Abstract
We studied the antimicrobial activity of fosfomycin against 960 strains of commonly encountered bacteria associated with urinary tract infection using standard agar dilution and disk diffusion methods. Species studied included 3 common species of Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia; methicillin-susceptible and -resistant Staphylococcus aureus; and vancomycin-susceptible and resistant Enterococcus faecalis and E. faecium. MICs and inhibition zone diameters were interpreted in accordance with both the currently recommended Clinical and Laboratory Standards Institute (CLSI) criteria for urinary tract isolates of Escherichia coli and Enterococcus faecalis and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria for Enterobacteriaceae. Tentative zone diameter interpretive criteria were developed for species not currently published by CLSI or EUCAST. Escherichia coli was uniformly susceptible to fosfomycin, as were most strains of Klebsiella pneumoniae and Enterobacter cloacae. A. baumannii was resistant to fosfomycin, while the prevalence of resistance in P. aeruginosa and S. maltophilia was greatly affected by the choice of MIC breakpoint. New tentative zone diameter criteria for K. pneumoniae, E. cloacae, S. aureus, and E. faecium were able to be set, providing some interim laboratory guidance for disk diffusion until further breakpoint evaluations are undertaken by CLSI and EUCAST.
INTRODUCTION
Fosfomycin tromethamine is a phosphonic acid antibacterial agent that inhibits bacterial cell wall formation by interfering with peptidoglycan synthesis (3, 33). This agent is indicated for single-dose treatment of uncomplicated urinary tract infection due to Escherichia coli and Enterococcus faecalis in women (1, 19). Many studies have reported high fosfomycin susceptibility rates for these two urinary pathogens (2, 20, 30, 34), and its treatment effect is comparable to the effects of other antimicrobial agents (1, 10, 32).
In recent years, the rapid emergence and spread of antibiotic resistance among commonly encountered bacteria causing a variety of clinical infections, especially in intensive care units and long-term care facilities, have been impressive (25, 34, 37). Moreover, infections caused by these multidrug-resistant (MDR) bacteria contributed to higher mortality rates in these facilities (34). Due to the low rate of introduction of new antibiotics effective against these MDR pathogens, old antibiotic agents, such as fosfomycin and the polymyxins, are now being considered potential treatment alternatives (17–19). Several nonrandomized and observational studies have demonstrated that fosfomycin is a promising agent, particularly in combination with other agents, for the treatment of various infections due to MDR Gram-positive and Gram-negative bacteria (17–19). However, there are limited studies related to the in vitro activities of fosfomycin against these commonly encountered bacteria, except for E. coli and E. faecalis isolates from the urinary tract (2, 11, 12, 17, 19, 21, 22, 28). The majority of clinical use of fosfomycin is based on the reported in vitro activity against the isolated pathogen of this agent determined by applying MIC criteria described by the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards [NCCLS]) (7) for E. coli and E. faecalis isolates. Interpretive criteria for Enterobacteriaceae are also available from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (14). Furthermore, the only recommended disk diffusion criteria for fosfomycin are those described by CLSI for E. coli and E. faecalis isolates from urine. Nevertheless, the disk diffusion susceptibility method is still widely used in most Asian countries, including Taiwan.
We report the in vitro activity of fosfomycin against nine commonly encountered bacterial species determined using the agar dilution and disk diffusion methods and an evaluation of the correlation between these two methods performed using methods described by the CLSI (8). Tentative disk diffusion resistant and susceptible zone diameter breakpoints are proposed on the basis of the current MIC interpretive criteria recommended by the CLSI and EUCAST (7, 14).
MATERIALS AND METHODS
Bacterial isolates.
A total of 960 consecutive nonduplicate isolates of commonly encountered bacterial species recovered from various clinical specimens taken from patients treated at the National Taiwan University Hospital were studied. The isolates included 100 isolates of each species or type, including methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Pseudomonas aeruginosa, Acinetobacter baumannii, vancomycin-susceptible Enterococcus spp. (50 isolates of E. faecalis and 50 isolates of E. faecium), and Stenotrophomonas maltophilia, collected from January 2008 to December 2008. Sixty isolates of vancomycin-resistant enterococci (VRE) (30 isolates of E. faecalis and 30 isolates of E. faecium) were collected from January 2007 to December 2008.
