
- Shen SH, Bastien L, Posner BI, Chretien P. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature. 1991;352:736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Simon MA, Carthew RW, Fortini ME, Gaul U, Mardon G, Rubin GM. Signal transduction pathway initiated by activation of the sevenless tyrosine kinase receptor. Cold Spring Harbor Symp Quant Biol. 1992;57:375–380. doi: 10.1101/sqb.1992.057.01.042. [DOI] [PubMed] [Google Scholar]
- Singh N, Han M. sur-2, a novel gene, functions late in the let-60 ras-mediated signaling pathway during Caenorhabditis elegans vulval induction. Genes & Dev. 1995;9:2251–2265. doi: 10.1101/gad.9.18.2251. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR. The combined action of two intercellular signaling pathways specifies three cell fates during vulval induction in C. elegans. Cell. 1989;58:679–693. doi: 10.1016/0092-8674(89)90103-7. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Tang TL, Freeman RM, Jr, O’Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- Tuck S, Greenwald I. lin-25, a gene required for vulval induction in Caenorhabditis elegans. Genes & Dev. 1995;9:341–357. doi: 10.1101/gad.9.3.341. [DOI] [PubMed] [Google Scholar]
- Waterston R, Ainscough R, Anderson K, Berks M, Blair D, Connell M, Cooper J, Coulson A, Craxton M, Dear S, et al. The genome of the nematode Caenorhabditis elegans. Cold Spring Harbor Symp Quant Biol. 1993;58:367–376. doi: 10.1101/sqb.1993.058.01.043. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Milarski KL, Saltiel AR, Pessin JE. Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc Natl Acad Sci. 1995;92:664–668. doi: 10.1073/pnas.92.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RR, Broeks A, van Meurs J, Groenen JT, Plasterk RH. Target-selected gene inactivation in Caenorhabditis elegans by using a frozen transposon insertion mutant bank. Proc Natl Acad Sci. 1993;90:7431–7435. doi: 10.1073/pnas.90.16.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]