All isolates were identified by conventional methods. Gram-negative bacteria were further confirmed by means of the API 20NE system (bioMérieux, Marcy l'Etoile, France) and the GNI system (Vitek systems; bioMérieux Vitek, Hazelwood, MO). All isolates were stored at −70°C in tryptic soy broth (Difco Laboratories, Detroit, MI) with 15% glycerol until they were tested against fosfomycin.
Antimicrobial susceptibility testing.
The susceptibilities of all isolates to fosfomycin (Sigma Chemical Co., St. Louis, MO) were determined concomitantly by the agar dilution and disk diffusion methods described by the CLSI (5–7). The inoculated plates were incubated in ambient air at 35°C for 16 to 18 h. For susceptibility testing by the agar dilution method, Mueller-Hinton agar (BBL Microbiology Systems, Cockeysville, MD) supplemented with 25 μg/ml of glucose-6-phosphate was used. The MIC of each antimicrobial agent was defined as the lowest concentration that inhibited visible growth of the organism. Control strains, including S. aureus ATCC 29213, E. coli ATCC 25922, and P. aeruginosa ATCC 27853, were included in each set of tests.
For testing of susceptibility to fosfomycin by the disk diffusion method (5), Mueller-Hinton agar (BBL Microbiology Systems) was used. Fosfomycin disks (200 μg) containing 50 μg of glucose-6-phosphate were used (BBL Microbiology Systems). S. aureus ATCC 25923, E. coli ATCC 25922, and P. aeruginosa ATCC 27853 were used as control strains. The susceptibility testing of each drug for each isolate was performed twice under the same conditions on the same day. Plates were read, and the mean of duplicate zone diameters of each drug for each isolate were determined after overnight incubation at 35°C in ambient air.
Interpretation of susceptibility results.
Epidemiological cutoff values (ECVs) of fosfomycin were calculated statistically as previously described (39). Interpretive criteria for susceptibility categories by MIC were applied using both the CLSI interpretive criteria for urinary tract isolates of E. coli and E. faecalis (7) and the EUCAST interpretive criteria for all isolates of Enterobacteriaceae (12) (Table 1).
Table 1.
Interpretive criteria of fosfomycin recommended by CLSI and EUCAST
| Standard and organism | MIC (μg/ml) for the following interpretive criteria: |
Resistance and susceptibility zone diam breakpoints (mm) for the following interpretive criteria: |
||||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| CLSI | ||||||
| Escherichia coli (urinary tract isolates only) | ≤64 | 128 | ≥256 | ≥16 | 13–15 | ≤12 |
| Enterococcus faecalis (urinary tract isolates only) | ≤64 | 128 | ≥256 | ≥16 | 13–15 | ≤12 |
| EUCAST | ||||||
| Enterobacteriaceae i.v.a | ≤32 | >32 | NAb | NA | NA | |
| Enterobacteriaceae (fosfomycin trometamol, uncomplicated UTIc only) | ≤32 | >32 | NA | NA | NA | |
| Pseudomonas species i.v.d | ≤32 | >32 | NA | NA | NA | |
| Staphylococcus species | ≤32 | >32 | NA | NA | NA | |
i.v., intravenous.
NA, not available.
UTI, urinary tract infection.
Intravenous fosfomycin may be used in combination with other antibiotics to treat P. aeruginosa infections.
Zone diameter analysis.
Tentative inhibition zone diameter interpretive criteria were developed using the error-rate-bounded methods recommended by the CLSI (7). For the tested species of Enterobacteriaceae, zone diameter criteria were analyzed both for pooled data and for separate species.
RESULTS
MIC distributions.
The MIC distributions of the 960 isolates are given in Table 2. Fosfomycin was highly active against E. coli, although a small number of strains appeared to have MICs that were about the calculated wild-type ECV of 1 μg/ml. This MIC distribution differed markedly from that published by EUCAST on its website (http://www.srga.org/eucastwt/WT_EUCAST.htm, accessed 14 February 2011). We found a modal value of 0.5 μg/ml, while the EUCAST website modal MIC is 4 μg/ml and has a greater spread of MICs and an ECV of 32 μg/ml. The reasons for this difference are not clear.
Table 2.
MIC distributions, epidemiological cutoff values, and susceptibility rates of the species examined
| Species | Subgroupa | No. of isolates with the following MIC (μg/ml): |
ECVb (μg/ml) | % isolates with MICless than or equal to current breakpointc |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | >512 | CLSI | EUCAST | |||
| Escherichia coli | 100 | 15 | 70 | 6 | 5 | 2 | 2 | 1 | 100 | 100 | ||||||||
| Klebsiella pneumoniae | 100 | 1 | 12 | 45 | 19 | 8 | 7 | 2 | 5 | 1 | 16 | 92 | 85 | |||||
| Enterobacter cloacae | 100 | 6 | 6 | 3 | 6 | 16 | 18 | 17 | 13 | 8 | 6 | 1 | 512 | 85 | 72 | |||
| Enterococcus faecalis | Vans | 50 | 7 | 37 | 5 | 1 | 64 | 99 | 94 | |||||||||
| Vanr | 30 | 1 | 28 | 1 | 64 | 100 | 96.7 | |||||||||||
| Combined | 80 | 8 | 65 | 6 | 1 | 64 | 98.8 | 91.3 | ||||||||||
| Enterococcus faecium | Vans | 50 | 1 | 11 | 33 | 5 | 128 | 95 | 62 | |||||||||
| Vanr | 30 | 8 | 18 | 4 | 128 | 86.7 | 26.7 | |||||||||||
| Combined | 80 | 1 | 19 | 51 | 9 | 128 | 88.8 | 25.0 | ||||||||||
| Staphylococcus aureus | Meths | 100 | 20 | 46 | 20 | 8 | 4 | 2 | 4 | 100 | 100 | |||||||
| Methr | 100 | 12 | 32 | 5 | 27 | 9 | 2 | 2 | 1 | 10 | 2 | 89 | 89 | |||||
| Combined | 200 | 32 | 78 | 25 | 35 | 9 | 6 | 4 | 1 | 10 | 2 | 94.5 | 94.5 | |||||
| Acinetobacter baumannii | 100 | 3 | 68 | 29 | 256 | 3 | 0 | |||||||||||
| Pseudomonas aeruginosa | 100 | 4 | 4 | 1 | 4 | 16 | 51 | 15 | 2 | 3 | 256 | 80 | 29 | |||||
| Stenotrophomonas maltophilia | 100 | 1 | 1 | 58 | 32 | 7 | 1 | 128 | 59 | 1 | ||||||||
Vans, vancomycin susceptible; Vanr, vancomycin resistant; Meths, methicillin susceptible; Methr, methicillin resistant.
Epidemiological (wild-type) cutoff values calculated using the statistical method (29).
The CLSI clinical breakpoint for E. coli and E. faecalis from the urinary tract is ≤64 μg/ml. The EUCAST breakpoint for Enterobacteriaceae is ≤32 μg/ml.
The activity of fosfomycin against K. pneumoniae and E. cloacae was lower than that against E. coli. Some strains (23%) of K. pneumoniae had MICs above the calculated wild-type value. Even so, 92% of K. pneumoniae isolates and 85% of E. cloacae isolates had MICs of less than or equal to 64 μg/ml, the current CLSI clinical breakpoint for susceptibility in E. coli. However, the wide range of MICs observed with E. cloacae resulted in a very high calculated ECV.
E. faecium had slightly higher MIC values than E. faecalis, and almost no strains appeared to have MICs above the calculated ECVs for those two species. Fosfomycin was equally active against vancomycin-susceptible and -resistant strains. Modal MICs (32 to 64 μg/ml) were higher than those observed for Enterobacteriaceae.
Fosfomycin showed good activity against S. aureus, with a modal MIC of 1 μg/ml against both methicillin-susceptible and -resistant strains. The MIC distribution for methicillin-resistant strains was trimodal, resulting in a paradoxically lower calculated ECV for methicillin-resistant strains than for methicillin-susceptible strains. Ten percent of methicillin-resistant strains had MICs well above those for the other strains and greater than 512 μg/ml.
The modal MICs for A. baumannii, P. aeruginosa, and S. maltophilia were high at 128, 64, and 64 μg/ml, respectively. Calculated ECVs were consequently very high.
Resistance rates.
Putative rates of susceptibility based on the currently available MIC interpretive criteria from CLSI and EUCAST are included in Table 2. These rates highlight the critical difference between the two breakpoints when they are applied to E. faecium, P. aeruginosa, and S. maltophilia, whose wild-type MIC distributions tend to straddle the two susceptible breakpoints. A. baumannii appears to be naturally resistant using either set of breakpoints, as noted previously by others (15).
Zone diameter interpretive criteria.
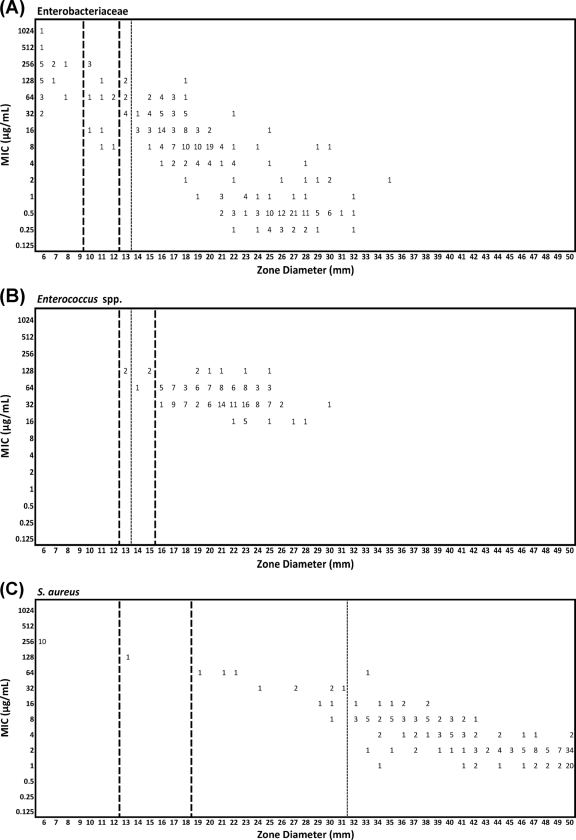
The tentative zone diameter breakpoints for most of the species tested were estimated using error-rate-bounded methods (Table 3). Figure 1 shows the scattergrams of MIC versus zone diameters for three organism groups (Enterobacteriaceae, Enterococcus species, and S. aureus), including proposed interpretive criteria recommended by the CLSI and the EUCAST. Using the currently listed MIC breakpoints from either standard, it was not possible to establish interpretive criteria for either A. baumannii or S. maltophilia effectively. The lower EUCAST breakpoint also meant that zone diameter interpretive criteria could not be set for P. aeruginosa using that standard.
Table 3.
Proposed resistance and susceptibility zone diameter breakpoints using the CLSI and EUCAST MIC breakpoints for urinary isolates of E. coli and Enterococcus faecalis
| Standard and species | Option | Diam (mm) for the following interpretive criteriad: |
Error rate (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| S≥ | I | R≤ | Major | Very major | Minor | Total | ||
| CLSI | ||||||||
| Enterobacteriaceaea | Current | 16 | 13–15 | 12 | 4 | 0 | 9 | 13.7 |
| Alternative | 13 | 10–12 | 9 | 2 | 0 | 5 | 8 | |
| E. coli | Current | 16 | 13–15 | 12 | 0 | 0 | 0 | 0 |
| Alternative | 13 | 10–12 | 9 | 0 | 0 | 0 | 0 | |
| K. pneumoniae | Current | 16 | 13–15 | 12 | 4 | 0 | 2 | 6 |
| Alternative | 13 | 10–12 | 9 | 3 | 0 | 2 | 5 | |
| E. cloacae | Current | 16 | 13–15 | 12 | 11 | 0 | 22 | 33 |
| Alternative | 13 | 10–12 | 9 | 6 | 0 | 16 | 19 | |
| Enterococcus spp. | Current | 16 | 13–15 | 12 | 0 | 0 | 4.4 | 4.4 |
| S. aureus | Current | 16 | 13–15 | 12 | 0 | 0 | 0 | 0 |
| Alternative | 19 | 13–18 | 12 | 0 | 0 | 0 | 0 | |
| P. aeruginosa | Current | 16 | 13–15 | 12 | 10 | 0 | 41 | 51 |
| Alternative | 13 | 10–12 | 9 | 2 | 0 | 17 | 19 | |
| EUCAST | ||||||||
| Enterobacteriaceaea | 2 breakpoints | 14 | 14 | 3.3 | 3.7 | 7 | ||
| 3 breakpoints | 14 | 11–13 | 11 | 1 | 3.7 | 5 | 9.7 | |
| E. coli | 2 breakpoints | 14 | 14 | 0 | 0 | 0 | 0 | |
| Alternativeb | 17 | 17 | 0 | 0 | 0 | 0 | ||
| K. pneumoniae | 2 breakpoints | 14 | 14 | 1 | 5 | 6 | ||
| 3 breakpoints | 16 | 14–15 | 14 | 1 | 3 | 9 | 13 | |
| E. cloacae | 2 breakpoints | 14 | 14 | 9 | 6 | 15 | ||
| 3 breakpoints | 14 | 12–13 | 12 | 3 | 6 | 17 | 26 | |
| E. faecalisc | 2 breakpoints | 14 | 14 | 0 | 4.4 | 4.4 | ||
| S. aureus | 2 breakpoints | 32 | 32 | 3 | 1 | 4 | ||
| 3 breakpoints | 32 | 29–31 | 29 | 0 | 1.0 | 6.0 | 7.0 | |
Includes E. coli, K. pneumoniae, and E. cloacae only.
Alternative with only 2 breakpoints, as no resistant strains were detected.
High rates of resistance in wild-type E. faecium precluded their inclusion.
S≥, diameter greater than or equal to the breakpoint for susceptibility; I, intermediate diameter; ≤R, diameter greater than or equal to the breakpoint for resistance.
Fig. 1.
Scattergrams of MICs versus zone diameters for three organism groups, including proposed interpretive criteria recommended by the CLSI and EUCAST. (A) Enterobacteriaceae (n = 300); (B) Enterococcus species (n = 160); (C) S. aureus (n = 200). Heavy dashed lines, proposed breakpoints using CLSI susceptible, intermediate, and resistant interpretive criteria; dashed line, proposed breakpoint using EUCAST susceptible and resistant interpretive criteria only.
The tentative zone diameter criteria using the CLSI MIC breakpoints were analyzed in two different ways. First, the currently listed zone diameter criteria (susceptible, ≤16 mm; intermediate, 13 to 15 mm; resistant, ≤12 mm) were applied to all species that could be analyzed. Second, alternative criteria that minimized the error rates while remaining practical for laboratory use were developed.
In the application of EUCAST MIC breakpoints, essentially a single value separating susceptible from nonsusceptible, zone diameter interpretive criteria were developed using two breakpoints (susceptible and resistant), one for susceptible and one for resistant, and three breakpoints (susceptible, intermediate, and resistant), which included an intermediate category. The exceptions to this were (i) E. coli, where no resistance was detected with the MIC breakpoint and instead two alternatives were proposed, and (ii) E. faecalis and E. faecium combined, where the MIC distributions required the use of three breakpoints.
DISCUSSION
Renewed interest in the therapeutic potential of fosfomycin for the treatment of MDR pathogens has brought a range of recent studies on its in vitro activity (12, 13, 15, 23, 24, 26, 36, 40, 41). The CLSI breakpoints have been the ones most widely applied in these studies, although the problem of which breakpoints are most appropriate has been highlighted (15) and remains. In the current M100 standard (7), CLSI MIC and zone diameter breakpoints are restricted to urinary tract isolates of E. coli and E. faecalis, while the current EUCAST breakpoints are for MIC values only but apply to isolates of Enterobacteriaceae from all sites (in theory). The documents describing the rationale for the selection of CLSI and EUCAST breakpoints have not yet been published. Besides the CLSI and EUCAST breakpoints, other breakpoints are extant: those of the British Society for Antimicrobial Chemotherapy (BSAC) (4) and the Comité de l'Antibiogramme de la Société Française de Microbiologie (CA-SFM) (9). In the former method, the medium used is Iso-Sensitest, not Mueller-Hinton, so the BSAC breakpoints may be different for that reason. The CA-SFM method does use Mueller-Hinton medium and CA-SFM appears to have applied the EUCAST MIC breakpoint but uses a lower-strength fosfomycin disk (50 μg) for disk diffusion testing, so the CA-SFM zone diameter breakpoints are not applicable to our study. The CA-SFM breakpoints do extend the EUCAST MIC breakpoints, however, beyond Enterobacteriaceae to include P. aeruginosa, Staphylococcus spp., and Streptococcus pneumoniae.
Our results demonstrate that fosfomycin is very active against S. aureus, including most of the large number of methicillin-resistant strains tested, although clearly resistant strains of MRSA (MICs > 512 μg/ml) were noted. These findings are consistent with those presented in previous publications (19). Fosfomycin was equally active against vancomycin-susceptible and vancomycin-resistant strains of both species of Enterococcus tested. Activity against vancomycin-resistant E. faecalis has previously been shown (19, 36). However, fosfomycin was less active against E. faecium than E. faecalis, resulting in a proportion of wild-type E. faecium isolates testing intermediate using the CLSI breakpoints and resistant using the EUCAST breakpoints. This has only a small influence on the ability to set zone diameter breakpoints using CLSI MIC breakpoint criteria and the combined data for the two species, but it resulted in the inability to include E. faecium in the zone diameter breakpoint setting using the EUCAST MIC criteria. Hence, we propose tentative zone diameter interpretive criteria based on correlation with EUCAST MIC interpretive criteria for E. faecalis only.
The wild-type MIC distribution of K. pneumoniae was some 32-fold higher than that of E. coli. Nevertheless, the calculated ECV was lower than either the CLSI or EUCAST susceptible breakpoint. Fosfomycin certainly appears to have considerable potential for treatment of MDR strains of this species, as also suggested by the findings of several other groups (11, 13, 16, 18, 23, 26, 40). The broad spread of the presumed wild-type distribution of E. cloacae was not expected and resulted in a high calculated ECV. Nevertheless, more than 70% had MICs below or at the EUCAST susceptible breakpoint and more than 80% had MICs below or at the CLSI susceptible breakpoint, and tentative zone diameter criteria were therefore able to be developed. By way of comparison, Marchese et al. reported that 60% of E. cloacae isolates were susceptible to fosfomycin (27). For E. cloacae isolates, a high major error rate (11%) was found for correlation of the disk diffusion method using the currently published CLSI zone diameter and MIC interpretive criteria. Our alternative proposal for zone diameter interpretive criteria was still associated with a high major error rate (6%), but that was the lowest rate that could be achieved.
Because we included only three major species of Enterobacteriaceae, albeit ones that are frequently multiresistant, we chose to develop tentative zone diameter interpretive criteria for both the pooled and separate species. However, we would advocate the use of the species-specific zone diameter interpretive criteria for E. coli, K. pneumoniae, and E. cloacae until further species can be added to the pool of data for Enterobacteriaceae.
We found that fosfomycin had no useful activity against A. baumannii but that a significant proportion of wild-type P. aeruginosa isolates have MICs below the CLSI susceptible breakpoint. However, the MIC distribution of P. aeruginosa species straddles the EUCAST susceptible breakpoint. Further work, including clinical studies, is needed to determine if wild-type P. aeruginosa strains are truly susceptible to fosfomycin (35). The MIC distribution of S. maltophilia is essentially above the EUCAST susceptible breakpoint and also straddles the CLSI susceptible breakpoint, calling into question whether this species will be truly susceptible in vivo.
Until further data are available, particularly data on the pharmacodynamics and target attainment rates of fosfomycin, it is likely that CLSI and EUCAST will not be able to reevaluate the breakpoint criteria for fosfomycin effectively. In the meantime, laboratories wishing to test strains from infections that clinicians may wish to treat with fosfomycin must default to the use of the currently published breakpoints while being aware of their limitations. We have attempted to provide some interim criteria for disk diffusion by using and extrapolating from the currently published MIC interpretive criteria of CLSI and EUCAST. The criteria may assist laboratories until such time as new interpretive criteria are established by the standards-setting bodies.
MIC results represent only in vitro susceptibility data for these commonly encountered pathogens. Because our current knowledge of the pharmacodynamics of fosfomycin is so limited, responses to fosfomycin in vivo could be different from those predicted by current interpretive criteria. Responses could also vary by tissue and through synergism with other frequently coadministered antimicrobial agents (28, 31, 32, 38). While fosfomycin is an alternative treatment choice for infections caused by multidrug-resistant pathogens, combination therapy is usually recommended when the fosfomycin MIC values are higher and because there is a tendency for resistance to develop during treatment with fosfomycin alone (15).
There are two main limitations of this study. First, isolates enrolled in this study were collected from only a single center from Taiwan, and the interpretive criteria, if they are to be adopted by other centers, should be considered tentative. It will be necessary to expand this work to other centers and geographic regions before formal breakpoint analysis is conducted. Second, the mechanisms of resistance to fosfomycin were not determined for these isolates. In developing interpretive criteria, it is useful to include isolates with known mechanisms of resistance to help in establishing the breakpoint categories.
In conclusion, our data suggested good in vitro activity of fosfomycin against MSSA, MRSA, vancomycin-susceptible enterococci, VRE, E. coli, and K. pneumoniae isolates. In addition, it appears to have useful activity against E. cloacae and possibly P. aeruginosa. Furthermore, the disk diffusion test can be considered an alternative method to determine the fosfomycin susceptibility of these species, depending on which method and MIC breakpoints are used (CLSI or EUCAST). More pharmacodynamic and clinical trial data are required to validate the suitability of the current breakpoints for a wider range of species than we have examined.
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Allerberger F., Klare I. 1999. In-vitro activity of fosfomycin against vancomycin-resistant enterococci. J. Antimicrob. Chemother. 43:211–217 [DOI] [PubMed] [Google Scholar]
- 2. Barry A. L., Fuchs P. C. 1991. In vitro susceptibility testing procedures for fosfomycin tromethamine. Antimicrob. Agents Chemother. 35:1235–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barry A. L., Brown S. D. 1995. Antibacterial spectrum of fosfomycin trometamol. J. Antimicrob. Chemother. 35:228–230 [DOI] [PubMed] [Google Scholar]
- 4. British Society for Antimicrobial Chemotherapy 2010. BSAC methods for antimicrobial susceptibility testing. British Society for Antimicrobial Chemotherapy, Birmingham, United Kingdom: http://www.bsac.org.uk/Resources/BSAC/Version_9.1_March_2010_final%20v2.pdf Accessed 10 March 2011 [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A10, 10th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2009. Methods for dilution susceptibility tests for bacteria that grow aerobically; approved standard. M7-A8, 8th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, Pa [Google Scholar]
- 8. Clinical and Laboratory Standards Institute/NCCLS 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 2nd ed NCCLS document M23-A2. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 9. Comité de l'Antibiogramme de la Société Française de Microbiologie 2010. Comite de l'Antibiogramme de la Société Française de Microbiologie Recommandations 2010 (edition de Janvier 2010). http://www.sfm-microbiologie.org/UserFiles/file/casfm_2010.pdf Accessed 10 March 2011 (In French.)
- 10. Crocchiolo P. 1990. Single-dose fosfomycin trometamol versus multiple-dose cotrimoxazole in the treatment of lower urinary tract infections in general practice. Multicenter Group of General Practitioners. Chemotherapy 36(Suppl. 1):37–40 [DOI] [PubMed] [Google Scholar]
- 11. de Cueto M., Lopez L., Hernandez J. R., Morillo C., Pascual A. 2006. In vitro activity of fosfomycin against extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob. Agents Chemother. 50:368–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jong Z., Pontonnier F., Plante P. 1991. Single-dose fosfomycin trometamol (Monuril) versus multiple-dose norfloxacin: results of a multicenter study in females with uncomplicated lower urinary tract infections. Urol. Int. 46:344–348 [DOI] [PubMed] [Google Scholar]
- 13. Endimiani A., et al. 2010. In vitro activity of fosfomycin against blaKPC-producing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob. Agents Chemother. 54:526–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Committee on Antimicrobial Susceptibility Testing 13 December 2009. European Committee on Antimicrobial Susceptibility Testing. http://www.eucast.org Accessed 20 June 2010
- 15. Falagas M. E., Giannopoulou K. P., Kokolakis G. N., Rafailidis P. I. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 46:1069–1077 [DOI] [PubMed] [Google Scholar]
- 16. Falagas M. E., et al. 2008. Antimicrobial susceptibility of multidrug-resistant Gram negative bacteria to fosfomycin. Eur. J. Clin. Microbiol. Infect. Dis. 27:439–443 [DOI] [PubMed] [Google Scholar]
- 17. Falagas M. E., Kastoris A. C., Karageorgopoulos D. E., Rafailidis P. I. 2009. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int. J. Antimicrob. Agents 34:111–120 [DOI] [PubMed] [Google Scholar]
- 18. Falagas M. E., et al. 2009. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int. J. Antimicrob. Agents 35:240–243 [DOI] [PubMed] [Google Scholar]
- 19. Falagas M. E., Roussos N., Gkegkes I. D., Rafailidis P. I., Karageorgopoulos D. E. 2009. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: a review of microbiological, animal and clinical studies. Expert Opin. Invest. Drugs 18:921–944 [DOI] [PubMed] [Google Scholar]
- 20. Fuchs P. C., Barry A. L., Brown S. D. 1999. Fosfomycin tromethamine susceptibility of outpatient urine isolates of Escherichia coli and Enterococcus faecalis from ten North American medical centres by three methods. J. Antimicrob. Chemother. 43:137–140 [DOI] [PubMed] [Google Scholar]
- 21. García-Rodríguez J. A., et al. 1997. In vitro activity of fosfomycin trometamol against pathogens from urinary tract infections: a Spanish multicenter study. J. Chemother. 9:394–402 [DOI] [PubMed] [Google Scholar]
- 22. Jean S. S., Teng L. J., Hsueh P. R., Ho S. W., Luh K. T. 2002. Antimicrobial susceptibilities among clinical isolates of extended-spectrum cephalosporin-resistant Gram-negative bacteria in a Taiwanese university hospital. J. Antimicrob. Chemother. 49:69–76 [DOI] [PubMed] [Google Scholar]
- 23. Knottnerus B. J., et al. 2008. Fosfomycin tromethamine as second agent for the treatment of acute uncomplicated urinary tract infections in adult female patients in The Netherlands? J. Antimicrob. Chemother. 62:356–359 [DOI] [PubMed] [Google Scholar]
- 24. Ko K. S., et al. 2007. In vitro activity of fosfomycin against ciprofloxacin-resistant or extended-spectrum β-lactamase-producing Escherichia coli isolated from urine and blood. Diagn. Microbiol. Infect. Dis. 58:111–115 [DOI] [PubMed] [Google Scholar]
- 25. Kuo L. C., et al. 2008. Antimicrobial resistance of bacterial isolates from respiratory care wards in Taiwan: a horizontal surveillance study. Int. J. Antimicrob. Agents 31:420–426 [DOI] [PubMed] [Google Scholar]
- 26. Maraki S., et al. 2009. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob. Agents Chemother. 53:4508–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marchese A., Gualco L., Debbia E. A., Schito G. C., Schito A. M. 2003. In vitro activity of fosfomycin against gram-negative urinary pathogens and the biological cost of fosfomycin resistance. Int. J. Antimicrob. Agents 22(Suppl. 2):53–59 [DOI] [PubMed] [Google Scholar]
- 28. Martinez-Martinez L., Rodriguez G., Pascual A., Suarez A. I., Perea E. J. 1996. In-vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii. J. Antimicrob. Chemother. 38:1107–1108 [DOI] [PubMed] [Google Scholar]
- 29. Metzler C. M., DeHaan R. M. 1974. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J. Infect. Dis. 130:588–594 [DOI] [PubMed] [Google Scholar]
- 30. Neu H. C. 1990. Fosfomycin trometamol versus amoxycillin—single-dose multicenter study of urinary tract infections. Chemotherapy 36(Suppl. 1):19–23 [DOI] [PubMed] [Google Scholar]
- 31. Okazaki M., et al. 2002. Effectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assay. J. Infect. Chemother. 8:37–42 [DOI] [PubMed] [Google Scholar]
- 32. Olay T., Rodriguez A., Oliver L. E., Vicente M. V., Quecedo M. C. 1978. Interaction of fosfomycin with other antimicrobial agents: in vitro and in vivo studies. J. Antimicrob. Chemother. 4:569–576 [DOI] [PubMed] [Google Scholar]
- 33. Patel S. S., Balfour J. A., Bryson H. M. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infection. Drugs 53:637–656 [DOI] [PubMed] [Google Scholar]
- 34. Rodríguez-Baño J., et al. 2008. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897–1902 [DOI] [PubMed] [Google Scholar]
- 35. Shimizu M., et al. 2000. Novel fosfomycin resistance of Pseudomonas aeruginosa clinical isolates recovered in Japan in 1996. Antimicrob. Agents Chemother. 44:2007–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Superti S., Dias C. A. G., d'Azevedo P. A. 2009. In vitro fosfomycin activity in vancomycin-resistant Enterococcus faecalis. Braz. J. Infect. Dis. 13:123–124 [DOI] [PubMed] [Google Scholar]
- 37. Tan C. K., Liaw S. J., Yu C. J., Teng L. J., Hsueh P. R. 2008. Extensively drug-resistant Stenotrophomonas maltophilia in a tertiary care hospital in Taiwan: microbiologic characteristics, clinical features, and outcomes. Diagn. Microbiol. Infect. Dis. 60:205–210 [DOI] [PubMed] [Google Scholar]
- 38. Tessier F., Quentin C. 1997. In vitro activity of fosfomycin combined with ceftazidime, imipenem, amikacin, and ciprofloxacin against Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 16:159–162 [DOI] [PubMed] [Google Scholar]
- 39. Turnidge J., Kahlmeter G., Kronvall G. 2006. Statistical characterization of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12:418–425 [DOI] [PubMed] [Google Scholar]
- 40. Wachino J.-I., Yamane K., Suzuki. S., Kimura K., Arakawa Y. 2010. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob. Agents Chemother. 54:3061–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J. L., Hsueh P. R. 2009. Therapeutic options for infections due to vancomycin-resistant enterococci. Expert Opin. Pharmacother. 10:785–796 [DOI] [PubMed] [Google Scholar]